Abstract
Objective
Periodontal infections have been linked to cardiovascular disease, including atherosclerosis, and systemic inflammation has been proposed as a possible mediator. Secretory phospholipase A2 (s-PLA2) and Lipoprotein-associated PLA2 (Lp-PLA2) are inflammatory enzymes associated with athero-sclerosis. No data are available on the association between oral microbiota and PLA2s. We studied whether a relationship exists between periodontal microbiota and the activities of these enzymes.
Methods
The Oral Infection and Vascular Disease Epidemiology Study (INVEST) collected subgingival biofilms and serum samples from 593 dentate men and women (age 68.7 ± 8.6 years). 4561 biofilm samples were collected in the two most posterior teeth of each quadrant (average 7/participant) for quantitative assessment of 11 bacterial species using DNA–DNA checkerboard hybridization. Mean concentration of s-PLA2 and activities of s-PLA2 and Lp-PLA2 were regressed on tertiles of etiologic dominance (ED). ED is defined as the level of presumed periodontopathic species/combined level of all eleven species measured, and represents the relative abundance of periodontopathic organisms. Analyses were adjusted for age, sex, race/ethnicity, education, smoking, BMI, diabetes, LDL cholesterol and HDL cholesterol, and systolic blood pressure.
Results
Higher levels of s-PLA2 activity were observed across increasing tertiles of etiologic dominance (0.66 ± 0.04 nmol ml−1 min−1, 0.73 ± 0.04 nmol ml−1 min−1, 0.89 ± 0.04 nmol ml−1 min−1; p < 0.001), with also a trend of association between Lp-PLA2 activity and ED (p = 0.07), while s-PLA2 concentration was unrelated to ED.
Conclusion
Increasingly greater s-PLA2 activity at higher tertiles of etiologic dominance may provide a mechanistic explanatory link of the relationship between periodontal microbiota and vascular diseases. Additional studies investigating the role of s-PLA2 are needed.
Keywords: Periodontitis, Phospholipases, Cardiovascular diseases, Atherosclerosis
1. Introduction
Chronic systemic inflammation has been repeatedly shown to predict incident clinical cardiovascular disease [35,28] which is possibly due to the involvement of inflammatory cells in the initiation and progression of atherosclerosis [25,1].
Human A2 phospholipases (including Secretory phospholipase A2 (s-PLA2) and Lipoprotein-associated phospholipase A2 (Lp-PLA2)) propagate inflammation by catalyzing the hydrolysis of glycerophospholipids, producing non-esterified fatty acids such as arachidonic acid. s-PLA2 is also expressed in the normal arterial wall, and its expression is readily up-regulated by inflammatory stimuli, suggesting a potential role for s-PLA2 in the early phases of the vessel response to injury [12]. Lp-PLA2 hydrolyzes oxidised phospholipids to yield pro-inflammatory products that are implicated in inflammatory responses in the arterial intima, and consequently vascular dysfunctions and diseases. Numerous studies have explored the role of s-PLA2 and Lp-PLA2 as risk markers of coronary diseases [35,16,18], and carotid artery disease [29].
Risk factors for elevated s-PLA2 and Lp-PLA2 are not well understood. One possible inflammatory trigger is adverse microbial exposures such as those commonly encountered in periodontal disease. Periodontal disease is a bacterially induced inflammatory disease of the soft and hard tissues supporting the tooth root. Sustained pathogenic periodontal bacterial infections can directly influence systemic inflammation and subclinical atherosclerotic development [9,8], Randomized controlled trials have demonstrated that anti-infective periodontal interventions can reduce systemic inflammation and improve vascular endothelial function [36,19,23].
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) was designed to explore the contribution of periodontal infections to atherosclerosis and subsequent vascular disease. We quantified a total of 11 subgingival bacterial species including 4 thought a-priori to be etiologically related to periodontal disease [3,31], 5 putatively associated with periodontal disease [3], and 2 associated with periodontal health [31]. We hypothesized that the four bacteria related to periodontal disease would differentially relate to novel inflammatory markers.
In this report, we investigated whether activity of s-PLA2 and Lp-PLA2 correlated cross-sectionally with (1) the specific organisms thought a-priori to be etiologically related to periodontal disease; and (2) the relative abundance of these four species among the eleven measured in this study.
2. Materials and methods
INVEST is a prospective cohort which aims to investigate the association between oral infections and both subclinical and clinical cardiovascular diseases. 1056 individuals living in Northern Manhattan were randomly selected from the Northern Manhattan Study (NOMAS) [26,8].
Residents of Northern Manhattan were contacted via randomdigit dialing (zip codes 10031, 10032, 10033, 10034, and 10040). Eligible participants were defined as: i) Hispanic, black, or white with residence of ≥3 years; ii) aged 55 years or older at time of first examination; iii) no baseline history of stroke, myocardial infarction, chronic inflammatory conditions or history of bacterial endocarditis.
2.1. Dental history and oral examination
After exclusion of edentulous participants, 841 individuals were enrolled at baseline. Because of missing clinical data (n = 248), 593 subjects were finally included in analyses. The Columbia University Institutional Review Board approved the study, and all subjects provided informed consent.
Details of the oral examination have been described previously [8]. Complete oral examinations were conducted by calibrated dental examiners. Participants were interviewed about oral hygiene habits (frequencies of tooth brushing and flossing as times/day and times/week respectively). For all teeth present, bleeding on probing, probing depth and gingival recession were measured in millimeters using a UNC-15 manual probe (Hu-Friedy) at six sites per tooth.
2.2. Oral plaque collection and laboratory processing
From the two most posterior teeth in each quadrant as available (mesiopalatal sites in the maxilla and mesiobuccal sites in the mandible), plaque samples were collected using sterile Gracey curettes by a single scaling stroke from the base of the periodontal pocket (n = 5369 plaque samples, analyses being conducted in 4561 samples after exclusion of participants with missing information on covariates). We chose a priori these specific teeth to avoid bias by collecting plaque samples in the most diseased sites [9,7].
Bacterial plaque samples were immediately transferred into the laboratory using Eppendorf tube containing 200 μL of sterile T-E buffer (10 mmol/L Tris HCl, 1.0 mm EDTA, pH 7.6). Then, plaque samples were resuspended and subjected to a vortex. 200 μL of a 0.5 mol/L NaOH solution was added before conserving the collected sampled at 4 °C. Finally, samples were immobilizated onto nylon membranes.
We used the High-Prime labeling kit (Roche/Boehringer-Mannheim) to prepare genomic digoxigenin-labeled probes to detect species currently considered to be (1) etiologically linked with periodontal diseases or frequently encountered in pathological periodontal conditions (Porphyromonas gingivalis [ATCC 33277], Tannerella forsythensis [ATCC 43037], Aggregatibacter actinomycetemcomitans [ATCC 43718], and Treponema denticola [ATCC 35404]) [31,3], (2) putatively associated with periodontal disease (Prevotella intermedia [ATCC 25611], Fusobacterium nucleatum [ATCC 10953], Parvimonas micra [ATCC 33270], Campylobacter rectus [ATCC 33238], and Eikenella corrodens [ATCC 23834]) [3] and (3) primarily associated with periodontal health (Veillonella parvula [ATCC 10790] and Actinomyces naeslundii [ATCC 49340]) [31].
The checkerboard DNA–DNA hybridization method was used to analyse bacterial plaque samples (sensitivity and specificity previously described [11]). A comparison between checkerboard hybridization and culture in the identification of subgingival microbiota has been published by our group [22]. After boiling, plaque samples were neutralized and transferred onto nylon membranes using a Minislot device (Immunetics), before being immobilized by UV light and baking at 120 °C. Hybridizations between the DNA probes and the plaque DNA were performed overnight in a Miniblotter device (Immunetics) at 42 °C. After a series of stringency washes, we used anti-digoxigenin antibodies conjugated with alkaline phosphatase to detect hybrid double-stranded DNA after incubation with a chemiluminescent substrate (CSPD, Roche/Boehringer- Mannheim). Chemiluminescent detection of DNA was performed in a LumiImager workstation with a chargecoupled device camera (Roche/Boehringer- Mannheim). We used LumiAnalyst software to convert the chemiluminescent signals into bacterial counts. The lower detection level of the method is between 103 and 104 bacterial cells.
Lp-PLA2 activity was measured and expressed in nmol/min per ml of plasma [15]. The within-assay variability was <10%. sPLA2 activity was measured on heparinated plasma samples as previously described [18]. The minimum detectable activity was 0.10 nmol/min/ml and the intra- and inter-assay CVs were <10%. Levels of sPLA2 protein in plasma were measured by an immunometric assay (Cayman Chemical Company, Ann Arbor, Michigan).
2.3. Risk factor assessment
Sociodemographic variables, medical status and risk factors for cardiovascular diseases were collected by trained assistants by using interviews in English or Spanish, and adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System [8]. Participants were categorized as never, former or current smokers [8]. Race/ethnicity was self-identified [26].
Study physicians measured systolic and diastolic blood pressures using a calibrated aneroid sphygmomanometer (Omron). We used the average of two measurements in all analyses [27,6].
Fasting glucose and cholesterol levels were measured from blood samples collected at enrollment [27,2,38]. Participants with a history of diagnosed diabetes, with insulin or hypoglycemic medication, or with a fasting glucose ≥126 mg/dL (≥7.7 mmol/L), were defined as having diabetes.
2.4. Statistical analysis
Laboratory analysis provided a relative quantity of bacteria per subgingival plaque sample by comparing all samples to known standards (see methods above). Bacterial values were first log-transformated. Standardized values for each bacterial species (SCbact) were calculated as the average of the ratio of mean within mouth (Mind) by the population standard deviation (SDpop) for the respective species (SCbact = Mean(Mind/SDpop)). Consequently, bacterial values are expressed in standard deviation units (SDU), and are equivalent across species. We used the consensus of the 1996 World Workshop in Periodontics that identified the periodontal pathogens associated with pathological periodontal conditions (P gingivalis, T forsythensis, and A actino-mycetemcomitans) [3] and the definition of Socransky's red complex [31], which led to the addition of T denticola, to generate an etiologic burden score (EB), which comprised the 4 pathogens (A actinomycetemcomitans, P gingivalis, T forsythensis, and T denticola) (EB = sum(SCAa + SCPg + SCTf + SCTd)). Similarly, putative and health-associated burden scores (respectively PB and HAB) were computed for respectively the five putatively associated and the two health-associated pathogens [3,31]. An etiologic dominance score (ED), which represents the predominance of the etiologic bacterial group in the whole ecological niche, was calculated as the ratio of the etiologic burden score by the sum of standardized values for the 11 periodontal pathogens analysed (ED = EB/(EB + PB + HAB)).
Clinical periodontal status was defined as the full-mouth mean clinical attachment level and mean periodontal probing depth.
Concentration of s-PLA2 and activity of both s-PLA2 and Lp-PLA2 (dependent variables) were regressed on tertiles of the aforementioned independent variables in multivariable regression models, first considering tertiles as a continuous variable to assess the linear pattern of the distribution (p for linear trend), and secondly considering tertiles as categories in case of a curvature distribution (p for statistical significance). All adjusted models included the following covariates: age, sex, race/ethnicity (Hispanic, black, or white), education (completed high school or not), smoking (never, former, and current smokers), diabetes, BMI, LDL and HDL cholesterol levels, and systolic blood pressure. Models for the s-PLA2 activity outcome were adjusted for s-PLA2 concentration to explore if bacterial burdens are associated with alterations in activity independently of variations in concentration. We finally ran models mutually adjusting for both bacterial burden and clinical periodontal data under the assumption that bacterial levels confound associations between clinical periodontal data and PLA2 outcomes, in accordance with the hypothesis that bacteria are the etiologic exposures. significance was set at p < 0.05. All analyses were performed with R Windows 2.14.0.
3. Results
3.1. General characteristics
The study population was triethnic, with 57% Hispanics, 23% non-Hispanic blacks and 18% non-Hispanic whites (the remaining 2% reported ‘other’). Nearly 60% of the studied participants were females. Concentration and activity of s-PLA2 were higher in females than males (Concentration: 4.67 ± 7.80 ng ml−1 vs 2.80 ± 3.39 ng ml−1;p < 0.001. Activity: 0.82 ± 0.59 nmol ml−1 min−1 vs 0.67 ± 0.52 nmol ml−1 min−1;p < 0.001). On the contrary, activity of Lp-PLA2 was higher in males than females (32.53 ± 11.49 nmol ml−1 min−1 vs 28.85 ± 10.19 nmol ml−1 min−1; p < 0.001). Clinical periodontal characteristics were worse in males (mean PPD (mm): 2.64 ± 0.76 vs 2.42 ± 0.69; p < 0.001. n = 583, 10 patients excluded because missing periodontal data). However, no difference exists between males and females for bacterial burdens except for health-associated burden, which is higher in females (25.74 ± 1.62 vs 25.46 ± 1.45; p = 0.03).
3.2. Validation of a-priori concepts
After adjustment for age and gender, lower tertiles of health-associated burden were inversely associated with better clinical periodontal conditions, in terms of percentage of sites with probing depth 3 mm and more and percentage of sites with bleeding on probing. On the contrary, worse periodontal conditions were observed with increasing levels of etiologic and putative periodontal pathogens [9] (Supplemental Table 1).
3.3. Periodontal bacteria and s-PLA2 concentration
In spite of an increase in s-PLA2 concentration between the two lower tertiles of etiologic dominance (ED), no significant independent association was found between s-PLA2 concentration and tertiles of etiologic dominance (p = 0.91).(Table 1) After multivariable adjustment, mean ± SE s-PLA2 concentrations across tertiles of etiologic burden (EB) were 3.94 ± 0.79 ng ml−1, 3.77 ± 0.70 ng ml−1, 3.52 ± 0.78 ng ml−1 (p = 0.92). Similarly, we observed no significant association between s-PLA2 concentration and putative and health-associated burdens. (Data not shown).
Table 1.
Crude and adjusted mean ± SE for s-PLA2 concentration, activity and Lp-PLA2 activity across tertiles of etiologic dominance.
| Crude model |
Adjusted model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile I (n = 197) |
Tertile II (n = 198) |
Tertile III (n = 198) |
p for trend |
p | Tertile I (n = 197) |
Tertile II (n = 198) |
Tertile III (n = 198) |
p for trend |
p | |
| s-PLA2 concentration ng ml−1 |
3.94 ± 0.35 | 4.16 ± 0.64 | 3.65 ± 0.32 | 0.66 | 0.73 | 3.76 ± 0.47 | 4.04 ± 0.46 | 3.96 ± 0.47 | 0.76 | 0.91 |
| s-PLA2 activity nmol ml−1 min−1 |
0.66 ± 0.04 | 0.73 ± 0.04 | 0.88 ± 0.04 | <0.001 | <0.001 | 0.66 ± 0.04 | 0.73 ± 0.04 | 0.89 ± 0.04 | <0.001 | <0.001 |
| Lp-PLA2 activity nmol ml−1 min−1 |
29.94 ± 0.75 | 29.17 ± 0.80 | 31.85 ± 0.76 | 0.08 | 0.04 | 29.88 ± 0.74 | 29.35 ± 0.73 | 31.71 ± 0.75 | 0.10 | 0.07 |
s-PLA2: Secretory phospholipase A2; Lp-PLA2: Lipoprotein-associated phospholipase A2. SE: Standard error. Adjusted model: Adjustment for Gender, Age, Race, Education, Diabetes, Smoking, BMI, mean systolic blood pressure, HDL-cholesterol, LDL-cholesterol.
3.4. Periodontal bacteria and activity of s-PLA2 and Lp-PLA2
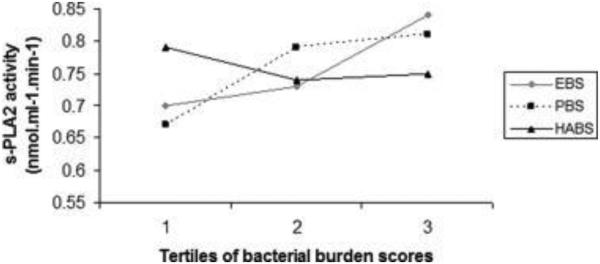
Fig. 1 shows mean adjusted s-PLA2 activity levels across tertiles of the three bacterial burdens, after adjustment for all covariates. Mean ± SE s-PLA2 activity levels across tertiles of EB were: 0.70 ± 0.05 nmol ml−1 min−1, 0.73 ± 0.04 nmol ml−1 min−1 and 0.84 ± 0.05 nmol ml−1 min−1, respectively.(Fig. 1) Also, s-PLA2 and Lp-PLA2 activities increased across tertiles of putative burden (PB).(Table 2 and Fig. 1)
Fig. 1.
Distribution of s-PLA2 activity across tertiles of etiologic, putative and health-associated burdens. s-PLA2: Secretory phospholipase A2. EB: Etiologic burden; PB: Putative burden; HAB: Health-associated burden. Adjustment for gender, age, race, education, diabetes, smoking, BMI, mean systolic blood pressure, HDL-cholesterol, LDL-cholesterol. Bacterial burdens are mutually adjusted. Tertile 1: n = 197; Tertile 2: n = 198; Tertile 3: n = 198. p for linear trend (1 d.f.): EB = 0.14; PB = 0.07; HAB = 0.43. p for global null hypothesis of any difference in outcome (2 d.f.): EB = 0.22; PB = 0.14; HAB = 0.60.
Table 2.
Adjusted mean ± SE for Lp-PLA2 activity (in nmol ml−1 min−1) across tertiles of etiologic, putative and health-associated bacterial burdens.
| Tertile I (n = 197) | Tertile II (n = 198) | Tertile III (n = 198) | p for trend | p | |
|---|---|---|---|---|---|
| Etiologic burden | 30.66 ± 0.91 | 29.23 ± 0.74 | 31.06 ± 0.95 | 0.84 | 0.21 |
| Putative burden | 29.04 ± 0.91 | 30.23 ± 0.74 | 31.68 ± 0.93 | 0.04 | 0.22 |
| Health-associated burden | 30.96 ± 0.78 | 28.97 ± 0.74 | 31.03 ± 0.76 | 0.97 | 0.09 |
Lp-PLA2: Lipoprotein-associated phospholipase A2. SE: Standard error. Adjusted for Gender, Age, Race, Education, Diabetes, Smoking, BMI, mean systolic blood pressure, HDL-cholesterol, LDL-cholesterol. Bacterial burdens were mutually adjusted.
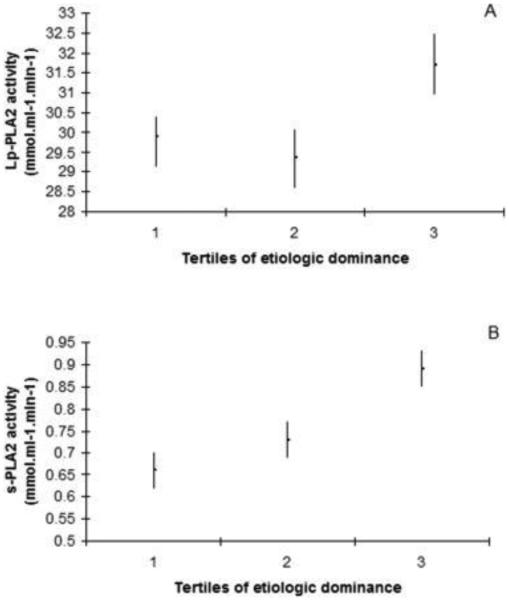
Similarly, when looking at bacterial etiologic dominance, s-PLA2 activity significantly increased in a dose-responsive manner as follows: 0.66 ± 0.04 nmol ml−1 min−1, 0.73 ± 0.04 nmol ml−1 min−1, and 0.89 ± 0.04 nmol ml−1 min−1 (p < 0.001). No significant association was observed for mean Lp-PLA2 activities across tertiles of etiologic dominance (p = 0.07).(Table 1 and Fig. 2)
Fig. 2.
Distribution of Lp-PLA2 and s-PLA2 activities across tertiles of etiologic dominance. s-PLA2: Secretory phospholipase A2; Lp-PLA2: Lipoprotein-associated phospholipase A2. Adjustment for gender, age, race, education, diabetes, smoking, BMI, mean systolic blood pressure, HDL-cholesterol, LDL-cholesterol. Tertile 1: n = 197; Tertile 2: n = 198; Tertile 3: n = 198. p for linear trend (1 d.f.): Lp-PLA2: 0.10; s-PLA2 < 0.001. p for global null hypothesis of any difference in outcome (2 d.f.): Lp-PLA2: 0.07; s-PLA2 < 0.001.
3.5. Clinical periodontal characteristics and levels of s-PLA2/Lp-PLA2 activity
After adjustment for medical, behavioral and sociodemographic confounders, s-PLA2 activity increased across increasing tertiles of mean probing depth and clinical attachment loss (respectively p < 0.001 and p = 0.02). On the contrary, no significant association was found for Lp-PLA2 activity after adjustment for same covariates (respectively p = 0.15 and p = 0.49). However, after additional adjustment for etiologic burden, statistical significance in the relationship between s-PLA2 activity and both mean clinical attachment loss and percentage of sites with probing depth 3 mm and more disappeared (respectively p = 0.25 and p = 0.06). Associations between clinical periodontal measures and Lp-PLA2 activity were not as evident.(Supplemental Tables 2–4)
4. Discussion
We report a positive independent relationship between activity of s-PLA2 and periodontal bacterial etiologic dominance. Mean clinical attachment loss and mean probing depth were also significantly associated with s-PLA2 activity. On the contrary, no significant association was found for s-PLA2 concentration and Lp-PLA2 activity with both microbiological and clinical data (except for Lp-PLA2 activity and percentage of sites with probing depth 3 mm and more).
Numerous studies have observed an association between periodontal diseases and incident cardiovascular diseases [4,5]. Studies of the association between periodontitis and well-established inflammatory markers, such as CRP and interleukins, or novel markers, such as s-PLA2 and Lp-PLA2, are of importance to understand (1) the mechanisms linking periodontal and cardiovascular diseases and (2) the potential systemic benefits of periodontal therapy. We previously observed that mean carotid intima-media thickness increased across tertiles of bacterial etiologic dominance from 0.84 ± 0.008 to 0.88 ± 0.008 mm (p = 0.002) [9]. Also, subjects with elevated levels of circulating antibodies against periodontal pathogens were at higher risk for coronary heart disease than controls with lower bacterial exposure (OR: 1.75 (95% CI, 1.32 to 2.34; p < 0.001)) [20]. On the other hand, and contrary to s-PLA2 concentration which has little prognostic utility, biological and epidemiologic evidence exists about the association between s-PLA2 activity and risk of coronary events and atherosclerosis [15–17]. This study could add to the discussion about explanatory mechanisms linking periodontal diseases to coronary events and atherosclerosis.
Our findings for s-PLA2 activity are consistent with previous studies showing an increase of several inflammatory markers across levels of periodontal bacteria [21,24]. We hypothesize the increase of s-PLA2 activity across tertiles of ED, independently of s-PLA2 concentration, could be due to an increase of s-PLA2 activator across levels of periodontal pathogens. Authors have shown levels of oxidized lipoproteins, which are activators of s-PLA2 [14], significantly increase in subjects with high levels of Aggregatibacter actinomycetmecomitans [13] and clinical diagnosis of periodontal diseases [33,34]. In this study we adjusted results for LDL and HDL cholesterol, but few studies investigated activators of s-PLA2 in humans and we suppose other non-identified activating factors, which levels increase with periodontal pathogens burdens, could be involved. Also, no significant association was found for Lp-PLA2 activity with bacterial burden or ED. Lp-PLA2 travels in blood mainly bound to LDL, and alterations of the binding could be due to acute phase proteins such as fibrinogen [10]. High levels of fibrinogen are found in periodontal diseases [39]. Consequently, our data of non-significant association between Lp-PLA2 activity and microbiological and clinical status could relate to the release of acute inflammatory markers, such as fibrinogen, which affects binding of Lp-PLA2 to LDL, and consequently the activity of Lp-PLA2.
As reported above, we found worse clinical periodontal conditions were observed in higher tertiles of etiologic dominance, and that significant association between levels of s-PLA2 activity and both mean clinical attachment loss and percentage of sites with probing depth 3 mm and more disappeared after we adjusted for confounders and etiologic burden. Such results confirm the hypothesis that bacteria are the common denominators, and that the etiologic exposures may be responsible for both periodontal breakdown and increasing levels of sPLA2-activity.
In the present study by INVEST design, we minimized the possibility of socioeconomic confounding by enrolling subjects from 5 different areas in northern Manhattan. Additionally, instead of simply selecting bacterial samples from the most periodontally diseased sites, we collected and analyzed an average of 7 subgingival plaque samples per mouth because we hypothesized that the overall subject's periodontal microbiota would likely be most relevant to systematic health in spite of an increased likelihood of positive findings when sampling from the most periodontally diseased sites. In addition to analyses performed on bacteria that are etiologically linked and putatively associated with periodontal diseases, plus bacteria primarily associated with healthy periodontal conditions [31], we performed supplemental analysis focused on the burden of those specific bacteria thought to be causally related to periodontal diseases relative to the overall bacterial burden in the periodontal niche assessed. This measure allows us to have internal bacterial controls, thus reducing the probability that bacterial levels might simply reflect poor oral and perhaps general health.
The present study naturally has some limitations. First, we found no significant association between s-PLA2 concentration and bacterial burdens, in spite of an increase from first to second tertiles of etiologic dominance. Desvarieux et al., in a previous report from the same cohort, found C-reactive protein concentration was not correlated with levels of EB [9]. Slade et al. showed BMI could be a modifying factor since relationship between CRP and periodontitis was only observed among adults with low BMI [30]. In INVEST, the average BMI is more than 28 kg/m2 which, if such a similar relationship exists between periodontal disease, s-PLA2 and BMI, could explain the non linear increase of s-PLA2 levels across EB tertiles. Moreover, because of the cross-sectional nature of our data, no inference can be made about a causal relationship. We must await the prospective results of INVEST and other studies to make firmer conclusions [37,7]. Finally, we have used race/ethnicity and educational level as crude surrogates for socioeconomic status. More robust constructs for socioeconomic status exist but are not available in INVEST. However, a particular strength of INVEST is the fact that participants are enrolled from neighborhoods in New York City where the socioeconomic status composition is relatively homogenous.
However, to our knowledge, this study provides the first evidence of a relationship between s-PLA2 activity and periodontal microbiota. Because of the previously described associations between s-PLA2 activity and atherosclerosis [35,16], and between periodontal conditions and vascular morbidity [9,8,32], these findings raise the possibility that s-PLA2 activity could mediate the link between periodontal infections and atherosclerosis.
Supplementary Material
Acknowledgments
We are indebted to Ewa Ninio, Inserm U937, Paris, France for measurement of Lp-PLA2 activity, the INVEST staff for their dedicated work and naturally the patients.
Sources of funding
This research is supported by NIH grants R01 DE-13094 (Dr. Desvarieux), NS-29993 (Dr. Sacco). Dr. Demmer is also supported by R00 DE-018739 and Dr. Rundek is also supported by NINDS R01 NS-047655, all from the NIH. This research was also partially supported by an INSERM Chair of Excellence from the Institut National de la Santé et de la Recherche Médicale (INSERM) and a Chair in Chronic Disease, École des Hautes Études en Santé Publique, France (both to Dr. Desvarieux); and a Mayo Chair Endowment, School of Public Health, University of Minnesota (Dr. Jacobs). Patients were seen at the Columbia University General Clinical Research Center, NIH grants UL1 TR000040 and 1UL1RR024156.
Footnotes
Conflict of interest
The authors declare they have no Conflict of interest.
Disclosures
None.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2015.07.039.
References
- [1].Andersson J, Libby P, et al. Adaptive immunity and atherosclerosis. Clin. Immunol. 2010;134(1):33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [2].Boden-Albala B, Cammack S, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the northern manhattan study (NOMAS) Diabetes Care. 2008;31(6):1132–1137. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- [3].Consensus report C. Periodontal diseases: pathogenesis and microbial factors. Ann. Periodontol. 1996;1(1):926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- [4].De Oliveira C, Watt R, et al. Toothbrushing, inflammation, and risk of cardiovascular disease: results from scottish health survey. BMJ. 2010;340:c2451. doi: 10.1136/bmj.c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DeStefano F, Anda RF, et al. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306(6879):688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Desvarieux M, Demmer RT, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST) J. Hypertens. 2010;28(7):1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Desvarieux M, Demmer RT, et al. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J. Am. Heart Assoc. 2013;2(6):e000254. doi: 10.1161/JAHA.113.000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Desvarieux M, Demmer RT, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the oral infections and vascular disease epidemiology study (INVEST) Stroke. 2003;34(9):2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Desvarieux M, Demmer RT, et al. Periodontal microbiota and carotid intimamedia thickness: the oral infections and vascular disease epidemiology study (INVEST) Circulation. 2005;111(5):576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elkind MS, Leon V, et al. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke. 2009;40(10):3233–3237. doi: 10.1161/STROKEAHA.109.552802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gunaratnam M, Smith GL, et al. Enumeration of subgingival species on primary isolation plates using colony lifts. Oral Microbiol. Immunol. 1992;7(1):14–18. doi: 10.1111/j.1399-302x.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- [12].Hurt-Camejo E, Andersen S, et al. Localization of nonpancreatic secretory phospholipase A2 in normal and atherosclerotic arteries. Activity of the isolated enzyme on low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1997;17(2):300–309. doi: 10.1161/01.atv.17.2.300. [DOI] [PubMed] [Google Scholar]
- [13].Jia R, Kurita-Ochiai T, et al. Periodontal pathogen accelerates lipid peroxidation and atherosclerosis. J. Dent. Res. 2013;92(3):247–252. doi: 10.1177/0022034513475625. [DOI] [PubMed] [Google Scholar]
- [14].Korotaeva AA, Samoilova EV, et al. Opposite effects of native and oxidized lipoproteins on the activity of secretory phospholipase A(2) group IIA. Prostagl. Other Lipid Mediat. 2009;90(1–2):37–41. doi: 10.1016/j.prostaglandins.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [15].Lind L, Simon T, et al. Circulating levels of secretory- and lipoprotein-associated phospholipase A2 activities: relation to atherosclerotic plaques and future all-cause mortality. Eur. Heart J. 2012;33(23):2946–2954. doi: 10.1093/eurheartj/ehs132. [DOI] [PubMed] [Google Scholar]
- [16].Mallat Z, Benessiano J, et al. Circulating secretory phospholipase A2 activity and risk of incident coronary events in healthy men and women: the EPIC-norfolk study. Arterioscler. Thromb. Vasc. Biol. 2007;27(5):1177–1183. doi: 10.1161/ATVBAHA.107.139352. [DOI] [PubMed] [Google Scholar]
- [17].Mallat Z, Lambeau G, et al. Lipoprotein-associated and secreted phospholipases A in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122(21):2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- [18].Mallat Z, Steg PG, et al. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J. Am. Coll. Cardiol. 2005;46(7):1249–1257. doi: 10.1016/j.jacc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- [19].Moura Foz A, Alexandre Romito G, et al. Periodontal therapy and biomarkers related to cardiovascular risk. Minerva Stomatol. 2010;59(5):271–283. [PubMed] [Google Scholar]
- [20].Mustapha IZ, Debrey S, et al. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J. Periodontol. 2007;78(12):2289–2302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- [21].Nibali L, Tonetti MS, et al. Interleukin-6 polymorphisms are associated with pathogenic bacteria in subjects with periodontitis. J. Periodontol. 2008;79(4):677–683. doi: 10.1902/jop.2008.070453. [DOI] [PubMed] [Google Scholar]
- [22].Papapanou PN, Madianos PN, et al. Checkerboard versus culture: a comparison between two methods for identification of subgingival microbiota. Eur. J. Oral Sci. 1997;105(5 Pt 1):389–396. doi: 10.1111/j.1600-0722.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- [23].Paraskevas S, Huizinga JD, et al. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008;35(4):277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- [24].Pitiphat W, Savetsilp W, et al. C-reactive protein associated with peri-odontitis in a Thai population. J. Clin. Periodontol. 2008;35(2):120–125. doi: 10.1111/j.1600-051X.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- [25].Ross R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- [26].Sacco RL, Boden-Albala B, et al. Stroke incidence among white, black, and hispanic residents of an urban community: the northern manhattan stroke study. Am. J. Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- [27].Sacco RL, Elkind M, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281(1):53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- [28].Saremi A, Anderson RJ, et al. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT) Atherosclerosis. 2009;203(2):610–614. doi: 10.1016/j.atherosclerosis.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sarlon-Bartoli G, Boudes A, et al. Circulating lipoprotein-associated phospholipase A2 in high-grade carotid stenosis: a new biomarker for predicting unstable plaque. Eur. J. Vasc. Endovasc. Surg. 2011;43(2):154–159. doi: 10.1016/j.ejvs.2011.10.009. [DOI] [PubMed] [Google Scholar]
- [30].Slade GD, Ghezzi EM, et al. Relationship between periodontal disease and C-reactive protein among adults in the atherosclerosis risk in communities study. Archives Intern. Med. 2003;163(10):1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- [31].Socransky SS, Haffajee AD, et al. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- [32].Spahr A, Klein E, et al. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the coronary event and periodontal disease (CORODONT) study. Archives Intern. Med. 2006;166(5):554–559. doi: 10.1001/archinte.166.5.554. [DOI] [PubMed] [Google Scholar]
- [33].Tamaki N, Tomofuji T, et al. Periodontal treatment decreases plasma oxidized LDL level and oxidative stress. Clin. Oral Investig. 2011;15(6):953–958. doi: 10.1007/s00784-010-0458-y. [DOI] [PubMed] [Google Scholar]
- [34].Tang K, Lin M, et al. Alterations of serum lipid and inflammatory cytokine profiles in patients with coronary heart disease and chronic periodontitis: a pilot study. J. Int. Med. Res. 2011;39(1):238–248. doi: 10.1177/147323001103900126. [DOI] [PubMed] [Google Scholar]
- [35].Thompson A, Gao P, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tonetti MS, D'Aiuto F, et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- [37].Van Dyke TE, Starr JR. Unraveling the link between periodontitis and cardiovascular disease. J. Am. Heart Assoc. 2013;2(6):e000657. doi: 10.1161/JAHA.113.000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Willey JZ, Xu Q, et al. Lipid profile components and risk of ischemic stroke: the Northern manhattan study (NOMAS) Archives Neurology. 2009;66(11):1400–1406. doi: 10.1001/archneurol.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu T, Trevisan M, et al. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am. J. Epidemiol. 2000;151(3):273–282. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.