Abstract
The Wnt/β-catenin signaling pathway is pathologically activated in cholangiocarcinoma (CCA). Here, we determined the expression profile as well as biological role of activated Wnt/β-catenin signaling in CCA. The quantitative reverse transcription polymerase chain reaction demonstrated that Wnt3a, Wnt5a, and Wnt7b mRNA were significantly higher in CCA tissues than adjacent non-tumor tissues and normal liver tissues. Immunohistochemical staining revealed that Wnt3a, Wnt5a, and Wnt7b were positive in 92.1, 76.3, and 100 % of 38 CCA tissues studied. It was noted that Wnt3 had a low expression in tumor cells, whereas a high expression was mainly found in inflammatory cells. Interestingly, a high expression level of Wnt5a was significantly correlated to poor survival of CCA patients (P=0.009). Membrane localization of β-catenin was reduced in the tumors compared to normal bile duct epithelia, and we also found that 73.7 % of CCA cases showed the cytoplasmic localization. Inflammation is known to be a risk factor for CCA development, and we tested whether this might induce Wnt/β-catenin signaling. We found that lipopolysaccharides (LPS) elevated the expression of Wnt3 both mRNA and protein levels in the macrophage cell line. Additionally, the conditioned media taken from LPS-induced activated macrophage culture promoted β-catenin accumulation in CCA cells. Furthermore, transient suppression of β-catenin by siRNA significantly induced growth inhibition of CCA cells, concurrently with decreasing cyclin D1 protein level. In conclusion, the present study reports the abundant expression of Wnt protein family and β-catenin in CCA as well as the effect of inflammatory condition on Wnt/β-catenin activation in CCA cells. Importantly, abrogation of β-catenin expression caused significant CCA cell growth inhibition. Thus, the Wnt/β-catenin signaling pathway may contribute to CCA cell proliferation and hence may serve as a prognostic marker for CCA progression and provide a potential target for CCA therapy.
Keywords: Activated macrophages, Wnt, β-Catenin, Cholangiocarcinoma
Introduction
Cholangiocarcinoma (CCA) is a malignant transformation of cholangiocytes, the epithelial cells lining the bile duct. CCA is a major public health problem in northeastern Thailand, where the highest recorded incidence rate in the world has been reported [1]. CCA is associated with Opisthorchis viverrini (Ov) infection that produces a chronic inflammation leading to CCA development based on both epidemiological and experimental studies [2–7].
We have recently reported the profile of activated protein kinases using human phospho-RTKs and phospho-kinases arrays for both CCA cell lines and human CCA tissues. Multiple protein kinases are activated in both CCA cell lines and CCA tissues. Of particular interest among the activated kinases was β-catenin which is a downstream signaling molecule of the Wnt signaling pathway [8]. Wnt proteins comprise a family of 19 secreted glycoproteins that act as ligands to activate a receptor-mediated signaling pathway through the Frizzled (Fz)/low-density lipoprotein receptor-related protein. Activation of the Wnt pathway leads to the accumulation of cytoplasmic β-catenin, which then enters the nucleus where β-catenin converts the TCF/LEF complex from transcriptional repression to a transcriptional activating complex, modulating the expression of several genes involved in cell proliferation, differentiation, migration, and apoptosis [9].
In normal and non-stimulated cells, the majority of β-catenin protein is present in cell–cell junctions but rare in cytoplasm or the nucleus [10]. The level of β-catenin is tightly regulated by the phosphorylation of β-catenin that is the function of adenomatous polyposis coli tumor suppressor protein, axin, and the glycogen synthase kinase-3β (GSK-3β) complex which leads to degradation of β-catenin by the ubiquitin–proteasome complex [11]. Activation of Wnt/β-catenin signaling is closely involved in the process of carcinogenesis in many types of cancer [12]. The nuclear β-catenin has an important function in various human malignancies by activating oncogenes such as cyclin D1 [13], c-Myc [14], and other downstream targets. Aberrant signaling involving the stabilization and nuclear translocation of β-catenin has been observed in cancer of the pancreas [15], colon [16], lung [17], and liver [18]. In addition, the role of macrophages in the promotion of Wnt/β-catenin activity in gastric tumorigenesis has been reported [19]. Furthermore, the conditioned medium of activated macrophages promoted Wnt/β-catenin signaling in gastric cancer cells [19].
In this study, we demonstrated that several Wnt genes were abundantly expressed in CCA cells as well as inflammatory cells. In addition, Wnt3 was upregulated in lipopolysaccharides (LPS)-activated macrophages promoting β-catenin nuclear translocation. Finally, abrogation of β-catenin expression induced growth inhibition of CCA cells, suggesting a potential target for CCA therapy.
Materials and methods
Human CCA specimens
The 38 paraffin-embedded and 48 frozen intrahepatic CCA tissues were selected from the specimen bank of the Liver Fluke and Cholangiocarcinoma Research Center, Khon Kaen University, Thailand. Informed consent was obtained from each subject before surgery, and the Human Research Ethics Committee, Khon Kaen University has approved the research protocol (#HE43201, #HE471214, and #HE521209).
Human CCA cell lines
Five respective CCA cell lines, KKU-M055, KKU-M139, KKU-M156, KKU-M213, and KKU-M214, were developed from primary tumors of patients who were admitted to Srinagarind Hospital with a primary CCA diagnosis. All cell lines were cultured in HAM-F12 (Gibco/BRL, Grand Island, NY) supplemented with 10 % inactivated fetal bovine serum, 2 mg/ml sodium bicarbonate, and 1 % antibiotic–antimycotic solution (Life Technologies, Inc., Gaithersburg, MD) and incubated at 37 °C in a humidified incubator maintained with an atmosphere of 5 % CO2. In addition, the U937 human macrophage cells were purchased from the American Type Culture Collection. The U937 cells were maintained in suspension culture in RPMI-1640 supplemented with 10 % (v/v) heat-inactivated fetal bovine serum in a humidified atmosphere containing 5 % CO2.
Antibodies
Antibodies used for immunohistochemical staining and western blotting were as follows: Wnt3 (Abcam, Cambridge, MA), Wnt3a (Abcam, Cambridge, MA), Wnt5a (Abcam, Cambridge, MA ), Wnt7b (Abcam, Cambridge, MA ), β-catenin (BD Biosciences, San Jose, CA), and β-actin (Sigma-Aldrich, St. Louis, MO).
Total RNA extraction and cDNA synthesis
Total RNA was isolated from liver tissues and cell lines using TRIZOL® Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. A 5-μg aliquot was reverse transcribed using RevertAid M-MuLV reverse transcriptase (Fermentas, Burlington, CA) and random hexamers (Promega, Madison, WI) which were mixed together and then heated at 70 °C for 5 min. After that, reaction mixture containing the first-stranded cDNA synthesis buffer (1×; 75 mM KCl, 50 mM Tris–Cl pH 8.3, 3 mM MgCl2), 0.5 mM each dNTPs, and 200 units of reverse transcriptase (Fermentas, Burlington, CA) was added. Reverse transcription was carried out using a DNA thermocycler (GeneAmp PCR system 2400, Applied Biosystems, CA). The thermal conditions were at 25 °C for 10 min, 42 °C for 1 h, and 70 °C for 10 min, respectively.
Relative quantification of Wnt mRNA expressions
Relative quantification of expression of Wnts and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was performed using a Taqman gene expression assay kit and the ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The TaqMan® Probes were labeled with quencher and reporter dyes. The reporter dye of targeted TaqMan (Probes in this experiment was labeled with FAM dye whereas the GAPDH probe was labeled with a VIC dye (Table 1). qPCR reactions were performed using a FastStart Universal Probe Master Mix (Roche, Switzerland) following the manufacturer's protocol in duplication for each sample. The relative quantification of gene expressions was done using the comparative cycle threshold (CT) method and GAPDH expression as the endogenous control.
Table 1.
Specific primers and probes for qRT-PCR
| Assay ID | Gene | Company |
|---|---|---|
| Hs00180529_m1 | Wnt1 | Applied Biosystems, USA |
| Hs00608224_m1 | Wnt2 | Applied Biosystems, USA |
| Hs00229135_m1 | Wnt3 | Applied Biosystems, USA |
| Hs00263977_m1 | Wnt3a | Applied Biosystems, USA |
| Hs00180103_m1 | Wnt5a | Applied Biosystems, USA |
| Hs01114990_m1 | Wnt7a | Applied Biosystems, USA |
| Hs00536497_m1 | Wnt7b | Applied Biosystems, USA |
| Hs00610126_m1 | Wnt8b | Applied Biosystems, USA |
| Hs00559664_m1 | Wnt10b | Applied Biosystems, USA |
| 4326317E | GAPDH | Applied Biosystems, USA |
Immunohistochemistry
Wnt3, Wnt3a, Wnt5a, Wnt7b, and β-catenin were detected on the formalin-fixed, paraffin-embedded sections using standard immunohistochemistry protocols. The paraffin sections were deparaffinized and then hydrated by submerging, respectively, in xylene and ethanol with stepwise decreasing concentration. The sections were treated with 3 % H2O2 in phosphate-buffered saline (PBS) to block the endogenous peroxidase. After blocking with 5 % skimmed milk, the sections were incubated with primary antibodies, including rabbit anti-Wnt3 antibody (1:500, Abcam, MA, USA), rabbit anti-Wnt3a antibody (1:200, Abcam, MA, USA), rabbit anti-Wnt5a antibody (1:50, Abcam, MA, USA), rabbit anti-Wnt7b antibody (1:100, Abcam, MA, USA), and mouse anti-β-catenin antibody (1;50, BD Biosciences, San Jose, CA), and then incubated at 4°C overnight. After that, sections were incubated with peroxidase-conjugated Envision™ secondary antibody (DAKO, Glostrup, Denmark). Peroxidase activity was observed using DAB as the substrate. The sections were counterstained with hematoxylin, dehydrated with stepwise increasing concentration of ethanol, cleared with xylene, and mounted with permount. The staining frequency of protein was semiquantitatively scored on the basis of the percentage of positive cells as: 0 %=negative; 1–25 %=+1; 26–50 %=+2; and >50 %=+3. The intensity of protein staining was scored as weak=1, moderate=2, and strong=3. For determination of biliary epithelial hyperplasia and dysplasia, hyperplastic duct was defined as bile duct with increasing cell numbers, enlargement of duct size, and pseudopapillary projection, while dysplastic duct was defined as bile duct composed of cells with multilayer nuclei, increasing nuclear–cytoplasm ratio, and containing micropapillary projection.
Localization of Wnt3 in macrophage cells was confirmed by double immunofluorescence staining. Slides were immunostained by using primary antibodies specific to Wnt3 or MAC387 (a macrophage marker), and anti-rabbit IgG Alexa 488 or anti-mouse IgG Alexa 555 (Invitrogen, CA, USA) was used as the secondary antibody. Stained sections were examined using a fluorescence microscope.
Production of LPS-stimulated U937 macrophage cells-conditioned media
The U937 macrophage cells were cultured in a 6-well plate 1.5×105 cells/well overnight. Then, cells were treated with 1 or 2 μg/ml LPS for 12, 24, and 48 h. PBS-treated cells were used as negative controls. After stimulation, Wnt3 mRNA expression was determined by qPCR as described above.
To produce macrophage cells conditioned media, the U937 macrophage cells were plated in 6-well culture plates 1×105 cells/well overnight and were left non-stimulated or stimulated with 2 μg/ml LPS for 48 h. After treatment, conditioned media were collected under sterile conditions, centrifuged (2,000 rpm, 5 min, 4 °C) to remove cell debris, and stored at −80 °C. The supernatant taken from the LPS-treated U937 macrophages was designated as activated macrophage-conditioned media (AMCM) and that taken from the untreated control cells as non-activated macrophage-conditioned media (NAMCM).
Stimulation of CCA cells with conditioned media from LPS-stimulated macrophages
CCA cell line, M214 was cultured in a 6-well plate 1×105 cells/well overnight. Then, cells were treated with NAMCM for 24 h and AMCM for 12 and 24 h, respectively. Untreated CCA cells were used as controls. After stimulation, cells were subjected to western blot analysis.
β-Catenin transient knockdown of CCA cells
High endogenous β-catenin expression, M214 CCA cells (2×105 cells) were seeded into a 6-well plate for 24 h before transfection. Cells were transfected either with siRNA specific for human β-catenin mRNA (siRNA ID: s438; Ambion, Foster City, CA) or validated non-targeting siRNAs (scramble controls) (Ambion, Foster City, CA) or no siRNA sequence at all, using Lipofectamine Transfection Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instruction. Specifically, 5 μl of lipofectamine was diluted in Opti-MEM I reduced serum media and then incubated for 5 min at room temperature. The siRNAwas added to diluted lipofectamine to a total volume of 500 μl. The solution was mixed gently and incubated for 20 min at room temperature. Subsequently, 500 μl of siRNA/lipofectamine complex was added to each well containing 2.5 ml of Opti-MEM I reduced serum medium and mix gently by rocking the plate back and forth. The siRNA-treated cells were incubated at 37 °C in 5 % CO2 incubator, and after 24 h of transfection, the medium was replaced with 2.5 ml of complete medium. The cells were cultured for further 24 h to achieve complete transfection for further analyses.
Western blot analysis
Fifty micrograms of proteins extracted from the cell lysates was solubilized in SDS buffer and boiled for 5 min. Samples were separated on 12.5 % polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5 % skim milk in Tris-buffered saline (TBS) at room temperature for 1 h or 4 °C overnight and incubated with a primary antibody for 1 h at room temperature. The blots were then probed with primary antibodies, including mouse anti-β-catenin antibody (1:1,000, BD Biosciences, San Jose, CA), mouse anti-cyclin D1 (1:1,000, Cell signaling, Danvers, MA), and mouse anti-β-actin (1:20,000, Sigma-Aldrich, St. Louis, MO, USA) as an internal control. After rinsing with TBS containing 0.1 % polyoxyethylene sorbitan monolaurate (Tween-20 or TBS-T), membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biochemical, Santa Cruz, CA) at room temperature for 1 h. After rinsing with TBS-T, membranes were exposed to the ECL Plus Western Blotting Detection System (GE Healthcare Bio-Science, UK) for 5 min. Human GAPDH was used as a loading control. The immunoblot and intensity were analyzed by an ImageQuant analysis system (GE Healthcare Bio-Science, UK).
Statistical analysis
Wnt mRNA levels in human CCA and normal liver tissues were reported as mean ± SD. Difference expression between groups was examined using a Mann–Whitney U test and Wilcoxon signed-rank test. Clinico-pathological characteristics and protein staining were compared using the χ2 or Fisher's exact probability test. The Kaplan–Meier method was used to calculate the survival curves, and the log-rank test was performed to compare differences in the survival rates of patients who were subjected to curative surgery. All analyses were done using SPSS software (version 15.0), and a P<0.05 was considered as statistically significant.
Results
Patient characteristics
Of the 38 intrahepatic CCA patients examined by immunohistochemistry staining, 28 (70.2 %) were males and 10 (29.8 %) were females. A ratio between male to female is 2.8:1.The mean age was 57±9 years (range, 37–74 years). Most of the patients were in an advanced stage, with 50.0 % metastasis. In this series, the histological type was classified as poorly differentiated (5.3 %), moderately differentiated (21.1 %), well differentiated (52.5 %), and papillary-type (21.1 %) CCA.
Wnt mRNA expression profiles in human CCA tissues
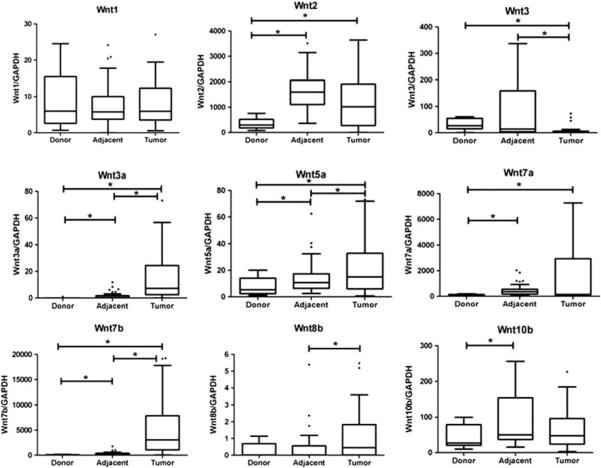
We studied the mRNA expression level of 9 Wnt genes that have been previously reported to alter cancer expression, Wnt1, Wnt2, Wnt3, Wnt3a, Wnt5a, Wnt7a, Wnt7b, Wnt8b, and Wnt10b, in 48 human CCA, adjacent non-tumorous tissues of the same patient and normal liver tissues of nine cadaveric donors. Relative Wnt mRNAs expression levels were determined using real time reverse transcription polymerase chain reaction (RT-PCR) and shown in Fig. 1. Our data demonstrated that there was no differential expression of Wnt1 among the studied groups, whereas the expression of Wnt2 and Wnt7a significantly increased in tumor and adjacent non-tumor tissues when compared with cadaveric tissues (P<0.05). In addition, Wnt3a, Wnt5a, and Wnt7b expressions significantly increased in tumor tissues when compared with adjacent non-tumor (P<0.05) and normal tissues (P<0.05), respectively. Conversely, Wnt3 expression significantly decreased in tumor tissues when compared with normal and adjacent tissues (P<0.05). The Wnt8b mRNA level in tumor tissues significantly increased compared to adjacent non-tumor tissues. Furthermore, the expression of Wnt10b in adjacent non-tumor tissues significantly increased when compared with normal liver tissues (P<0.05). However, there was no significant correlation observed between Wnt mRNA expression and clinico-pathological as well as survival data.
Fig. 1.
Quantitative real time RT-PCR was performed to determine Wnt mRNA levels. GAPDH expression was used as the endogenous control. Difference of expression between groups was examined using Mann–Whitney U test and Wilcoxon signed-rank test. *P<0.05
Immunohistological analysis of Wnt protein and β-catenin in human CCA tissues and their clinico-pathological significance
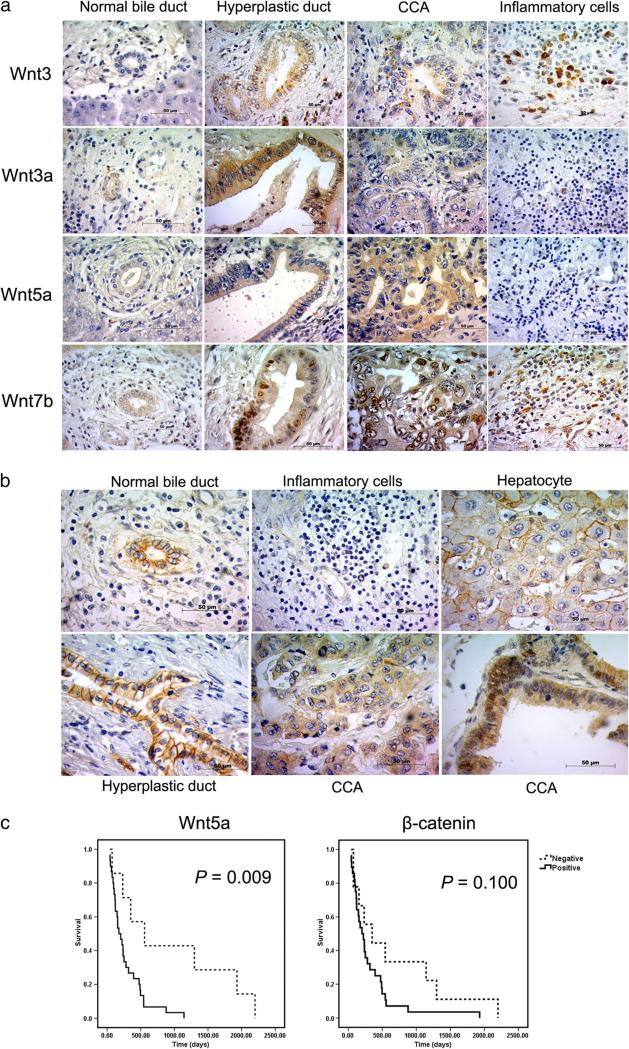
For a better understanding of the Wnt/β-catenin signaling pathway that is activated in CCA, immunohistochemistry analysis was performed to examine the expression and localization of Wnt proteins and β-catenin in human CCA tissues. According to real-time RT-PCR results, we chose Wnt3, which was downregulated in CCA and three Wnt genes, namely Wnt3a, Wnt5a, and Wnt7b, which significantly increased in tumor tissues when compared with adjacent and normal liver tissues.
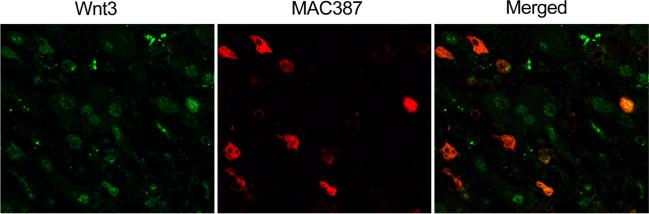
As shown in Fig. 2a, the normal bile duct taken from cadaveric donors showed the negative staining of Wnt3. All normal bile duct epithelia surrounding tumor sections and hepatocytes showed no staining. Of 38 intrahepatic CCA patients, 22 (57.9 %) showed no staining of Wnt3 protein, whereas weakly to moderately positive staining were detectable in tumor cells of 16 patients (42.1 %). In addition, Wnt3 staining was found in hyperplastic bile ducts. Interestingly, strongly positive staining of Wnt3 was seen in Kupffer cells and inflammatory cells residing in tumor and adjacent non-tumor tissues. We then investigated the type of inflammatory cells, which expressed Wnt3 in CCA tissues. Double immunofluorescence analysis was performed to co-localized Wnt3 and MAC387 which is a macrophage marker which demonstrated a part of Wnt3-positive cells expressed MAC387 (Fig. 3). This result suggested that some of inflammatory cells expressing Wnt3 are macrophages. However, no statistical significant correlation was observed between Wnt3 expression in tumor cells or inflammatory cells with clinical–pathological findings or patient survival data.
Fig. 2.
a The expression of Wnt3, Wnt3a, Wnt5a, Wnt7b, and b β-catenin in human liver tissues by immunohistochemistry staining. c The survival curves calculated for Wnt5a and β-catenin according to Kaplan–Meier method
Fig. 3.
Co-expression of Wnt3-positive cells with MAC387 detected by double immunofluorescence in CCA tissue. Original magnification is ×400
Immunohistocemical staining demonstrated that Wnt3a proteins were present in the cytoplasm of tumor cells in 35 of 38 CCA cases (92.1 %) (Fig. 2a). Strongly positive staining of Wnt3a was found in bile duct with hyperplasia as well as cancer cells. Normal bile duct in non-tumor area showed low expression of Wnt3a, while hepatocytes, Kupffer cells, and inflammatory cells showed negative staining. No significant correlation was observed between Wnt3a expression and clinical–pathological findings or survival data.
Furthermore, the normal bile duct from a cadaveric donor showed barely positive staining of Wnt5a. Of the 38 intrahepatic CCA patients explored, 9 (23.7 %) were negative stained for Wnt5a and 29 (76.3 %) were weakly to strongly positive for tumor cells. Normal bile duct epithelia showed a weak cytoplasm staining for Wnt5a, whereas in hepatocytes and inflammatory cells there were no staining. Interestingly, the log-rank analysis indicated that CCA patients with Wnt5a positive had significantly lower survival than those with Wnt5a negative (P=0.009) as demonstrated in Fig. 2c.
All of CCA patients exhibited cytoplasmic Wnt7b staining of tumor cells (Fig. 2a). Low and high cytoplasmic Wnt7b expression were observed in 25 (65.8 %) and 13 cases (34.2 %) of CCA patients, respectively. Moreover, nuclear staining was mostly found in the bile ducts with hyperplasia as well as tumor cells. Wnt7b staining was also seen in the cytoplasm of hepatocytes and some infiltrating cells. No significant correlation was observed between Wnt7b expression and clinico-pathological findings as well as survival data.
β-Catenin expression in CCA is presented in Fig. 2b. Membrane expression of β-catenin was observed in hepatocytes and normal bile duct epithelium in non-tumor area as well as hyperplastic ducts. β-Catenin expression was not found in inflammatory and stromal cells. In the tumor area, immunohistochemical analysis revealed the reduced membranous expression of β-catenin. The remaining 28 (73.7 %) tumors had a cytoplasmic β-catenin expression. Moreover, aberrant nuclear expression for β-catenin was observed in CCA cells. No significant correlation was observed between β-catenin expression and clinico-pathological findings, while the log-rank analysis indicated that there was a trend toward a correlation of cytoplasmic β-catenin expression and poor survival rate of Fig. 2c. Moreover, significantly, β-catenin expression was correlated with Wnt3, Wnt5a, and Wnt7b expression (P=0.047, 0.014, and 0.005, respectively), indicating the upstream regulatory molecules of β-catenin in CCA (Table 2).
Table 2.
Correlation of β-catenin expression and clinico-pathological data of CCA patients
| Variable | No. of patients | β-Catenin score |
||
|---|---|---|---|---|
| Low | High | P value | ||
| Age (years) | ||||
| <56 | 20 | 15 | 5 | 0.179 |
| >56 | 18 | 9 | 9 | – |
| Gender | ||||
| Female | 10 | 8 | 2 | 0.268 |
| Male | 28 | 16 | 12 | – |
| Histological grading | ||||
| Papillary | 8 | 6 | 2 | 0.684 |
| Non-papillary | 30 | 18 | 12 | – |
| Metastasis stage | ||||
| Negative | 19 | 11 | 8 | 0.737 |
| Positive | 19 | 13 | 6 | – |
| Wnt3 expression | ||||
| Negative | 22 | 17 | 5 | 0.047* |
| Positive | 16 | 7 | 9 | – |
| Wnt3a expression | ||||
| Negative | 3 | 3 | 0 | 0.283 |
| Positive | 35 | 21 | 14 | – |
| Wnt5a expression | ||||
| Negative | 9 | 9 | 0 | 0.014* |
| Positive | 29 | 15 | 14 | – |
| Wnt7b expression | ||||
| Low expression | 25 | 20 | 5 | 0.005* |
| High expression | 13 | 4 | 9 | – |
P value equal to or less than 0.05 was considered statistically significant
LPS-induced Wnt3 expression in human macrophage cell line
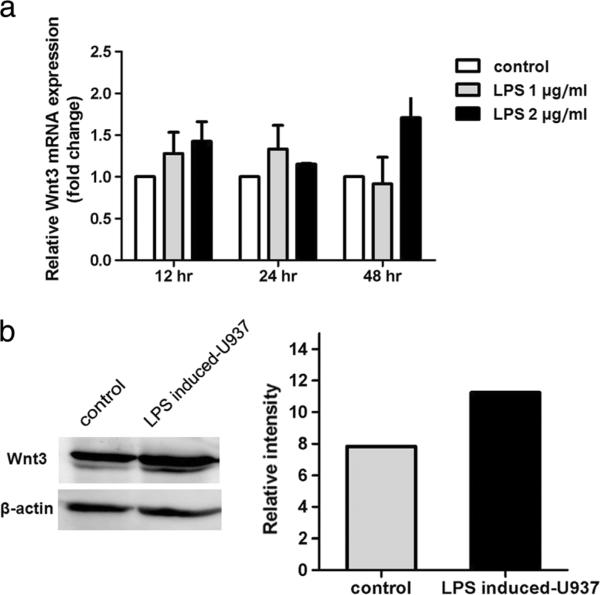
As demonstrated by immunohistochemistry, Wnt3 is predominantly expressed in Kupffer cells and inflammatory cells residing in tumor and adjacent non-tumor tissues. We therefore hypothesized that an inflammatory reaction via these specialized cells could mediate β-catenin activation. To test this hypothesis, we investigated the expression of Wnt3 in a macrophage cell line upon LPS stimulation. Quantitative RT-PCR (qRT-PCR) assay revealed that the Wnt3 mRNA level in LPS-induced cells increased when compared to their corresponding untreated cells and reached the highest expression level at 48 h of induction with 2 μg/ml of LPS as shown in Fig. 4a. Moreover, western blot analysis demonstrated that the protein expression of Wnt3 in LPS induced-cells was elevated when compared to non-treated control cells (Fig. 4b).
Fig. 4.
Wnt3 mRNA and protein expression in LPS-treated macrophage cell line. a Relative real-time RT-PCR was performed to detect Wnt3 mRNA level upon being treated with LPS. The data represented the results of two independent experiments. Each bar represents the mean ± SD. b Western blot analysis showed increased Wnt3 protein level in LPS-induced U937 cells. Apparent intensity of bands on the membranes was analyzed by ImageQuant analysis software
AMCM-induced β-catenin nuclear translocation in CCA cell lines
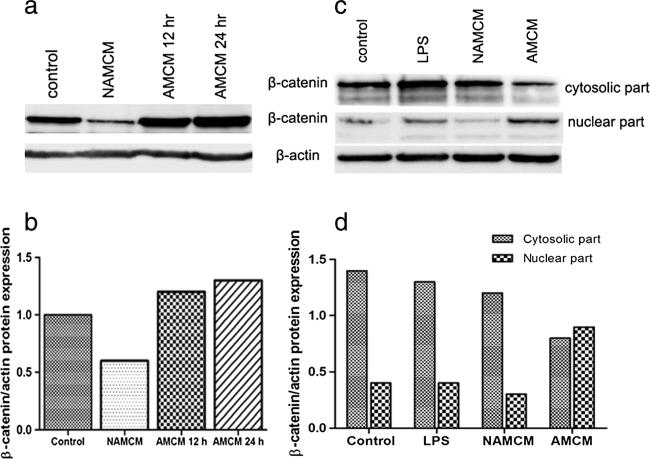
In order to confirm that factors which are secreted from activated macrophages (probably including Wnt3) that could promote β-catenin activation in CCA cell lines, U937 human macrophage cells were untreated or treated with 2 μg/ml of LPS for 48 h to become activated macrophages. Conditioned media from both non-activated macrophages (NAMCM) and conditioned media from activated macrophages (AMCM) were collected. The CCA cell line (M214) was treated with RPMI (negative control), 2 μg/ml of LPS, NAMCM, and AMCM for 12 and 24 h, respectively. Western blot analysis showed that treatment with AMCM resulted in increasing of β-catenin protein levels at 12 and 24 h after treatment (Fig. 5a, b). In contrast, no change of β-catenin expression was observed in CCA cells after LPS or NAMCM treatment. Moreover, cytosolic and nuclear β-catenin in M214 was detected by using western analysis (Fig. 5c, d). The result revealed that treatment of M214 with AMCM for 24 h resulted in elevation of β-catenin level in the nucleus of cells. This change correlated with decreasing level of cytosolic β-catenin.
Fig. 5.
β-Catenin activation in CCA cell lines after treatment with AMCM. CCA cell line (M214) was treated with 2 μg/ml LPS, conditioned media from untreated macrophages (NAMCM), LPS-activated macrophages (AMCM) or left untreated (control) at the indicated time. Western blot analysis was performed to determine the protein levels of β-catenin from whole cell lysates of CCA cell line (a, b). Cytosolic and nuclear β-catenin was investigated in M214 cells (c, d). β-Actin was used to check for equal loading of the gel. AMCM activated macrophage conditioned media, NAMCM non-activated macrophage condition media
Knocking down of β-catenin by siRNA reduced CCA cell growth
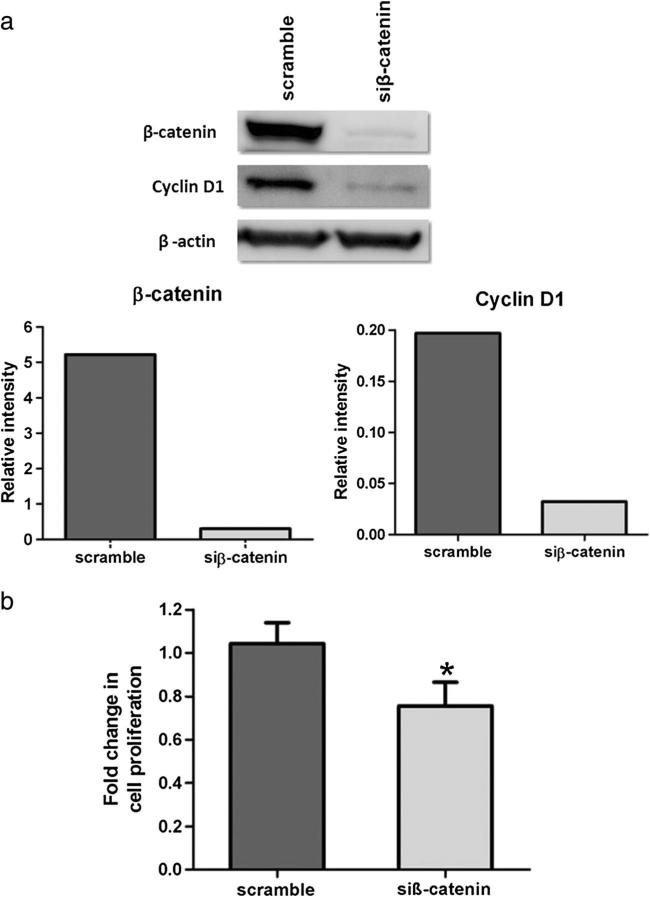
We subsequently evaluated whether β-catenin was involved in CCA cell growth. The M214 CCA cell line was transiently transfected with either β-catenin siRNA to suppress β-catenin protein translation or with the scrambled siRNA as a control. After 48 h of transfection, western blot analysis revealed 90 % suppression of β-catenin protein by siRNA (Fig. 6a). The effect of β-catenin knockdown on cell viability was determined by trypan blue counting. The results showed that β-catenin suppression caused a significant decrease in the growth rate of M214 cells when compared with scrambled siRNA-treated cells (P=0.02, Fig. 6b).
Fig. 6.
Effect of siRNA-mediated knockdown of β-catenin in M214 CCA cell line. Western blot analysis showed a markedly decreased of β-catenin protein level after 48 h of transfection (a). β-Catenin siRNA transfection significantly inhibited CCA cell proliferation (b). *P<0.05
We also evaluated whether the decrease in β-catenin protein in M214 cells following siRNA treatment could affect the transcriptional regulatory function of β-catenin. Western blot analysis was performed to determine the effect of downregulation of β-catenin on its targets, cyclin D1 in M214 cells. Our results showed that siRNA-mediated downregulation of β-catenin expression caused decreased level of cyclin D1 (Fig. 6a).
Discussion
Accumulated evidence indicates that the Wnt/β-catenin signaling pathway is associated with many tumor types [9]. In humans, the Wnt family is composed of 19 structurally related molecules. The members exhibit unique expression patterns and distinct functions in development [20]. There are several studies which have shown aberrant expression of Wnt molecules in various types of human cancers [21–23]. In this study, we profiled Wnt genes by qRT-PCR and revealed that three Wnt genes, Wnt3a, Wnt5a, and Wnt7b, were significantly overexpressed in CCA patients compared with their adjacent non-tumor tissues and normal liver tissues. We also showed that Wnt3 expression was downregulated in the malignant cells of CCA tumors.
We then investigated the expression and localization of four Wnt proteins in CCA patients. In the 40 cases of human CCA examined, most showed positive staining of Wnt3a, Wnt5a, and Wnt7b in tumor cells. In contrast, Wnt3 was predominantly expressed in Kupffer cells as well as inflammatory cells, while only 40 % of cancer cells showed positive Wnt3 staining. The predominant Wnt3 expression in macrophage cells might hint that the interactions between neoplastic and stromal cells contribute to tumor progression as similar to the previous report which demonstrated the critical of Wnt5a signaling for macrophage-induced invasion of breast cancer cell lines [24]. The cumulative survival analysis demonstrated that CCA patients with Wnt5a positive expression had a significantly shorter survival time than those without Wnt5a expression. A similar finding was reported in ovarian cancer [25]. This indicates that Wnt5a expression in tumor cells might serve as a prognostic marker for CCA. Conversely, it has been reported that recombinant Wnt 5a decreased cell proliferation in vitro [26]. Therefore, Wnt5a might have double faces of the functions which depend on their microenvironment.
β-Catenin is a key mediator of the Wnt/β-catenin pathway. In normal inactivated tissues, β-catenin is primarily located in the membrane and the cytoplasm of cells and the expression is kept at low levels [27]. The role of free cytoplasmic β-catenin has been revealed to be the co-activator for a family of transcription factors, TCF/LEF, which results in activating expression of TCF/LEF-regulated target genes [28]. The present study revealed the immunolocalization of β-catenin in CCA patients. Our results showed that loss of membranous β-catenin in tumors. Moreover, most of CCA patients had a cytoplasmic β-catenin expression and nuclear translocation of β-catenin was also found in cancer cells. These results support other studies which have reported the accumulation of cytoplasmic and nuclear β-catenin in CCA [29, 30]. Sugimashi and coworkers demonstrated that the reduced membranous expression of β-catenin is associated with non-papillary intrahepatic CCA, which have a more malignant behavior and that nuclear translocation of β-catenin results in oncogenic events [27]. These results suggest that the aberrant cytoplasm and nuclear expression of β-catenin might be involved in carcinogenesis and progression of CCA. In addition, we also found a significant correlation of abnormal β-catenin expression with Wnt3, Wnt5a, and Wnt7b expression. Our findings suggested that Wnt3, Wnt5a, and Wnt7b might be the key upstream molecules that activate the Wnt/β-catenin signaling pathway in CCA.
A number of studies have established that inflammation contributes to several types of malignancies, including CCA [2–7]. Previous study of CCA has demonstrated that the presence of Ov antigen was associated with heavy inflammatory cell infiltration, particularly with mononuclear cells [31]. In the present study, immunohistochemistry results showed that inflammatory cells surrounding tumor cells had strong Wnt3 expression. Therefore, we postulated that inflammatory conditions could modulate β-catenin activation in CCA. In this study, we used the human U937 macrophage cell line to evaluate whether LPS can induce Wnt3 expression. The result showed that treatment with 2 μg/ml of LPS for 48 h stimulated Wnt3 mRNA and protein expression in the macrophage cell line. Therefore, inflammation induced by LPS could induce Wnt3 expression in macrophages.
Hence, we examined whether LPS-induced Wnt3 expression in macrophages could mediate β-catenin activation in CCA cells. There are many studies that have demonstrated that the crosstalk between the tumor cells and macrophages was mediated by soluble factors [32]. Therefore, CCA cells were exposed to conditioned media from LPS-induced macrophages, and then β-catenin activation was investigated by western blot analysis. We demonstrated that conditioned media from LPS-induced macrophages was able to increase β-catenin protein level of CCA cells, whereas conditioned media from non-activated macrophages did not, suggesting that factors secreted by activated macrophages were responsible for β-catenin activation in CCA cells. These observations are supported by our study showing that activated macrophages increased in Wnt3 expression. It implies that Wnt3 could be one factor which is secreted from activated macrophages and mediates cross-talking between carcinoma cells and stromal cells resulting in β-catenin activation in CCA cells. Although, other cytokines which can be secreted by LPS-activated macrophage [33] might also activate β-catenin nuclear translocation observed in the present study. Additionally, an inflammation condition, as well as activated macrophages-promoted condition which induces β-catenin activation, has been reported [19]. Vice versa, the activation of Wnt/ β-catenin signaling pathway triggers and inflammatory reaction has also been documented [34].
The increased levels of β-catenin frequency found in both premalignant and malignant cells are associated with increased rates of cellular proliferation [35, 36]. In this study, we demonstrated that β-catenin was involved in regulating of CCA cell proliferation. The M214 CCA cell line transfected with siRNA targeting β-catenin showed less cell viability. Moreover, our current data revealed that siRNA-mediated downregulation of β-catenin inhibited the expression of growth-related gene, cyclin D1, which is a specific target gene of β-catenin. Our results are consistent with previous studies showing that suppressed β-catenin by siRNA could successfully induce growth inhibition in a variety of cancers, both in vitro and in vivo [37, 38]. Nonetheless, our results demonstrated that suppression of β-catenin resulted in a 20 % reduction in CCA cell growth, which implies that inhibition of only β-catenin may not be enough to inhibit CCA cell growth. This was consistent with a previous study which indicated that other signaling pathways participate in controlling of CCA cell growth [39]. Therefore, blocking only β-catenin may result in activation of an alternative pathway giving rise to some advantage to cell growth so blocking two or more pathways would be a more effective therapeutic approach to treatment of CCA. Recently, an inhibition of Wnt/β-catenin signaling which downregulates P-glycoprotein and reverses multi-drug resistance of CCA cell has been reported [40].Additionally, the RAR gamma playing an important role in the proliferation, metastasis, and chemoresistance of CCA through simultaneous activation of the Akt/NF-kappaB and Wnt/β-catenin pathways which might be serving as a potential molecular target for CCA treatment has been demonstrated [41].
In summary, our results indicate that many Wnt genes were abundantly expressed in CCA. The expression can also be found in inflammatory cells, and LPS can induce Wnt upregulation in macrophages. It implies that Wnt/β-catenin pathway may be involved in inflammation-associated CCA. In addition, abrogation of β-catenin expression induced growth inhibition of CCA cells. Therefore, suppression of Wnt/β-catenin signaling could be a potential target for inhibiting CCA cell growth.
Acknowledgments
This study is supported by the grant of the Thailand Research Fund (grant no. MRG5400834) and the Research Assistantship Grant of the Faculty of Medicine, Khon Kaen University (grant no. AS55203) to WL. PB is supported by the scholarship of the Research Strengthening Grant from BIOTEC-NSTDA. GJR is supported in part by the Irving J. Sherman Research Professorship in Neurosurgery Research and the Virginia and D.K. Ludwig Fund for Cancer Research. We thank Professor Ross H Andrews for his editorial assistance.
Footnotes
Conflicts of interest None
Contributor Information
Watcharin Loilome, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Pornpan Bungkanjana, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Anchalee Techasen, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Nisana Namwat, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Puangrat Yongvanit, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Anucha Puapairoj, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Narong Khuntikeo, Department of Biochemistry and Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Gregory J. Riggins, Department of Neurosurgery, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
References
- 1.Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32(Suppl):S82–91. doi: 10.1093/jjco/hye134. [DOI] [PubMed] [Google Scholar]
- 2.Dechakhamphu S, Pinlaor S, Sitthithaworn P, Bartsch H, Yongvanit P. Accumulation of miscoding etheno-DNA adducts and highly expressed DNA repair during liver fluke-induced cholangiocarcinogenesis in hamsters. Mutat Res. 2010;691(1–2):9–16. doi: 10.1016/j.mrfmmm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dechakhamphu S, Pinlaor S, Sitthithaworn P, Nair J, Bartsch H, Yongvanit P. Lipid peroxidation and etheno DNA adducts in white blood cells of liver fluke-infected patients: protection by plasma alpha-tocopherol and praziquantel. Cancer Epidemiol Biomarkers Prev. 2010;19(1):310–8. doi: 10.1158/1055-9965.EPI-09-0849. [DOI] [PubMed] [Google Scholar]
- 4.Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25(8):1535–42. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 5.Pinlaor S, Yongvanit P, Hiraku Y, Ma N, Semba R, Oikawa S, et al. 8-nitroguanine formation in the liver of hamsters infected with Opisthorchis viverrini. Biochem Biophys Res Commun. 2003;309(3):567–71. doi: 10.1016/j.bbrc.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38(12):4634–9. [PubMed] [Google Scholar]
- 7.Thanan R, Murata M, Pinlaor S, Sithithaworn P, Khuntikeo N, Tangkanakul W, et al. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol Biomarkers Prev. 2008;17(3):518–24. doi: 10.1158/1055-9965.EPI-07-2717. [DOI] [PubMed] [Google Scholar]
- 8.Dokduang H, Juntana S, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, et al. Survey of activated kinase proteins reveals potential targets for cholangiocarcinoma treatment. Tumour Biol. 2013;34(6):3519–28. doi: 10.1007/s13277-013-0930-9. [DOI] [PubMed] [Google Scholar]
- 9.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 10.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4(8):653–71. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 11.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13(14):4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 13.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 14.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 15.Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8(4):279–89. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugh TJ, Dillon SA, Taylor BA, Pignatelli M, Poston GJ, Kinsella AR. Cadherin-catenin expression in primary colorectal cancer: a survival analysis. Br J Cancer. 1999;80(7):1046–51. doi: 10.1038/sj.bjc.6690461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal AS, Kennedy CH, Narayan S. A correlation of APC and cmyc mRNA levels in lung cancer cell lines. Oncol Rep. 1999;6(6):1253–6. doi: 10.3892/or.6.6.1253. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Fabre M, Branchereau S, Gauthier F, Perilongo G, Buendia MA. Activation of beta-catenin in epithelial and mesenchymal hepatoblastomas. Oncogene. 2000;19(4):498–504. doi: 10.1038/sj.onc.1203356. [DOI] [PubMed] [Google Scholar]
- 19.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27(12):1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CL, Liu D, Ishikawa S, Nakashima T, Nakashima N, Yokomise H, et al. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer. 2008;44(17):2680–8. doi: 10.1016/j.ejca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Uraguchi M, Morikawa M, Shirakawa M, Sanada K, Imai K. Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res. 2004;83(4):327–32. doi: 10.1177/154405910408300411. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Oue N, Sato A, Hasegawa Y, Matsubara A, Yasui W, et al. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29(14):2036–46. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 24.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, et al. Wnt5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103(14):5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21(2):280–8. doi: 10.1097/IGC.0b013e31820aaadb. [DOI] [PubMed] [Google Scholar]
- 26.DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S, et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2008;295(6):G1150–8. doi: 10.1152/ajpgi.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 29.Lim K, Han C, Xu L, Isse K, Demetris AJ, Wu T. Cyclooxygenase-2-derived prostaglandin E2 activates beta-catenin in human cholangio-carcinoma cells: evidence for inhibition of these signaling pathways by omega 3 polyunsaturated fatty acids. Cancer Res. 2008;68(2):553–60. doi: 10.1158/0008-5472.CAN-07-2295. [DOI] [PubMed] [Google Scholar]
- 30.Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, et al. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14(9):900–5. doi: 10.1038/modpathol.3880409. [DOI] [PubMed] [Google Scholar]
- 31.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30(6):735–40. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 32.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28(44):3892–902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Techasen A, Loilome W, Namwat N, Dokduang H, Jongthawin J, Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):115–8. [PubMed] [Google Scholar]
- 34.Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122(2):586–99. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 36.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 37.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9(4):1291–300. [PubMed] [Google Scholar]
- 38.Wang JS, Zheng CL, Wang YJ, Wen JF, Ren HZ, Liu Y, et al. Gene silencing of beta-catenin by RNAi inhibits cell proliferation in human esophageal cancer cells in vitro and in nude mice. Dis Esophagus. 2009;22(2):151–62. doi: 10.1111/j.1442-2050.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 39.Loilome W, Juntana S, Namwat N, Bhudhisawasdi V, Puapairoj A, Sripa B, et al. PRKAR1A is overexpressed and represents a possible therapeutic target in human cholangiocarcinoma. Int J Cancer. 2011;129(1):34–44. doi: 10.1002/ijc.25646. [DOI] [PubMed] [Google Scholar]
- 40.Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/beta-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104(10):1303–8. doi: 10.1111/cas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang GL, Luo Q, Rui G, Zhang W, Zhang QY, Chen QX, et al. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol Cell Biol. 2013;33(17):3416–25. doi: 10.1128/MCB.00384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]