Abstract
The longitudinal muscle layer in gut is the functional opponent to the circular muscle layer during peristalsis. Differences in innervation of the layers allow for the contraction of one layer concurrently with the relaxation of the other, enabling the passage of gut contents in a controlled fashion. Differences in development have given the cells of the two layers differences in receptor populations, membrane lipid handling, and calcium handling profiles/behaviors. The contractile activity of the longitudinal muscle is largely mediated by cholinergic neural input from myenteric plexus. Activation of muscarinic receptors leads to rapid activation of several kinases including MLC kinase, ERK1/2, CaMKII and Rho kinase. Phosphorylation of myosin light chain (MLC20) by MLC kinase (MLCK) is a prerequisite for contraction in both circular and longitudinal muscle cells. In rat colonic longitudinal muscle strips, we measured muscarinic receptor-mediated contraction following incubation with kinase inhibitors. Basal tension was differentially regulated by Rho kinase, ERK1/2, CaMKII and CaMKK. Selective inhibitors of Rho kinase, ERK1/2, CaMKK/AMPK, and CaMKII each reduced carbachol-induced contraction in the innervated muscle strips. These inhibitors had no direct effect on MLCK activity. Thus unlike previously reported for isolated muscle cells where CaMKII and ERK1/2 are not involved in contraction, we conclude that the regulation of carbachol-induced contraction in innervated longitudinal muscle strips involves the interplay of Rho kinase, ERK1/2, CaMKK/AMPK, and CAMKII.
Keywords: smooth muscle, gastrointestinal tract, motility
Introduction
Gastrointestinal motility requires coordinated activity of the two muscular layers of the gut, the circular muscle layer and the longitudinal muscle layer (1,2,3,4). The circular muscle layer consists of muscle cells oriented around the circumference of the gut lumen, forming adjacent rings of muscle that extend throughout the gut. Upon local activation of these cells, the circular muscle tissue contracts, and these muscular rings decrease their diameter and therefore the caliber of the gut lumen. The longitudinal muscle layer is comprised of cells oriented along the long axis of the gut, parallel to the overall direction of the movement of contents. Upon local activation of the peristaltic reflex, contraction of the longitudinal muscle causes the gut to shorten while the relaxation of the circular muscle layer causes an increase in the diameter of the lumen. Thus, the two muscular layers are not normally activated at the same location simultaneously (5,6,7); however, when these opposing actions occur in the longitudinal and circular muscle layers at contiguous locations, the contents of the lumen can be propelled distally in a controlled fashion. Functional differences are evident between the two muscle layers and reflect distinct receptor expression, calcium handling, and signaling pathways involved in muscle contraction (7,8,9,10,11,12,13,14).
Muscle activity is driven by the interaction of the proteins actin and myosin, which facilitates the inherent ATPase activity of the heavy chains of myosin. The phosphorylation of the 20 kDa myosin regulatory light chain (MLC20) at a serine residue at position 19 is sufficient to cause cross-bridge formation and cycling, ATP hydrolysis, and muscle contraction (15, 16). Myosin light chain kinase (MLCK) is a dedicated serine-threonine kinase that has MLC20 as its sole substrate. Upon binding with the calcium-calmodulin complex, MLCK phosphorylates MLC20 at serine 19 (15, 16, 17). Myosin light chain phosphatase (MLCP) is the opposing enzyme to MLCK, removing the phosphate from Serine 19 on MLC20. It is a trimer composed of protein phosphatase 1c-delta, the myosin phosphatase target subunit 1 (MYPT1), and a small 20kDa subunit MP20. MYPT1 targets the phosphatase subunit to MLC20, while increasing the specificity of PP1c-delta through a conformational change that occurs when bound to MYPT1. The MP20 subunit is of unknown function (18, 19).
Many studies have shown that MLC20 phosphorylation can be regulated, either directly or through the regulation of MLCP activity and associated proteins, by several other kinases including Rho kinase, ERK1/2, CaMKII, PAK and CaMKK (20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35). This regulation is important during the agonist-induced sustained contraction as MLCK activity is transient and in synchronization with the transient nature of increase in Ca2+ levels (16, 36, 37). The activity of MLCK itself can be affected by its phosphorylation at several site(s); kinases that phosphorylate can increase or decrease MLCK activity, and may do so by either altering the rate of enzyme activity or the affinity of MLCK for Ca-CaM (38,39,40,41,42).
Our previous studies have shown that in both circular and longitudinal intestinal smooth muscle, contraction in response to G protein-coupled receptor agonists is biphasic (36, 37). However, the mechanisms that mobilize Ca2+ for initial contraction and regulate MLCP activity for sustained contraction are distinct in these types of muscle (8, 13). The aim of the present study is to characterize the role of various kinases that are activated in response to contractile agonists in the regulation of MLCK activity and muscle contraction in response to muscarinic receptor activation in innervated longitudinal muscle strips. Our results demonstrate a distinct pattern of regulation. Similar to studies in isolated muscle cells, inhibition of both Rho kinase and ERK1/2 leads to diminished contraction in innervated muscle strips, but unlike isolated cells, inhibition of CaMKK/AMPK and CaMKII also diminished contraction.
Materials and Methods
Materials
Y27632, PD98059, STO-609, and KN62 were purchased from Calbiochem (La Jolla, CA). 15% Tris-HCl Ready Gels and DC Protein Assay Kit were products of Bio-Rad (Hercules, CA), Myelin Basic Protein was purchased from Upstate Biotechnology (now Millipore, Billerica, MA) and [γ-32P]ATP from Perkin Elmer Life Sciences (Boston, MA). Antibody for MLCK and protein A/G agarose beads are products of Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Sprague-Dawley Rats were purchased from Charles River Laboratories and housed in the animal facility of the Division of Animal Resources, Virginia Commonwealth University. All procedures followed guidelines of and were in accordance with the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Animal Preparation
Rats were euthanized by CO2 asphyxiation under approved protocols. The colon was dissected out, emptied of contents and placed in a warmed (37 °C) oxygenated Krebs solution of the following composition (in mM): 118 NaCl, 4.75 KCl, 1.19 KH2PO4, 1.2 MgSO4, 2.54 CaCl2, 25 NaHCO3, 11 mM glucose (pH 7.4). 2–3 cm sections of colon were removed and threaded onto a glass rod, where the longitudinal muscle/myenteric plexus (LM-MP) was removed by radial abrasion with a lab wipe. The resultant strip of muscle was freed of excess fat and mesenteric attachments and held in oxygenated Krebs buffer until use for tension recording or molecular assay.
Muscle Strip Preparation
Strips destined for recordings of contractile behavior were tied at both ends with surgical silk so that one end had a simple loop for attachment to a glass hook and the other end had a length of silk tied to a brass ring. The strip was then placed in a vertical orientation with the loop secured to a glass hook and the brass ring to a Model FT03C Force Transducer (Grass Technologies, Quincy, MA). An organ bath (Radnoti, Monrovia, CA) was raised to submerge the strip in 5 mL of continuously oxygenated and warmed Krebs solution. Force recordings were amplified by a 15A12 model amplifier (contained within a Model 15LT Amplifier System), relayed to a PVA-16 Polyview Adaptor Unit (A/D-D/A converter), and displayed/stored by a PC running Polyview Version 1.3 (Grass Technologies, Quincy, MA). Force was recorded in grams.
Strips destined for molecular assay were treated as those intended for recordings and the time scales for inhibitor incubation and agonist exposure were identical. Following prescribed incubation times, tissue strips were submerged in a bath of Krebs with 1 µM carbachol. After 60 seconds of immersion in carbachol/Krebs (corresponding to a normal vigorous response seen in strips), strips were flash frozen in liquid nitrogen and placed into TPER (Tissue Protein Extraction Reagent, Pierce, Rockford, IL) or lysis buffer with composition 50 mM Tris-HCl (pH 7.5), 150 mM NaCl. 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 10 mM sodium pyrophosphate. In addition, a protease/phosphatase cocktail (100 µg/mL PMSF, 10 µg/mL leupeptin, 30 mM sodium fluoride and 3 mM sodium vanadate) was added at a concentration of 2 µL/mL. Tissue was homogenized and solubilized in the above solutions. Following centrifugation at 20000 g for 15 min at 4 °C, the protein concentration of the supernatant was assessed with a DC protein assay kit. These supernatant lysates were stored at –80 °C until needed for immunokinase assay.
Isometric force measurement
Force experiments were conducted in the following manner. Following hanging of the strip and submersion in the organ bath, strips were subjected to approximately 1 gram of pre-tension via the mounting rack-and-pinion. Strips were allowed to equilibrate for no less than 30 min before experiments were conducted and data collected. Exposure to inhibitors, blockers, and carbachol occurred within the organ bath. Concentrations were appropriate and in agreement with current literature and are noted in the results. Following an experiment, strip data were reviewed and analyzed from within the Polyview software suite. One way ANOVA and paired t-tests were conducted in GraphPad (GraphPad Software, La Jolla, CA), and significance set at P<0.05. All tests of significance were done by comparison of raw data between control and experimental groups.
Data Analysis
Contractile data was viewed from three perspectives as to the effects of kinase inhibition: changes to basal tone upon kinase inhibitor administration, peak contractile amplitude following agonist exposure, and as area under the curve measurement. The latter was used to quantify the contraction as viewed during the first two minutes of the contraction in an effort to determine differences in force development/decay. All numerical values are expressed as mean ± S.E.M.
Basal tension was measured as the mean tension during a 3 min period following at least 30 min of equilibration (control conditions) or 10 min of inhibitor incubation (basal recording obtained during the interval proceeding carbachol administration). Such measurements were made in multiple strips from multiple animals, and paired t-tests conducted to determine a significant effect. Peak contraction (amplitude) was defined to be the greatest amplitude of tone above basal during the two minute period following agonist administration. This two minute period is taken to begin when the contractile response began to rise from basal value. Area under curve (AUC) for first two minutes of exposure reflects the development and maintenance of the tension and was expressed as gram-seconds.
Kinase Assay
Activity of MLCK activity was measured by an immunokinase assay as described previously (37, 43, 44). One hundred µg of protein was transferred from the supernatant of prepared tissue sample to a designated tube, 1 µg of MLCK goat antibody was added, and this mixture incubated for 2 h. Protein A/G agarose beads were added to each sample, and the mixture again incubated at 4 °C overnight. Following centrifugation, supernatants were withdrawn and the beads/protein washed with lysis buffer three times. The bead/protein pellets were resuspended in kinase buffer with composition (in mM): 50 KH2PO4, 15 dithiothreitol (DTT), 10 NaF, 1 PMSF, 0.5% Triton X-100 and 10 µg/ml aprotinin. Twenty microliters of supernatant were added to a mixture containing (in mM) 0.1 Ca2+, 50 Mops, 15 DTT, 10 magnesium acetate, and 0.3 µM calmodulin and 18 µM smooth-muscle MLC20. The reaction was initiated with 1 mM [γ-32P]ATP. Aliquots were spotted on Whatman filter paper, rinsed successively with 75 mM phosphoric acid, 95% (v/v) ethanol and 100% (v/v) diethyl ether and were dried for measurement of radioactivity.
Results
Muscarinic m3 receptor-mediated contraction
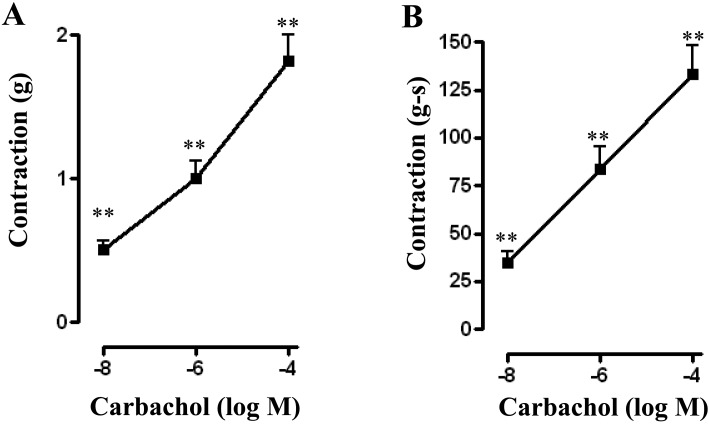
Longitudinal muscle strips exposed to carbachol (CCh) at concentrations of 10 nM, 1 µM, and 100 µM demonstrated contraction in a concentration-dependent manner and the maximal response was obtained with 100 µM of CCh. Peak contractile responses were 0.51 ± 0.06 grams at 10 nM CCh (n=40), 1.00 ± 0.12 grams at 1 µM CCh (n=50) and 1.80 ± 0.18 grams at 100 µM CCh (n=54). Contractile responses calculated as area under curve (AUC) for first 2 min were 34.44 ± 5.84 gram-seconds at 10 nM CCh (n=34), 83.62 ± 11.35 gram-seconds at 1 μM CCh (n=42), and 133.25 ± 14.67 gram-seconds at 100 μM CCh (n=45) (Fig. 1A and 1B).
Fig. 1.
Contractile response of longitudinal muscle strips to carbachol. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, different concentrations of carbachol (CCh, 10 nM to 100 µM) was added. Contractile response with maximum force was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Carbachol elicited dose-dependent increase in both peak and total contraction. Values are means ± SEM of 6–7 experiments and each experimental value was derived from several strips. **P<0.05 significant contraction.
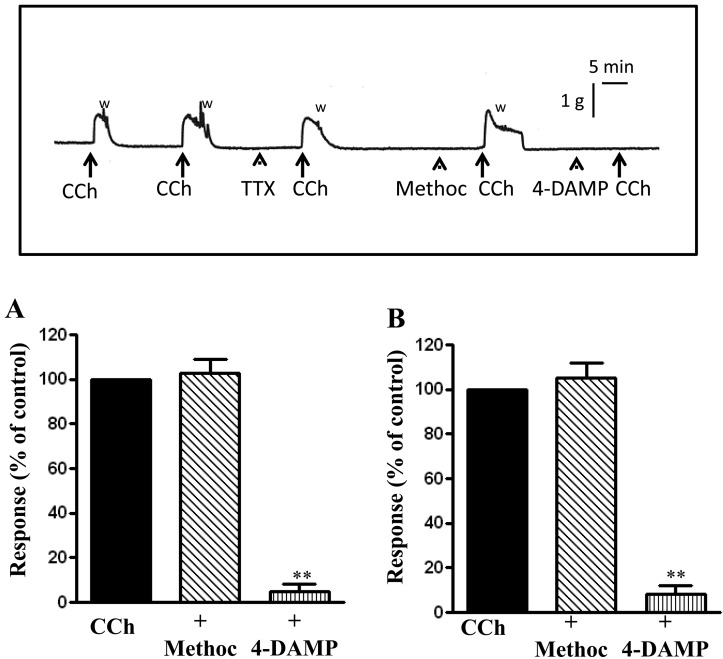
Repeated measurements of peak contraction and area under the curve in response to 1 µM CCh were conducted on strips following wash for 15 min in Krebs buffer and contractions were calculated as percentage of initial contraction before wash. There were no significant differences in either peak contraction or AUC with repeated measurements. Following a 15 min incubation after initial contraction, peak contraction was 98.29% ± 3.99% (n=13, 5 animals) and the AUC for 2 min was 102.1% ± 1.88% (n=13, 5 animals) of the initial contraction (Fig. 2A and 2B).
Fig. 2.
Contractile response to carbachol is mediated via m3 receptors. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with 1 µM of the m2 receptor antagonist methoctramine or 1 µM of the m3 receptor antagonist 4-DAMP and then with CCh (1 µM). Contractile response with maximum force was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Carbachol elicited contraction was selectively blocked by 4-DAMP. Values are means ± SEM of 4 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction. The inset illustrates an original tracing showing abolition of carbachol-induced contraction by 1 µM 4-DAMP but not by 1 µM tetrodotoxin (TTX) or 1 µM methoctramine. "W" indicates when the preparation was washed with fresh Kreb's buffer after peak contraction. Horizontal bar indicates time in minutes and the vertical bar illustrates force in grams.
Smooth muscle expresses both m2 and m3 muscarinic receptors (45,46,47,48). As shown previously in circular muscle, CCh-induced contraction in longitudinal muscle is mediated primarily by muscarinic m3 receptors. Muscle strips were pretreated with m2 receptor antagonist, methoctramine (1 µM) or m3 receptor antagonist 4-DAMP (1 µM) and the response to 1 µM CCh was measured. Incubation with methoctramine caused no significant changes to peak contraction or AUC in response to CCh (P>0.05, n=7 from 4 animals). Incubation with 4-DAMP, however, abolished responses to CCh (Fig. 2). These results indicate that contraction in response to CCh is mediated mainly by activation of m3 receptors.
To further examine the smooth muscle muscarinic m3 receptors, muscle strips were incubated with 1 μM tetrodotoxin (TTX), a Na+ channel blocker, and the contractile response to different concentrations of CCh was measured. Consistent with the blockade of CCh-induced contraction with m3 receptor antagonist, incubation of muscle strips with 1 μM tetrodotoxin (TTX), had no significant changes to peak contraction or AUC in response to different concentrations of CCh (P>0.05, n=7 from 4 animals). Peak contractions in the presence of TTX exposure were 102.8% ± 5.98% of the control value at 1 μM CCh (n=7), 113.2 ± 8.6% at 10 μM CCh (n=7), and 110.5 ± 9.1% at 100 μM CCh (n=7) and the AUC were 112.1 ± 7.3% of the control value at 1 µM, 110.4 ± 4.5% at 10 µM and 97.7 ± 8.3% at 100 µM CCh (n=7).
Regulation of CCh-induced contraction by kinases
Previous studies have shown that activation of m3 receptors leads to stimulation of various kinases such as Rho kinase, ERK1/2, CaMKII and AMPK and these kinases in turn, as shown in isolated cells, regulate phosphorylation of MLC20 levels (37, 43, 44, 49). To examine the role of these kinases, muscle strips were pre-incubated with inhibitors of Rho kinase (Y27632), ERK1/2 (PD98059), CaMKII (KN62) or CaMKK (STO-609) and the response to CCh was measured.
1) Regulation by Rho kinase
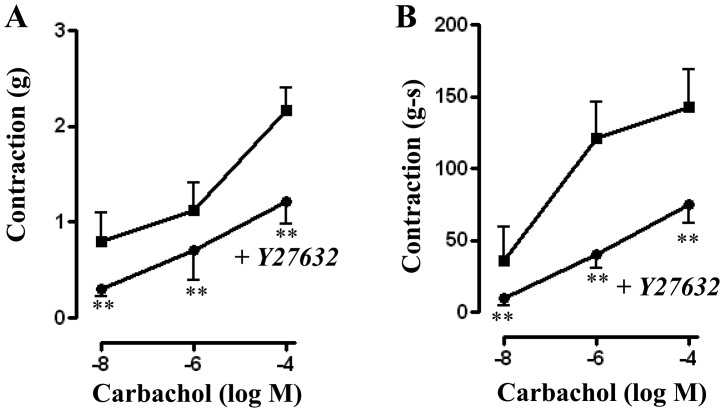
Treatment of muscle strips with Rho kinase inhibitor Y27632 (10 µM) for 10 min led to a significant decrease in basal tone from 0.82 ± 0.07 grams to 0.73 ± 0.06 grams (P<0.01, n=15). Y27632 also significantly reduced contraction in response to different concentrations of CCh. Peak contractions (in grams) for control versus with Y27632 were 0.80 ± 0.30 versus 0.30 ± 0.08 (P<0.05, n=4) at 10 nM CCh; 1.13 ± 0.28 versus 0.71 ± 0.19 (P<0.01, n=15) for 1 μM CCh; and 2.17 ± 0.38 versus 1.21 ± 0.23 (P<0.05, n=16) for 100 µM (Fig. 3A). A similar inhibition of contraction was also observed when AUC was calculated and the values (in gram-seconds) were: 35.54 ± 24.26 versus 9.50 ± 5.22 (P<0.05, n=3) for 10 nM CCh; 120.8 ± 25.15 versus 39.90 ± 9.42 (P<0.01, n=10) for 1 µM CCh; and 142.7 ± 26.03 versus 74.87 ± 13.31 (P<0.01, n=15) for 100 µM (Fig. 3B).
Fig. 3.
Effect of Rho kinase inhibitor, Y27632, on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective Rho kinase inhibitor Y27632 (10 µM) and then with different concentrations of CCh (10 nM to 100 µM). Contractile response with maximum force was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 4 experiments and each experimental value was derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
2) Regulation by ERK1/2
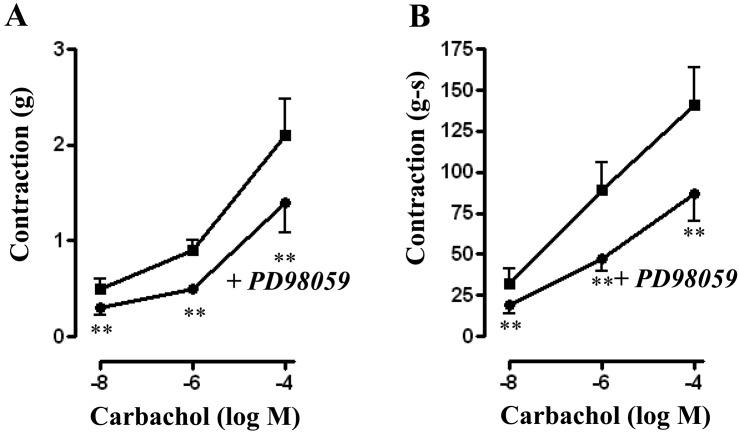
Treatment of muscle strips with ERK1/2 inhibitor PD98059 (10 µM) for 10 min had no significant effect on basal tone (0.79 ± 0.06 grams versus 0.81 ± 0.05 grams with PD98059). PD98059, however, significantly reduced contraction in response to different concentrations of CCh. Peak contractions (in grams) for control versus with PD98059 were 0.47 ± 0.13 versus 0.28 ± 0.08 (P<0.05, n=6) at 10 nM CCh; 0.86 ± 0.13 versus 0.49 ± 0.09 (P<0.01, n=9) for 1 μM CCh; and 2.06 ± 0.38 versus 1.40 ± 0.32 (P<0.001, n=9) for 100 µM (Fig. 4A). A similar inhibition of contraction was also observed when AUC was calculated and the values (in gram-seconds) were 31.22 ± 9.69 versus 18.78 ± 5.57 (P>0.05, n=7) for 10 nM CCh; 87.82 ± 17.61 versus 47.16 ± 6.56 (P<0.01, n=7) for 1 µM CCh; and 141.3 ± 23.86 versus 87.66 ± 16.69 (P<0.01, n=9) for 100 µM CCh (Fig. 4B).
Fig. 4.
Effect of ERK1/2 inhibitor PD98059 on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective ERK1/2 inhibitor PD98059 (10 µM) and then with different concentrations of CCh (10 nM to 100 µM). Contractile response with maximum force was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 4 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
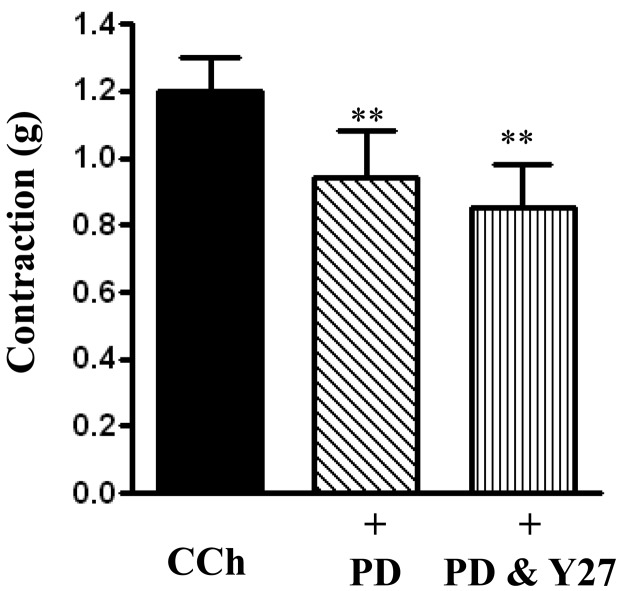
The inhibitory effect of Y27632 in combination with PD98059 was not significantly greater than the effect obtained with PD98059 alone: 1.17 ± 0.19 grams for 1 µM CCh, 0.96 ± 0.14 grams CCh plus PD98059 alone (P<0.05), 0.85 ± 0.13 grams for CCh plus PD98059 and Y27632 (P<0.05) (Fig. 5).
Fig. 5.
Effect of PD98059 and Y27632 in combination on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective ERK1/2 inhibitor PD98059 (10 µM) alone or in combination with Rho kinase inhibitor Y27632 (10 µM) and then with CCh (1 µM). Contractile response was measured as peak contraction. Values are means ± SEM of 4–7 experiments and each experimental value was derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
3) Regulation by CaMKK
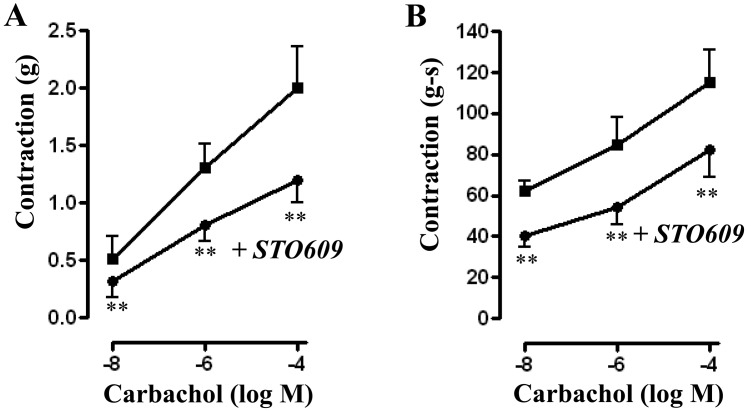
Treatment of muscle strips with CaMKK inhibitor STO-609 (10 µM) for 10 min had no significant effect on basal tone (0.76 ± 0.10 grams versus 0.74 ± 0.10 grams with STO-609). STO-609, however, significantly reduced contraction in response to different concentrations of CCh. Peak contractions (in grams) for control versus with STO-609 were 0.51 ± 0.16 versus 0.32 ± 0.14 (P<0.05, n=6) at 10 nM CCh; 1.31 ± 0.20 versus 0.81 ± 0.15 (P<0.01, n=23) for 1 μM CCh; and 1.96 ± 0.37 versus 1.22 ± 0.20 (P<0.05, n=16) for 100 µM (Fig. 6A). A similar inhibition of contraction was also observed when AUC was calculated and the values (in gram-seconds) were 62.64 ± 5.40 versus 40.46 ± 4.61 (P<0.01, n=7) for 10 nM CCh; 85.42 ± 13.77 versus 54.67 ± 7.92 (P<0.01, n=15) for 1 µM CCh; and 114.6 ± 16.23 versus 81.29 ± 13.01 (P<0.01, n=9) for 100 µM CCh (Fig. 6B).
Fig. 6.
Effect of CaMKK inhibitor, STO-609 on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective CaMKK inhibitor STO-609 (10 µM) and then with different concentrations of CCh (10 nM to 100 µM). Contractile response with maximum force was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 4–6 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
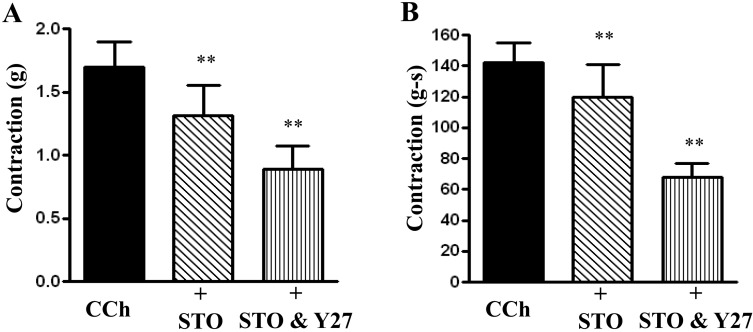
The inhibitory effect of Y27632 in combination with STO-609 was significantly greater than the effect obtained with STO-609. Peak contractions were 1.57 ± 0.32 grams for CCh alone, 1.31 ± 0.24 grams for CCh plus STO-609 (P<0.05), and 0.89 ± 0.18 grams for CCh plus STO-609 and Y27632 (Fig. 7A). For AUC the values (in grams-seconds) were 142.6 ± 13.86 for CCh alone, 122.4 ± 26.22 for CCh plus STO-609 (P<0.05, n=7), and 68.36 ± 9.72 for CCh plus STO-609 and Y27632 (P<0.05, n=7) (Fig. 7B). Thus, there is a significant (P<0.05 for both peak and AUC contraction) additive effect of CaMKK inhibition and Rho Kinase inhibition on both peak contraction and AUC.
Fig. 7.
Effect of STO-609 and Y27632 in combination on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective CaMKK inhibitor STO-609 (10 µM) alone or in combination with Rho kinase inhibitor Y27632 (10 µM) and then with CCh (1 µM). Contractile response was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 8 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
4) Regulation by CaMKII
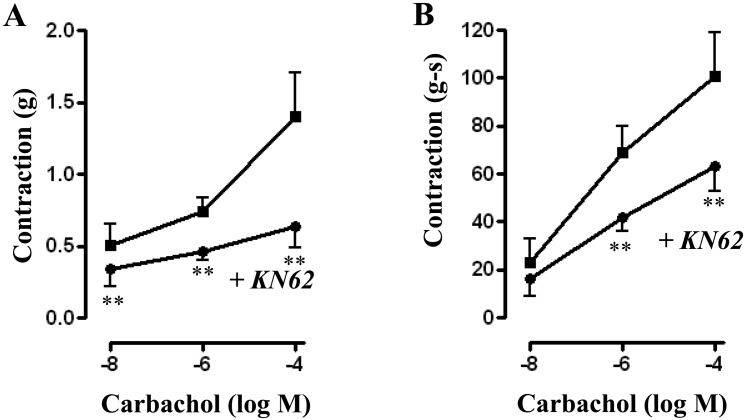
Treatment of muscle strips with CaMKII inhibitor KN-62 (10 µM) for 10 min caused a significant decrease in basal tone from 0.61 ± 0.07 grams to 0.53 ± 0.07 grams (P<0.05). KN-62 also significantly reduced contraction in response to different concentrations of CCh. Peak contractions (in grams) for control versus with KN62 were 0.52 ± 0.14 versus 0.34 ± 0.12 (P<0.05, n=7) at 10 nM CCh; 0.74 ± 0.10 versus 0.46 ± 0.05 (P<0.01, n=13) at 1 μM CCh; and 1.35 ± 0.32 versus 0.64 ± 0.15 (P<0.05, n=7) at 100 µM (Fig. 8A). A similar inhibition of contraction was also observed when AUC was calculated and the values (in gram-seconds) were 22.13 ± 10.10 versus 16.14 ± 7.43 for 10 nM; 69.38 ± 11.85 versus 42.11 ± 5.99 (P<0.05, n=12) for 1 µM; and 100.7 ± 18.58 versus 63.46 ± 10.27 (P<0.05, n=5) for 100 µM (Fig. 8B).
Fig. 8.
Effect of CaMKII inhibitor, KN62 on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective CaMKII inhibitor KN62 (10 µM) and then with different concentrations of CCh (10 nM to 100 µM). Contractile response was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 4 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
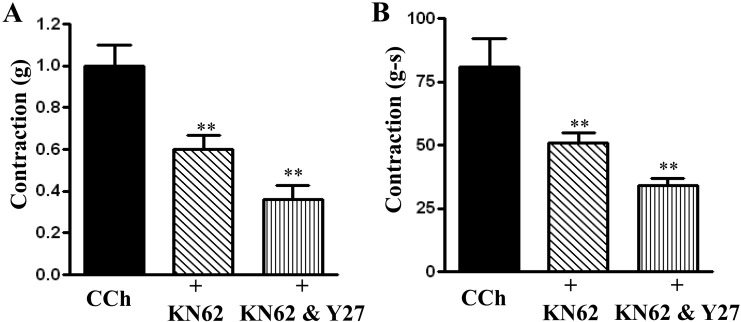
The inhibitory effect of Y27632 in combination with KN-62 was significantly greater than the effect obtained with KN-62 alone. Peak contractions were 0.92 ± 0.09 grams for CCh alone, 0.60 ± 0.05 grams for CCh plus KN-62 (P<0.05), and 0.36 ± 0.06 grams for CCh plus KN-62 and Y27632 (P<0.01) (Fig. 9A). For AUC the values (in grams-seconds) were 80.97 ± 11.84 for CCh alone, 51.43 ± 4.00 for CCh plus KN-62 (P<0.05, n=8), and 34.70 ± 3.18 for CCh plus KN-62 and Y27632 (P<0.05, n=8) (Fig. 9B). Thus, there is a significant (P<0.05 for both peak and AUC contraction) additive effect of CaMKII inhibition and Rho Kinase inhibition on both peak contraction and AUC.
Fig. 9.
Effect of KN62 and Y27632 in combination on carbachol-induced contraction. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min with the selective CaMKII inhibitor KN62 (10 µM) alone or in combination with Rho kinase inhibitor Y27632 (10 µM) and then with CCh (1 µM). Contractile response was measured as peak contraction (A) and total response for first 2 min, measured as area under curve (AUC), was considered as total contraction (B). Values are means ± SEM of 8 experiments and each experimental value derived from several strips. **P<0.05 significant inhibition of CCh-induced contraction.
5) Effect of kinase inhibitors on MLCK activity
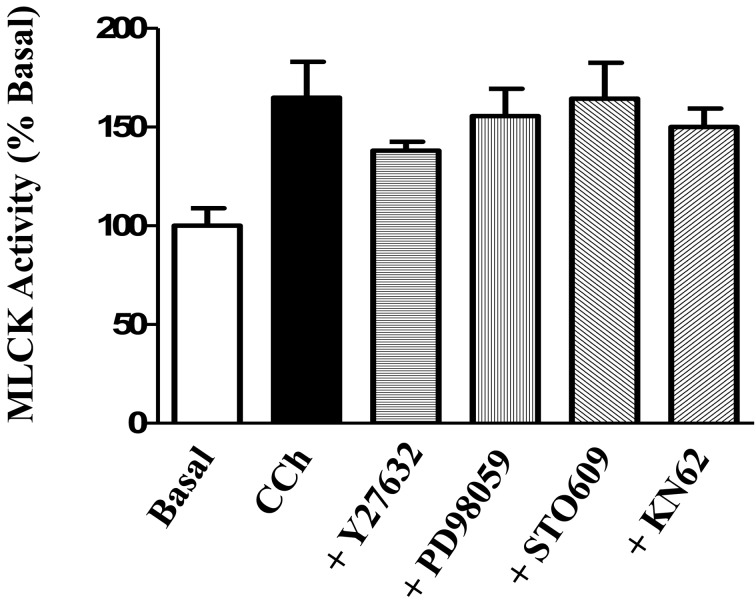
The effect of each kinase inhibitor was examined to determine if it had a direct effect on MLCK activity induced by 1 µM CCh. CCh caused a significant increase in MLCK activity (65 ± 18% above basal). There was no significant effect of any of the kinase inhibitors on the MLCK kinase activity induced by 1 µM CCh (P>0.05 vs 1 µM CCh alone for each kinase) (Fig. 10).
Fig. 10.
Effect of kinase inhibitors on carbachol-induced MLCK activity. Longitudinal muscle strips of rat colon were placed in an organ bath and subjected to 1 g of basal tension. After 30 min of equilibration, strips were incubated for 15 min in the absence of any agonist or inhibitor (Basal), in the presence of 1 µM carbachol (CCh) alone, or in the presence of a selective inhibitor plus 1 µM CCh. Separate strips were used to test the effect of the selective Rho kinase inhibitor Y27632 (10 µM), the selective ERK1/2 inhibitor PD98059 (10 µM), the selective CaMKK inhibitor STO-609 (10 µM) and the selective CaMKII inhibitor KN-62 (10 µM). Strips were flash frozen in liquid nitrogen and MLCK activity was measured as described in the methods using MLC20 as substrate and [32P]ATP. MLCK activity was measured as cpm and values are expressed as percent of basal activity. Values are means ± SEM of 7–13 experiments and each experimental value derived from several strips. CCh significantly increased (P<0.05 vs basal) MLCK activity in the presence and absence of each inhibitor. The effect of CCh on MLCK activity was not significantly inhibited (P>0.05 vs CCh) in the presence of any of the inhibitors.
Discussion
Gastrointestinal motility is mediated by the coordinated contractile activity of smooth muscle cells of both circular and longitudinal muscle layer. The main excitatory neurotransmitters in the gastrointestinal tract are acetylcholine and substance P (1,2,3). This study demonstrated that contraction in longitudinal muscle strips, both peak contraction and total contraction (measured as AUC), in response to the acetylcholine mimetic, carbachol, was solely dependent on activation of m3 receptors. Similar m3 receptor-dependent contraction was also reported in smooth muscle from circular muscle layer (37). The results are consistent with the concept that m3 receptors are known to play a dominant role in eliciting smooth muscle contraction and m2 receptors are considered to play a minor role despite their abundant expression (45,46,47,48, 50). Physiologically in vivo activation of the m2 receptor augments smooth muscle contractions mediated by m3 receptors. This is consistent with the concept of the conditional role of the m2 receptors in the smooth muscle (45, 46). Studies by Unno et al. (48), using m2 and m3 receptor knockout mice and pertussis toxin (PTx) to block m2-mediated contractions, have demonstrated that both m2 and m3 receptor activation induces ileal muscle contraction and the contribution of m2 receptors to contraction depends on the concentration of carbachol; at less than 1 µM carbachol, nearly 80% of the contractions are PTx sensitive and at concentrations more than 10 µM carbachol, PTx had no significant effect suggesting that the contribution of m2 receptors to CCh-induced contraction is significant only at low CCh concentrations and decreases with increasing concentrations of CCh. The notion that the effect of CCh in innervated longitudinal muscle strips could be due to activation of neuronal receptors was excluded as blockade of neuronal activation with tetrodotoxin had no effect on CCh-induced peak and total contraction.
Previous studies in isolated muscle cells from circular and longitudinal muscle layer have shown in circular muscle that treatment with CCh induced activation of Rho kinase downstream of RhoA, although the upstream mechanism of RhoA are distinct in circular versus longitudinal muscle cells. M3 receptors are coupled to G12 to activate RhoA via RhoGEF, LARG in longitudinal muscle cells, whereas m3 receptors are coupled to G13 to activate RhoA via RhoGEF, p116RhoGEF in circular muscle cells (37, 43, 44). One of the downstream targets of RhoA is serine/threonine kinase Rho kinase, which plays an important role in the regulation of sustained contraction. In vivo studies demonstrated the phosphorylation at Thr696/853 of MYPT1, the regulatory subunit of MLCP, and in vitro studies demonstrated phosphorylation at Thr38 of CPI-17, an endogenous inhibitor of MLCP; phosphorylation of both substrates leads to inhibition of MLCP activity and an increase in MLC20 phosphorylation and muscle contraction (18,19,20, 51). Inhibition of both basal tone and CCh-induced peak and total contraction by blockade of Rho kinase with Y27632 supports the role of Rho kinase in not only maintenance of tone but also agonist-induced contraction and may reflect stimulation of basal and disinhibition of agonist-induced inhibition of MLCP activity. Studies by Hagerty et al., offers an alternative explanation whereby Rho kinase increases the activity of ZIP kinase, a putative MLC kinase (52). This is supported by Ihara and MacDonald, who demonstrated a direct phosphorylation of MLC20 by ZIP kinase as well as phosphorylation of MYPT1 by ZIP kinase, both lead to increased contraction (53). A direct phosphorylation of MLC20 by Rho kinase on MLC20 has also been demonstrated in in vitro studies (24). Regulation of multiple proteins involved in the regulation of MLCP by Rho kinase is also indicative of a stronger inhibitory effect of Y27632 on total contraction than peak contraction. It is noteworthy that as compared to inhibition of other kinases, inhibition of Rho kinase resulted in greater inhibition of peak and total contraction. The effect of Y27632 on high K+-induced smooth muscle contraction was demonstrated in several studies. In rat thoracic aorta and mesenteric artery, inhibition of K+-induced contraction by Y27632 was attributed to disruption of actin filament network, but not to changes in MLCK and MLCP activities (54). In rat caudal artery, Y27632 had no effect on K+-induced increase in Ca2+, but abolished sustained contraction (55). In chicken gizzard, a typical phasic muscle, inhibition of K+-induced contraction was not significant even in the presence of 10 µM of Y27632 (56). These results suggest that the effect of Y27632 is variable depending on the species and muscle type. The variable responses of Y-27632 on high K+-induced contraction may be also caused by expression of Ca2+-dependent PI3 kinase that mediates RhoA activation (57).
Extracellular signal-regulated kinases (ERK1/2) play an important role in the regulation of smooth muscle contraction via phosphorylation of caldesmon or regulation of kinases such as MLCK or CaMKII (21, 58,59,60). The involvement of ERK1/2 was shown to be dependent on the type of smooth muscle and agonist. In rat aorta, PD98059 had no effect on K+-induced contraction, but inhibited lysophosphtidylcholine-induced augmentation of K+-induced contraction (61). In lower esophageal sphincter of rat or cat, PD98059 reduced agonist-induced contraction (62, 63). In swine carotid arteries, PD98059 had no effect on histamine-induced contraction (64), whereas in ferret aorta PD98059 had no effect on K+-induced contraction, but significantly inhibited phenylephrine-induced contraction in the absence, but not in the presence of extracellular Ca2+ (59). Activation of ERK1/2 in response to CCh has been demonstrated previously in muscle cells isolated from circular and longitudinal muscle layers; in both cell types activation of ERK1/2 induces stimulation of cytosolic phospholipase A2 (cPLA2) activity, which plays an important role in Ca2+ mobilization mechanisms in longitudinal muscle cells (8, 13, 49, 65). Ca2+ mobilization in these muscle cells is mediated by activation of cPLA2 and generation of arachidonic acid (AA), leading to Ca2+ influx and stimulation of cyclic ADP ribose (cADPR); both Ca2+ and cADPR act in concert to induce Ca2+ release via ryanodine receptors (RYR2)/Ca2+ channels (9, 13). Inhibition of ERK1/2 activity caused reduction in both peak and total contraction and the degree of inhibition second only to Rho kinase inhibition. Like Rho kinase, ERK1/2 targets multiple proteins to regulate MLC20 phosphorylation and contraction. Previous studies have shown that activation of integrin-linked kinase, another putative MLC kinase, and MYPT1 kinase is dependent on ERK1/2 (28, 60), suggesting that ERK1/2 can regulate contraction either as MLC kinase or an inhibition of MLCP activity. A direct stimulatory effect of ERK1/2 on MLCK was also demonstrated (22). Inhibition of both Rho kinase and ERK1/2 had no additive effect suggesting that the pathways activated by Rho kinase and ERK1/2 to regulate MLC20 phosphorylation and contraction may not line in parallel.
Previous studies in muscle cells isolated from circular and longitudinal muscle layers show that MLCK activity in response to muscarinic receptor activation is negatively regulated via phosphorylation of MLCK at Ser815 by AMPK, which is activated downstream of CaMKKβ upon phosphorylation at Thr172 (32, 43). STO609, an inhibitor of CaMKKβ (66), blocked muscarinic receptor-induced phosphorylation of AMPK and MLCK resulting in attenuation of AMPK activity and augmentation of MLCK activity, MLC20 phosphorylation and muscle contraction in circular muscle. In contrast, in longitudinal strips as shown in the present study, inhibition of CaMKK activity was found to reduce peak and total contraction but had no effect on MLCK activity. The distinct role of CaMKKβ in the regulation of MLCK activity and muscle contraction in the innervated muscle strips compared to isolated muscle awaits further work.
Inhibition of another Ca2+/CaM-dependent enzyme CaMKII also reduced CCh-induced peak and total contraction. These results are also in conflict with the previous studies in tracheal smooth muscle demonstrating that CaMKII phosphorylates MLCK and inhibits its activity by decreasing affinity for Ca2+/CaM (38). Kim et al, however, demonstrated direct phosphorylation of MLC20 with CaMKII activation in ferret aorta. CaMKII also has positive modulatory effects on Ca2+ channels that may lead to increase in contraction. Another possibility could be inhibition of ERK1/2 activity and ERK1/2-dependent contractile process as CaMKII was shown to be an upstream effector of ERK1/2 (58, 67). Thus blockade of CaMKII would affect contraction in a manner similar to ERK1/2 inhibition.
Award
DMK was supported by an Institutional Research and Academic Career Development Award (K12GM093857) to Virginia Commonwealth University from the National Institute of General Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by grants from the National Institutes of Diabetes, and Digestive and Kidney Diseases DK34153 (JRG) and DK28300 (KSM).
References
- 1.Pandol SJ, Raybould HE, Hal FY. Textbook of gastroenterology. Hoboken: Blackwell; 2009. Integrative responses of the gastrointestinal tract and liver to a meal; p. 3–14. [Google Scholar]
- 2.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008; 42(5): 610–9. doi: 10.1097/MCG.0b013e31816b444d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunze WAA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999; 61: 117–42. doi: 10.1146/annurev.physiol.61.1.117 [DOI] [PubMed] [Google Scholar]
- 4.Brookes SJ, Song ZM, Steele PA, Costa M. Identification of motor neurons to the longitudinal muscle of the guinea pig ileum. Gastroenterology. 1992; 103(3): 961–73. doi: 10.1016/0016-5085(92)90030-3 [DOI] [PubMed] [Google Scholar]
- 5.Grider JR. Regulation of excitatory neural input to longitudinal intestinal muscle by myenteric interneurons. Am J Physiol. 1998; 275(5 Pt 1): G973–8. [DOI] [PubMed] [Google Scholar]
- 6.Grider JR. Reciprocal activity of longitudinal and circular muscle during intestinal peristaltic reflex. Am J Physiol Gastrointest Liver Physiol. 2003; 284(5): G768–75. doi: 10.1152/ajpgi.00384.1998 [DOI] [PubMed] [Google Scholar]
- 7.Young HM. On the outside looking in: longitudinal muscle development in the gut. Neurogastroenterol Motil. 2008; 20(5): 431–3. doi: 10.1111/j.1365-2982.2008.01127.x [DOI] [PubMed] [Google Scholar]
- 8.Kuemmerle JF, Murthy KS, Makhlouf GM. Longitudinal smooth muscle of the mammalian intestine. A model for Ca2+ signaling by cADPR. Cell Biochem Biophys. 1998; 28(1): 31–44. doi: 10.1007/BF02738308 [DOI] [PubMed] [Google Scholar]
- 9.Kuemmerle JF, Murthy KS, Makhlouf GM. Agonist-activated, ryanodine-sensitive, IP3-insensitive Ca2+ release channels in longitudinal muscle of intestine. Am J Physiol. 1994; 266(5 Pt 1): C1421–31. [DOI] [PubMed] [Google Scholar]
- 10.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol. 1996; 50(4): 870–7. [PubMed] [Google Scholar]
- 11.Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor-mediated signaling in smooth muscle. Activation of phospholipase C-beta3 by Gbetagamma and inhibition of adenylyl cyclase by Galphai1 and Galphao. J Biol Chem. 1996; 271(38): 23458–63. doi: 10.1074/jbc.271.38.23458 [DOI] [PubMed] [Google Scholar]
- 12.Misra S, Mahavadi S, Grider JR, Murthy KS. Differential expression of Y receptors and signaling pathways in intestinal circular and longitudinal smooth muscle. Regul Pept. 2005; 125(1-3): 163–72. doi: 10.1016/j.regpep.2004.08.020 [DOI] [PubMed] [Google Scholar]
- 13.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006; 68: 345–74. doi: 10.1146/annurev.physiol.68.040504.094707 [DOI] [PubMed] [Google Scholar]
- 14.Murthy KS, Grider JR, Makhlouf GM. InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol. 1991; 261(6 Pt 1): G937–44. [DOI] [PubMed] [Google Scholar]
- 15.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001; 276(7): 4527–30. doi: 10.1074/jbc.R000028200 [DOI] [PubMed] [Google Scholar]
- 16.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 17.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008; 135(2): 610–20. doi: 10.1053/j.gastro.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: Many roles in cell function. Biochem Biophys Res Commun. 2008; 369(1): 149–56. doi: 10.1016/j.bbrc.2007.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004; 279(36): 37211–4. doi: 10.1074/jbc.R400018200 [DOI] [PubMed] [Google Scholar]
- 20.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997; 410(2-3): 356–60. doi: 10.1016/S0014-5793(97)00657-1 [DOI] [PubMed] [Google Scholar]
- 21.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005; 288(5): G849–53. doi: 10.1152/ajpgi.00530.2004 [DOI] [PubMed] [Google Scholar]
- 22.Morrison DL, Sanghera JS, Stewart J, Sutherland C, Walsh MP, Pelech SL. Phosphorylation and activation of smooth muscle myosin light chain kinase by MAP kinase and cyclin-dependent kinase-1. Biochem Cell Biol. 1996; 74(4): 549–57. doi: 10.1139/o96-459 [DOI] [PubMed] [Google Scholar]
- 23.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006; 291(5): G830–7. doi: 10.1152/ajpgi.00130.2006 [DOI] [PubMed] [Google Scholar]
- 24.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996; 271(34): 20246–9. doi: 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- 25.Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000; 475(3): 197–200. doi: 10.1016/S0014-5793(00)01654-9 [DOI] [PubMed] [Google Scholar]
- 26.Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey JA, Singer HA, Morgan KG. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol. 2000; 526(Pt 2): 367–74. doi: 10.1111/j.1469-7793.2000.00367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald JA, Eto M, Borman MA, Brautigan DL, Haystead TA. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 2001; 493(2-3): 91–4. doi: 10.1016/S0014-5793(01)02277-3 [DOI] [PubMed] [Google Scholar]
- 28.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J. 2002; 367(Pt 2): 517–24. doi: 10.1042/bj20020522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murányi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J. 2002; 366(Pt 1): 211–6. doi: 10.1042/bj20020401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J. 2006; 396(1): 193–200. doi: 10.1042/BJ20051772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca(2+)-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem. 2001; 276(31): 29567–74. doi: 10.1074/jbc.M102753200 [DOI] [PubMed] [Google Scholar]
- 32.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008; 283(27): 18505–12. doi: 10.1074/jbc.M802053200 [DOI] [PubMed] [Google Scholar]
- 33.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004; 279(33): 34496–504. doi: 10.1074/jbc.M405957200 [DOI] [PubMed] [Google Scholar]
- 34.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009; 75(5): 1031–41. doi: 10.1124/mol.108.049858 [DOI] [PubMed] [Google Scholar]
- 35.Cain AE, Tanner DM, Khalil RA. Endothelin-1--induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension. 2002; 39(2 Pt 2): 543–9. doi: 10.1161/hy0202.103129 [DOI] [PubMed] [Google Scholar]
- 36.Murthy KS, Grider JR, Kuemmerle JF, Makhlouf GM. Sustained muscle contraction induced by agonists, growth factors, and Ca(2+) mediated by distinct PKC isozymes. Am J Physiol Gastrointest Liver Physiol. 2000; 279(1): G201–10. [DOI] [PubMed] [Google Scholar]
- 37.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J. 2003; 374(Pt 1): 145–55. doi: 10.1042/bj20021274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca(2+)-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem. 1994; 269(13): 9912–20. [PubMed] [Google Scholar]
- 39.Hashimoto Y, Soderling TR. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+/calmodulin-dependent protein kinase II: comparative study of the phosphorylation sites. Arch Biochem Biophys. 1990; 278(1): 41–5. doi: 10.1016/0003-9861(90)90228-Q [DOI] [PubMed] [Google Scholar]
- 40.Conti MA, Adelstein RS. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3′:5′ cAMP-dependent protein kinase. J Biol Chem. 1981; 256(7): 3178–81. [PubMed] [Google Scholar]
- 41.Nishikawa M, Shirakawa S, Adelstein RS. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J Biol Chem. 1985; 260(15): 8978–83. [PubMed] [Google Scholar]
- 42.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem. 2000; 275(24): 18366–74. doi: 10.1074/jbc.M001339200 [DOI] [PubMed] [Google Scholar]
- 43.Nalli AD, Kumar DP, Mahavadi S, Al-Shboul O, Alkahtani R, Kuemmerle JF, Grider JR, Murthy KS. Hypercontractility of intestinal longitudinal smooth muscle induced by cytokines is mediated by the nuclear factor-κB/AMP-activated kinase/myosin light chain kinase pathway. J Pharmacol Exp Ther. 2014; 350(1): 89–98. doi: 10.1124/jpet.113.212522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Shboul O, Nalli AD, Kumar DP, Zhou R, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Jun kinase-induced overexpression of leukemia-associated Rho GEF (LARG) mediates sustained hypercontraction of longitudinal smooth muscle in inflammation. Am J Physiol Cell Physiol. 2014; 306(12): C1129–41. doi: 10.1152/ajpcell.00021.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehlert FJ, Thomas EA, Gerstin EH, Griffin MT. Muscarinic receptor subtypes in smooth muscle. Boca Raton, CRC Press; 1997. Muscarinic Receptors and Gastrointestinal Smooth Muscle; p. 87–145. [Google Scholar]
- 46.Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 2003; 74(2-3): 355–66. doi: 10.1016/j.lfs.2003.09.023 [DOI] [PubMed] [Google Scholar]
- 47.Unno T, Matsuyama H, Sakamoto T, Uchiyama M, Izumi Y, Okamoto H, Yamada M, Wess J, Komori S. M(2) and M(3) muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol. 2005; 146(1): 98–108. doi: 10.1038/sj.bjp.0706300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stengel PW, Yamada M, Wess J, Cohen ML. M(3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol. 2002; 282(5): R1443–9. doi: 10.1152/ajpregu.00486.2001 [DOI] [PubMed] [Google Scholar]
- 49.Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol. 2003; 284(3): G472–80. doi: 10.1152/ajpgi.00345.2002 [DOI] [PubMed] [Google Scholar]
- 50.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002; 22(24): 10627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori D, Hori M, Murata T, Ohama T, Kishi H, Kobayashi S, Ozaki H. Synchronous phosphorylation of CPI-17 and MYPT1 is essential for inducing Ca(2+) sensitization in intestinal smooth muscle. Neurogastroenterol Motil. 2011; 23(12): 1111–22. doi: 10.1111/j.1365-2982.2011.01799.x [DOI] [PubMed] [Google Scholar]
- 52.Hagerty L, Weitzel DH, Chambers J, Fortner CN, Brush MH, Loiselle D, Hosoya H, Haystead TA. ROCK1 phosphorylates and activates zipper-interacting protein kinase. J Biol Chem. 2007; 282(7): 4884–93. doi: 10.1074/jbc.M609990200 [DOI] [PubMed] [Google Scholar]
- 53.Ihara E, MacDonald JA. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can J Physiol Pharmacol. 2007; 85(1): 79–87. doi: 10.1139/y06-103 [DOI] [PubMed] [Google Scholar]
- 54.Sakamoto K, Hori M, Izumi M, Oka T, Kohama K, Ozaki H, Karaki H. Inhibition of high K+-induced contraction by the ROCKs inhibitor Y-27632 in vascular smooth muscle: possible involvement of ROCKs in a signal transduction pathway. J Pharmacol Sci. 2003; 92(1): 56–69. doi: 10.1254/jphs.92.56 [DOI] [PubMed] [Google Scholar]
- 55.Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J. 2002; 364(Pt 2): 431–40. doi: 10.1042/bj20020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anabuki J, Hori M, Hayakawa K, Akahane S, Ozaki H, Karaki H. Muscarinic stimulation does not induce rhoA/ROCK-mediated Ca2+ sensitization of the contractile element in chicken gizzard smooth muscle. Pflugers Arch. 2000; 441(2-3): 189–99. doi: 10.1007/s004240000425 [DOI] [PubMed] [Google Scholar]
- 57.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003; 93(6): 548–56. doi: 10.1161/01.RES.0000090998.08629.60 [DOI] [PubMed] [Google Scholar]
- 58.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res. 2005; 97(6): 541–9. doi: 10.1161/01.RES.0000182630.29093.0d [DOI] [PubMed] [Google Scholar]
- 59.Dessy C, Kim I. Sougnez, Laporte R, Morgan KG. A role for MAP kinase in differentiated smooth muscle contraction evoked by α-adrenoreceptor stimulation. Am J Physiol Cell Physiol. 1998; 275(4): C1081–6. [DOI] [PubMed] [Google Scholar]
- 60.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005; 288(3): G407–16. doi: 10.1152/ajpgi.00398.2004 [DOI] [PubMed] [Google Scholar]
- 61.Suenaga H, Kamata K. Lysophosphatidylcholine activates extracellular-signal-regulated protein kinase and potentiates vascular contractile responses in rat aorta. J Pharmacol Sci. 2003; 92(4): 348–58. doi: 10.1254/jphs.92.348 [DOI] [PubMed] [Google Scholar]
- 62.Cao W, Sohn UD, Bitar KN, Behar J, Biancani P, Harnett KM. MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003; 285(1): G86–95. doi: 10.1152/ajpgi.00156.2002 [DOI] [PubMed] [Google Scholar]
- 63.Puri RN, Fan YP, Rattan S. Role of pp60(c-src) and p(44/42) MAPK in ANG II-induced contraction of rat tonic gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2002; 283(2): G390–9. doi: 10.1152/ajpgi.00025.2002 [DOI] [PubMed] [Google Scholar]
- 64.Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinase does not alter smooth muscle contraction in swine carotid artery. Am J Physiol. 1998; 275(1 Pt 2): H131–8. [DOI] [PubMed] [Google Scholar]
- 65.Murthy KS, Kuemmerle JF, Makhlouf GM. Agonist-mediated activation of PLA2 initiates Ca2+ mobilization in intestinal longitudinal smooth muscle. Am J Physiol. 1995; 269(1 Pt 1): G93–102. [DOI] [PubMed] [Google Scholar]
- 66.Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem. 2002; 277(18): 15813–8. doi: 10.1074/jbc.M201075200 [DOI] [PubMed] [Google Scholar]
- 67.Lu KK, Armstrong SE, Ginnan R, Singer HA. Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005; 289(5): C1343–50. doi: 10.1152/ajpcell.00064.2005 [DOI] [PubMed] [Google Scholar]