Abstract
Background
Obtaining high quality genomic DNA safely and economically is vital for diverse studies of large populations aimed at evaluating the role of genetic factors in susceptibility to disease.
Aim
This study was to test a protocol for the extraction of high quality genomic DNA from saliva samples obtained with mouthwash and taken from patients with periodontal disease.
Methods
Saliva samples were taken from 60 patients and then stored at room temperature. DNA extraction was carried out at distinct post-sampling times (10, 20 and 30 days). Evaluation of genomic DNA was performed with spectrophotometry, electrophoresis, and PCR genotyping and sequencing.
Results
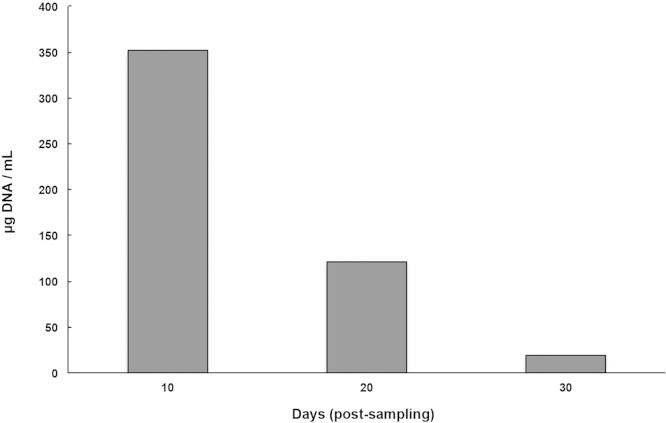
The greatest concentration of DNA obtained was 352 μg at 10 days post-sampling, followed by 121.025 μg and 19.59 μg at 20 and 30 days, respectively. When determining the purity of DNA with the spectrophotometric ratio of 260/230, the relations of 1.20, 1.40 and 0.781 were obtained for 10, 20 and 30 days, respectively. In all samples, it was possible to amplify the product of 485 bp and the sequence of the amplicons showed 95% similarity to the reference sequence.
Conclusion
The present protocol represents an easy, safe and economical technique for obtaining high quality genomic DNA.
Keywords: Genomic DNA, Chronic periodontitis, Saliva, Mouthwash, PCR
1. Introduction
It is of vital importance to carry out genetic analyses of the most prevalent diseases in large populations. This need has fueled research into techniques for obtaining high quality genomic DNA in an easy, safe and economical manner. Although blood samples provide an excellent means of obtaining DNA of sufficient quantity and quality for such genetic analyses, this procedure is invasive and unpleasant for many people, making it difficult for studies of large populations. Consequently, there is a search for alternatives.
An easier and less invasive way to obtain DNA is with saliva samples. Saliva provides a great number of nucleated cells, such as epithelial cells, leukocytes, and Langerhans cells. Additionally, it contains bacteria, virus, fungus, salts, and food residues.1, 2 The presence of bacteria represents a disadvantage, because these microorganisms can degrade human DNA. Moreover, if the sample is maintained at room temperature, these organisms can easily grow and proliferate.3
Different protocols have been developed for obtaining DNA from saliva. The commonly used methods employ polyethylene swabs or brushes, treated Guthrie-type cards, rinses with saline solution or 3% sucrose.4, 5, 6, 7, 8, 9, 10, 11, 12, 13 However, these techniques also have their disadvantages since the sample must be frozen or processed immediately so as to assure the quantity and quality of DNA. This requirement represents a difficulty for their use in studies that require transportation of the sample and its maintenance at room temperature. Additionally, Rogers et al. documented that the use of cotton swabs and polyethylene brushes results in the worst quantity and quality of DNA.14
Garcia-Closas et al. documented in an epidemiological study of cancer that with this technique the average percentage of bacteria is 50.5% than that found in saliva samples obtained without mouthwash.15 Feigelson et al. reported that only 40% percent of the bacteria remained in saliva samples after using mouthwash with alcohol, and that the quantity and quality of DNA was stable during one week at room temperature.16 Lum and Le Marchand proposed and documented the utilization of a mouthwash with alcohol (Listerine®, Johnson & Johnson) for obtaining high quality genomic DNA from saliva samples, demonstrating diminished bacterial growth at a temperature of 37 °C for one week. This technique can be used for self-collection of samples sent by mail.17, 18 The drawback of using this technique for extracting DNA is that it employs phenol–chloroform, which is a known toxic, mutagenic and carcinogenic agent.19 There are kits on the market for the extraction of DNA that are non-toxic and easy to use, but the cost represents a problem for studies involving large populations. Therefore, there is the need to search for an easy, safe and economical manner to obtain high quality genomic DNA.
The aim of the present study was to evaluate a technique for the extraction of high quality genomic DNA that fulfills these requirements. For this purpose, we designed a model for obtaining saliva samples with a mouthwash, and tested it under the difficult buccal conditions of patients with severe chronic periodontitis that were not under treatment. This infection is a complex and multifactorial disease that results from the interaction of the host defense mechanisms with the plaque microorganisms. This interaction leads to the primary clinical features of periodontitis, which are gingival inflammation, attachment loss periodontal pocketing and alveolar bone loss.20, 21, 22 The oral conditions of these patients give us an ideal scenario for assessing our protocol.
2. Materials and methods
This study was registered on https://clinicaltrials.gov/ as NCT02523326.
2.1. Patients
The study was conducted with 60 patients diagnosed with severe chronic periodontitis in the Periodontic Clinic of the Interdisciplinary Health Sciences Center (Santo Tomás Unit), Instituto Politécnico Nacional. The protocol of this study was approved by the Research and Bioethics Committee of the Escuela Superior de Medicina, Instituto Politécnico Nacional. Informed consent was obtained from all participants included in the study, who signed the appropriate form allowing for the collection of saliva samples and the extraction of genomic DNA. Ethical norms for research on human beings, established by the Declaration of Helsinki (1975; updated most recently in 2008) were strictly followed. Each patient was instructed how to prepare for saliva sampling, which involved brushing their teeth at least 2 h previously and abstaining from eating or drinking anything.
2.2. Taking the sample
In a tube of 50 ml was placed 10 ml mouthwash (Listerine Cool Mint, Johnson & Johnson, 21.6% alcohol). Patients were instructed to rinse their mouth vigorously with this quantity of mouthwash during 30 s, then spit it into the tube. Once collected, the samples were stored at room temperature (20–24 °C). The 60 samples were divided into 3 groups (n = 20), and the extraction of DNA was performed at distinct times: group A at 10 days, group B at 20 days and group C at 30 days.
2.3. DNA extraction
To separate the mouthwash, the tube was centrifuged during 10 min at 10,750 × g. The supernatant was poured out and the cellular pellet washed with 1 ml of PBS, resuspended in the vortex for 30 s, and transferred to a new 2 ml tube. It was then centrifuged for 5 min at 2000 × g, the supernatant poured out, and the pellet washed with 1.5 ml of PBS and resuspended in the vortex for 30 s. To this solution was added a 1 ml solution of lysis of nucleated cells (Wizard® Genomic DNA Purification Kit) before being shaken for 20 s, followed by incubation at 37 °C for 10 min. After adding 300 μL of protein precipitator solution (Wizard® Genomic DNA Purification Kit), the mixture was placed on ice for 5 min and then centrifuged at 15,000 × g for 3 min. The supernatant was put in a 1.5 ml tube containing 600 μL cold Isopropanol and homogenized gently, then centrifuged at 15,000 × g for 3 min. The supernatant was poured out, and 600 μL of 70% ethanol was added to a fresh preparation, which was centrifuged at 15,000 × g for 3 min. The supernatant was poured out, and the tube was completely dried at room temperature. Finally, 100 μL rehydration solution (Wizard® Genomic DNA Purification Kit) was added and the mixture was incubated at 64 °C for 1 h before storing the samples at −20 °C to await further processing.
2.4. DNA evaluation
Evaluation of the concentration and purity of DNA was determined by spectrophotometry with an ACT-GENE ASP-3700 spectrometer. The concentration of DNA was evaluated at 260 nm, while purity was estimated with the ratios of 260/230 and 260/280. The integrity of genomic DNA was assessed in a 0.8% agarose gel in 1% TBE (89 mM Tris Borate, 89 mM boric acid, 2 mM EDTA) by electrophoresis, and then the DNA was visualized with Gel-Red and read under UV light. DNA extracted from blood samples was used as the positive control.
2.5. PCR
The PCR reaction was carried out in an Eppendorf Mastercycler® Personal apparatus with the HotStarTaq® Master Mix Qiagen kit (No. de Cat 203445), following the manufacturer's instructions. To verify the quality and species (human) of the DNA extracted, primers were designed for the rs679620 polymorphism (forward 5′-GGG GCT TAA GGC ACA TGA GT-3; reverse 5′-ACT TCG GGA TGC CAG GAA AG-3′; Tm: 56 °C, 40 cycles) that amplified a product of 485 bp. The final PCR product was evaluated with a 2% agarose gel. DNA extracted from blood samples was used as a positive control.
2.6. Sequencing
To further verify the quality and species of the DNA obtained, the product resulting from PCR was sequenced with an Applied Biosystems 3130 sequencer and the BigDye® Terminator v3.1 Cycle Sequencing kit, following the manufacturer's instructions. Data were analyzed with Geneious version 7.1.3 software23 using the NC_000011.10 sequence as the reference.
3. Results
The average age of study participants was 38 years (range 30–60) for the 40 women and 20 men. The mean total concentration of DNA in group A (after 10 days) was 352 μg (with a range of 138–506 μg), in group B (after 20 days) 121.025 μg (ranging from 90 to 193 μg), and in group C (after 30 days) 19.59 μg (ranging from 11.7 to 31.9 μg) (Fig. 1). Although the quantity of DNA diminished over time, it remained in optimal condition for performing PCR genotyping and sequencing.
Fig. 1.
Mean concentration of DNA, expressed in μg, obtained by spectrophotometry for the three different groups (at 10, 20 and 30 days post-sampling).
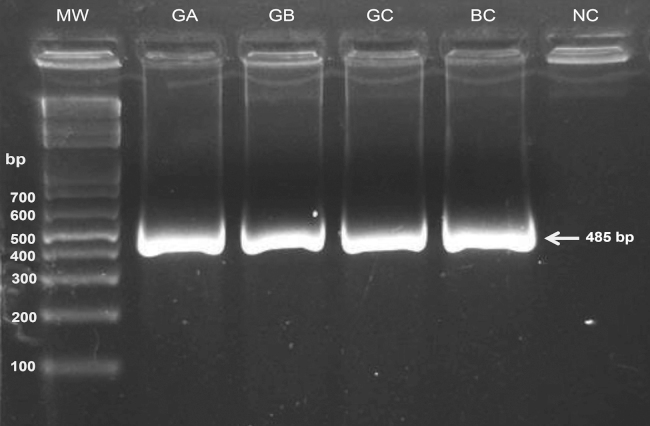
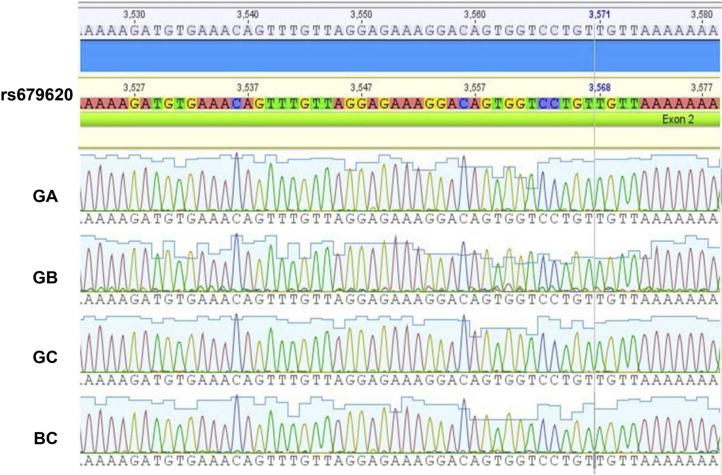
The result of the determination of purity with the spectrophotometric ratio of 260/230 was 1.20, 1.40 and 0.781 (for 10, 20 and 30 days post-sampling, respectively). For the ratio 260/280, the result was 1.50, 1.51 and 1.57. The integrity of DNA was determined by PCR, using agarose gel to visualize the presence of DNA of high molecular weight. In all samples, it was possible to amplify a product of 485 bp without the presence of unspecific products (Fig. 2). In the genotyping, the sequence of amplicons showed 95% similarity to the reference sequence (NC_000011.10) for all samples (Fig. 3).
Fig. 2.
PCR product. The image is representative of the amplification of rs679620 in DNA obtained from saliva samples and stored for different times: group A (GA) for 10 days, group B (GB) for 20 days, and group C (GC) for 30 days. DNA blood sample control (BC) and the negative control (NC).
Fig. 3.
An electropherogram representative of rs679620 in DNA extracted from a control blood sample (BC) and from saliva samples of the three distinct groups: group A (GA) at 10 days, group B (GB) at 20 days, and group C (GC) at 30 days post-sampling. A close similarity can be observed between all samples.
4. Discussion
It is important to control the presence of bacteria in a saliva sample that is to be used for obtaining genomic DNA, because the growth and proliferation of these microorganisms can degrade the desired product. A mid-range percentage of bacteria (34% and 50.5%) has been reported 15, 16 in saliva samples from patients using mouthwash with alcohol, which highlights the importance of this medium for obtaining saliva samples in order to prevent bacterial growth and protect human DNA.
In order to evaluate a new protocol, a model was designed with a mouthwash used to obtain saliva samples from patients having severe chronic periodontitis that was not yet treated. The inflammation and higher than normal concentration of bacteria of these patients provide excellent buccal conditions for testing the new protocol. All of the samples could be sequenced and genotyped, which suggests that the technique could possibly be used in the same manner in healthy people or patients with other diseases.
Various authors report that saliva samples obtained after the use of mouthwash can be stored at room temperature for 1–2 weeks without affecting the quality or quantity of DNA.17, 18, 24, 25 In the present study, we found that the samples stored at room temperature in Mexico City (20–24 °C) could be maintained stable and viable for up to 30 days. Although the DNA concentration was sharply reduced during this period, the quality and quantity of genomic DNA were still in optimal conditions for performing genotyping and sequencing.
There are reports of a wide range of DNA obtained from saliva samples. Tobal et al. reported that a range of 10–240 μg of DNA was extracted with phenol–chloroform from saliva samples in saline solution.13 Harty et al. reported a mean of 25.9 μg of DNA (a range of 2.0–204.5 μg) extracted from saliva samples obtained with sterile water mixed immediately with a transportation medium and stored at −70 °C for 3–36 months before DNA extraction.6 Garcia-Closas et al. reported a mean of 57.7 μg, 35.2 μg and 52.5 μg of DNA extracted from saliva samples obtained with mouthwash.15 In the present study after 10 days post-sampling we obtained a mean of 352 μg of DNA, after 20 days 121.025 μg, and after 30 days 19.59 μg. These mean quantities of DNA were extracted from saliva samples obtained with a mouthwash. This can be attributed to the buccal condition of the patients, the technique of extraction, and the mouthwash with alcohol.
The determination of DNA quality was done with spectrophotometry. A proportion of 1.7 to 1.8 is generally preferred for the 260/280 ratio, as this indicates limited protein and low organic contamination.14 We found a mean of 1.50, 1.51 and 1.57 corresponding to 10, 20 and 30 days post-sampling, respectively, which indicates moderate contamination. For the 260/230 ratio, on the other hand, values higher than 1.7 to 1.8 are preferred, as this indicates little salt and low contamination of alcohol.14 We found a mean of 1.20, 1.40 and 0.781 for the three different post-sampling times at which DNA was extracted, which indicates that the greatest contamination occurred at 30 days.
Buccal cells are becoming an important source of genomic DNA in epidemiological studies, but little is known about the effect of different sampling conditions on DNA quality and yield. We used a mouthwash protocol to collect buccal cell samples from volunteers diagnosed with severe chronic periodontitis.
Chronic periodontitis represents an extreme scenario of oral conditions: high amounts of bacteria, increased inflammation, and poor hygiene, which contribute to high readings DNA. The relevance of this protocol is that although different types of DNA present in the samples of buccal cells can be isolated human DNA quality, as shown in the electropherogram. Although several recent studies have focused on the best way to collect buccal cells as a source of genomic DNA, some unresolved issues remain. There is general agreement that buccal cells can provide high molecular weight DNA of sufficient quality for PCR-based analyses. However, the variations in performance of buccal cells by oral conditions have not been addressed in specific pathologies.
The medium that we utilized to obtain the sample was Listerine Cool Mint® (Johnson & Johnson), which is an antiseptic for daily use in the treatment of caries and periodontal disease. It is a combination of two phenols related to the essential oils thymol and eucalyptol, mixed with menthol and methyl salicylate in a hydroalcoholic vehicle at 26.9%.26, 27 The concentration of alcohol present prevents the growth and proliferation of bacteria, thus maintaining the sample in optimal conditions at room temperature.
The essential oils in the mouthwash have a high bactericidal activity against the majority of oral gram-positive and gram-negative bacteria.28, 29 Their bactericidal activity in situ allows for the destruction upon contact of bacteria such as those found in biofilm.30, 31, 32 Additionally, these essential oils have antifungal activity against species of Candida and antiviral activity against herpes simplex virus type 1 (HSV-1)33, 34 and their cytoprotective and antioxidant properties are well documented.35, 36 These conditions allow for the safe use of this product to obtain a saliva sample relatively low in bacteria.
The technique herein proposed allows for optimizing the quantity of DNA in the sample. Moreover, it is safe for the researcher, because phenol–chloroform is not used, which is carcinogenic and can cause liver and kidney damage when lodged in these organs.19 The laboratory procedure that we used for processing the samples involved a relatively safe colorant known as GelRed, which is an alternative to ethidium bromide (the colorant most commonly used for nucleic acids). The latter compound is a strongly mutagenic agent that represents a danger for the environment and the researcher.
Whereas Lum and Le Marchand reported that samples remained stable for 1–2 weeks (using a mouthwash with alcohol), sample stability in the present study lasted for up to 30 days. Hence, the present study supports the use of mouthwash with alcohol as an easy, safe and economical alternative for obtaining genomic DNA from epithelial cells in saliva samples. Which can be stored at −80 °C for up to 1 year did not significantly deplete the amount of DNA in the samples.15
The present results indicate a considerable difference in the total concentration of genomic DNA obtained from saliva at 10, 20 and 30 days post-sampling. Even though this concentration diminished over time, the DNA remained in optimal condition for performing PCR genotyping and sequencing, thus making it possible to search for a polymorphism of the simple rs679620 nucleotide. Saliva samples obtained with mouthwash represent a good alternative to blood samples for extracting sufficient quantities of high quality DNA. The methodology used presently is non-toxic and allows for storage of samples at room temperature. Hence, the technique herein reported is easy, safe, economical and reliable. It should certainly be of great utility in long-term cohort studies, epidemiological studies involving distant geographic sites, and in cases or zones, where obtaining blood samples is unsafe or inconvenient.
Funding
This study was supported by the through a grant (#287789) by the Consejo Nacional de Ciencia y Tecnología (CONACYT)).
Conflicts of interest
The authors have none to declare.
Ethical approval
The protocol of this study was approved by the Research and Bioethics Committee of the Escuela Superior de Medicina, Instituto Politécnico Nacional. Informed consent was obtained from all participants included in the study, who signed the appropriate form. Ethical norms for research on human beings, established by the Declaration of Helsinki (1975; updated most recently in 2008) were strictly followed.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This study was supported by the through a grant (#287789) by the Consejo Nacional de Ciencia y Tecnología (CONACYT). The authors express their gratitude to COTEBAL, Instituto Politécnico Nacional for excellent attention and superb support. We also are deeply grateful to the patients for their cooperation in donating saliva samples for the current research.
References
- 1.Terasaki P., Chia D., Sugich I. Saliva as DNA source for HLA typing. Hum Immunol. 1998;59:597–598. doi: 10.1016/s0198-8859(98)00057-3. [DOI] [PubMed] [Google Scholar]
- 2.Saftlas A.F., Waldschmidt M., Logsden-Sackett N., Triche E., Field E. Optimizing buccal cell DNA yields in mothers and infants for human leukocyte antigen genotyping. Am J Epidemiol. 2004;160:77–84. doi: 10.1093/aje/kwh171. [DOI] [PubMed] [Google Scholar]
- 3.Harty L.C., Shields P.G., Winn D.M., Caporaso N.E., Hayes R.B. Self-collection of oral epithelial cell DNA under instruction from epidemiologic interviewers. Am J Epidemiol. 2000;151:199–205. doi: 10.1093/oxfordjournals.aje.a010188. [DOI] [PubMed] [Google Scholar]
- 4.King I.B., Satia-Abouta J., Thornquist M.D., Bigler J., Patterson R.E., Kristal A.R. Buccal cell DNA yield, quality, and collection costs: comparison of methods for large-scale studies. Cancer Epidemiol Biomark Prev. 2002;11:1130–1133. [PubMed] [Google Scholar]
- 5.Holland N.T., Smith M.T., Eskenazi B., Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat Res. 2003;543:217–234. doi: 10.1016/s1383-5742(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 6.Harty L.C., Garcia-Closas M., Rothman N., Reid Y.A., Tucker M.A., Hartge P. Collection of buccal cell DNA using treated cards. Cancer Epidemiol Biomark Prev. 2000;9:501–506. [PubMed] [Google Scholar]
- 7.Meulenbelt I., Droog S., Trommelen G.J., Boomsma D.I., Slagboom P.E. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. Am J Hum Genet. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- 8.Greer C.E., Wheeler C.M., Manos M.M. Sample preparation and PCR amplification from paraffin-embedded tissues. PCR Methods Appl. 1994;3:113–122. doi: 10.1101/gr.3.6.s113. [DOI] [PubMed] [Google Scholar]
- 9.Freeman B., Powell J., Ball D., Hill L., Craig I., Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- 10.Andrisin T.E., Humma L.M., Johnson J.A. Collection of genomic DNA by the noninvasive mouthwash method for use in pharmacogenetic studies. Pharmacotherapy. 2002;22:954–960. doi: 10.1592/phco.22.12.954.33598. [DOI] [PubMed] [Google Scholar]
- 11.Cao W., Hashibe M., Rao J.Y., Morgenstern H., Zhang Z.F. Comparison of methods for DNA extraction from paraffin-embedded tissues and buccal cells. Cancer Detect Prev. 2003;27:397–404. doi: 10.1016/s0361-090x(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 12.Cozier Y.C., Palmer J.R., Rosenberg L. Comparison of methods for collection of DNA samples by mail in the Black Women's Health Study. Ann Epidemiol. 2004;14:117–122. doi: 10.1016/S1047-2797(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 13.Tobal K., Layton D.M., Mufti G.J. Non-invasive isolation of constitutional DNA for genetic analysis. Lancet. 1989;2:1281–1282. doi: 10.1016/s0140-6736(89)91893-x. [DOI] [PubMed] [Google Scholar]
- 14.Rogers N.L., Cole S.A., Lan H.C., Crossa A., Demerath E.W. New saliva DNA collection method compared to buccal cell collection techniques for epidemiological studies. Am J Hum Biol. 2007;9:319–326. doi: 10.1002/ajhb.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Closas M., Egan K.M., Abruzzo J., Newcomb P.A., Titus-Ernstoff L., Franklin T. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomark Prev. 2001;10:687–696. [PubMed] [Google Scholar]
- 16.Feigelson H.S., Rodriguez C., Robertson A.S., Jacobs E.J., Calle E.E., Reid Y.A. Determinants of DNA yield and quality from buccal cell samples collected with mouthwash. Cancer Epidemiol Biomark Prev. 2001;10:1005–1008. [PubMed] [Google Scholar]
- 17.Lum A., Le Marchand L. A simple mouthwash method for obtaining genomic DNA in molecular epidemiological studies. Cancer Epidemiol Biomark Prev. 1998;7:719–724. [PubMed] [Google Scholar]
- 18.Le Marchand L., Lum-Jones A., Saltzman B., Visaya V., Nomura A.M., Kolonel L.N. Feasibility of collecting buccal cell DNA by mail in a cohort study. Cancer Epidemiol Biomark Prev. 2001;10:701–703. [PubMed] [Google Scholar]
- 19.Schwetz B.A., Leong B.K., Gehring P.J. Embryo- and fetotoxicity of inhaled chloroform in rats. Toxicol Appl Pharmacol. 1974;28:442–451. doi: 10.1016/0041-008x(74)90229-4. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Periodontology informational paper: the pathogenesis of periodontal diseases. J Periodontol. 1999;70:457–470. doi: 10.1902/jop.1999.70.4.457. [DOI] [PubMed] [Google Scholar]
- 21.Page R.C., Kornman K.S. The pathogenesis of human periodontitis: an introduction. Periodontol. 1997;2000(14):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 22.Zambon J.J. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 23.Kearse M., Moir R., Wilson A. Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxf, Engl) 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayney M.S., Poland G.A., Lipsky J.J. A noninvasive “swish and spit” method for collecting nucleated cells for HLA typing by PCR in population studies. Hum Hered. 1996;46:108–111. doi: 10.1159/000154335. [DOI] [PubMed] [Google Scholar]
- 25.Heath E.M., Morken N.W., Campbell K.A., Tkach D., Boyd E.A., Strom D.A. Use of buccal cells collected in mouthwash as a source of DNA for clinical testing. Arch Pathol Lab Med. 2001;125:127–133. doi: 10.5858/2001-125-0127-UOBCCI. [DOI] [PubMed] [Google Scholar]
- 26.Charles C.H., Mostler K.M., Bartels L.L., Mankodi S.M. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004;31:878–884. doi: 10.1111/j.1600-051X.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma N., Charles C.H., Lynch M.C. Adjunctive benefit of an essential oil-containing mouthrinse in reducing plaque and gingivitis in patients who brush and floss regularly: a six-month study. J Am Dent Assoc. 2004;135:496–504. doi: 10.14219/jada.archive.2004.0217. [DOI] [PubMed] [Google Scholar]
- 28.Sekino S., Ramberg P. The effect of a mouth rinse containing phenolic compounds on plaque formation and developing gingivitis. J Clin Periodontol. 2005;32:1083–1088. doi: 10.1111/j.1600-051X.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 29.Filoche S.K., Soma K., Sissons C.H. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol. 2005;20:221–225. doi: 10.1111/j.1399-302X.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 30.Albert-Kiszely A., Pjetursson B.E., Salvi G.E. Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis – a six month randomized controlled clinical trial. J Clin Periodontol. 2007;34:658–667. doi: 10.1111/j.1600-051X.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 31.Fine D.H., Furgang D., Sinatra K., Charles C., Mcguire A., Kumar L.D. In vivo antimicrobial effectiveness of an essential oil-containing mouth rinse 12 h after a single use and 14 days’ use. J Clin Periodontol. 2005;32:335–340. doi: 10.1111/j.1600-051x.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 32.Charles C.H., Pan P.C., Sturdivant L., Vincent J.W. In vivo antimicrobial activity of an essential oil-containing mouthrinse on interproximal plaque bacteria. J Clin Dent. 2000;11:94–97. [PubMed] [Google Scholar]
- 33.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 34.Meiller T.F., Silva A., Ferreira S.M., Jabra-Rizk M.A., Kelley J.I., Depaola L.G. Efficacy of listerine antiseptic in reducing viral contamination of saliva. J Clin Periodontol. 2005;32:341–346. doi: 10.1111/j.1600-051X.2005.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 36.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]