Abstract
Background:
There is a strong relationship between physical inactivity and low-grade inflammation and its adverse health outcomes, particularly cardiovascular disease. The level of anti-inflammatory and pro-inflammatory cytokines may be changed by exercise.
Objectives:
The aim of the present study was to determine the response of certain inflammatory biomarkers to exercise with differences in maximal oxygen consumption (VO2max). These biomarkers were IL-1β, TNF-α, hs-CRP, IL-6, sICAM-1, IL-10, and ratios of TNF-α/IL-10 and IL-6/IL-in circulating peripheral blood (PB).
Materials and Methods:
In a semi-experimental study, twenty male students who performed regular football exercise at least three days a week, for two years, were selected by easy sampling at Shahid Chamran university of Iran. Subjects were then randomly assigned to two groups: the protocol of the first group was 30 minutes of running at a speed of 65% of VO2max, and the second group performed six periodic repetitions with three minutes at a speed of 85% of VO2max with a 90-second rest between the repetitions. Blood samples were taken at baseline, immediately after the exercise and at rest. Cytokine levels were quantified by the Sandwich enzyme-linked immunosorbent assay (ELISA) method.
Results:
The first protocol resulted in a decrease of serum IL-1β to 3.77 ± 0.28 pg/mL at rest, from 4.33 ± 0.28 at baseline and 4.32 ± 0.34 immediately after exercise (P = 0.008 and P = 0.013, respectively). There was also a decrease in the level of sICAM-1 to 260.11±15.64 ng/mL at rest, from 329.58 ± 20.82 at baseline and 302.7 ± 20.49 post exercise (P = 0.013 and P = 0.038, respectively). On the other hand, IL-6 and ratio of IL-6/IL-10 increased to 6.55±0.84 pg/mL and 2.12 ± 0.37 immediately after exercise from baseline (2.73 ± 0.58 and 1.16 ± 0.33) and rest (2.49 ± 0.45 and 0.95 ± 0.19) in the second protocol (P = 0.001 and P = 0.001, respectively for IL-6, and P = 0.047 and P = 0.024, respectively for IL-6/IL-10).
Conclusions:
The data of the present study demonstrated that a single bout of exercise with higher-intensity induces a transient increase in some proinflammatory markers, and lower-intensity can reduce these biomarkers.
Keywords: Exercise, Aerobic, Inflammation, Cytokines
1. Background
Sub-clinical inflammation due to the increase of inflammatory biomarkers is an important risk factor for aging-related disability and several chronic diseases (1). It is believed that a strong association exists between physical inactivity and low-grade inflammation and its adverse health outcomes, particularly cardiovascular disease (CVD) (2). Some studies have shown that exercise probably changes the level of anti-inflammatory and pro-inflammatory cytokines such as C-reactive Protein (CRP), Interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), Interleukin-10 (IL-10), Interleukin-1β (IL-1β), soluble intercellular adhesion molecule-1 (sICAM-1) (3, 4).
Historically, the first study demonstrating that level of circulating cytokines can change in response to exercise was published in 1983 by Cannon and Kluger. These authors injected the plasma obtained from human subjects after exercise to rats intraperitoneally, leading to an elevation in the rat’s rectal temperature. Plasma collected before exercise failed to show such response (5).
VO2 is a part of O2 that is consumed by muscles while exercising. Maximal energy output during aerobic processes can be determined by assessment of maximal oxygen consumption (VO2max) (6).
While several studies have shown that a single exercise session induces a transient increase in inflammatory status (7, 8), different studies have reported different results and this increase ranges from mild to severe (1, 9). For example, plasma concentration of IL-6 can be increased up to 100 times during high intensity exercise (10). These differences are due to changes in the intensity or type of the exercise. However, the effect of one session of aerobic exercise with different intensities on inflammatory markers in people that exercise regularly (e.g. soccer players) has been poorly studied.
2. Objectives
The present study aimed to determine the effect of a single bout of treadmill running on inflammatory markers immediately after the exercise and at rest (24 hours after exercise) in different VO2max (65% and 85%); to compare between these inflammatory markers at different intensities; and to determine which of these markers changes immediately after exercise and which changes at rest.
3. Materials and Methods
3.1. Subjects
This semi-experimental study was performed from June 2011 to January 2013. Twenty eligible male students were selected by easy sampling from the students at Shahid Chamran university of Iran. The students were then divided randomly to two groups (10 for the first protocol and 10 for the second protocol). The sample size was calculated with preliminary data from the study of Gray et al. (2009) in which type I error was 0.05 with the power of test was equal to 0.8 (11). Body weights were measured to the nearest 0.1 kg and the heights to the nearest 0.1 cm, using a standard balance and stadiometer (Seca, Germany). Fat-free mass (FFM, Kg) and fat mass (FM, %) were established by the direct segmental multi-frequency bioelectrical impedance method (inbody230, Biospace, Korea). The inclusion criteria were limited to non-smoking students who performed regular football exercise at least three days a week, for two years. Those who had an acute illness or infection, and those who reported a history of inflammatory, cardiovascular and autoimmune disorders, blood disease, allergy conditions, or had taken any medication for the last four weeks were excluded from the study.
The institutional review board, which is the ethics committee of Ahvaz Jundishapur University of medical sciences, approved the study protocol (Eth.No.036 dated December 2010). A written informed consent was obtained from all cases before their participation in the study.
3.2. Exercise Protocols
The aerobic fitness level of each soccer player was determined by measuring VO2max. The VO2max was measured during a graded incremental exercise test to symptom-limited exhaustion on a treadmill (Track master TMX425CP, Full Vision Inc., Newton, KS), using a modified Bruce protocol (12).
Seven days after the VO2max test, the exercise sessions were performed on treadmills. The first protocol consisted of 30 minutes of running at a speed of 65% of VO2max (submaximal), while the other one consisted of six periodic repetitions with three minutes at a speed of 85% of VO2max (maximal) with 90 seconds of rest among each repetition.
3.3. Cytokines Assay
Fifteen minutes before starting exercise, 5 mL of blood sample was taken from the peripheral vein of each participant. Venous blood was also drawn immediately after the exercise and 24 hours after exercise. Serum samples were then separated and stored at -80˚C until use in the assay.
The secreted cytokine levels in the serum samples were determined using commercially available Sandwich enzyme-linked immunosorbent assay (ELISA) kits (IL-6, IL-1β, TNF-α and IL-10 by Koma BioTec INC., Source Korea and; hs-CRP and sICAM-1 by BioVendor, Czech Republic), according to the manufacturer’s instructions (13). The sample or standard is added to the wells and incubated to allow target proteins to bind. The wells are then washed to remove unbound material. After washing the plates, detected antibodies were added to all wells. This was followed by addition of Horseradish peroxidase HRP-conjugate and washing, according to the manufacturer’s protocols. Color was developed with the 3,3′,5,5′-Tetramethylbenzidine TMB substrate and the reaction was stopped using the stop solution (H2SO4), and optical density (OD) reading was taken at 450 nm (ELISA reader, TECAN, Sunrise Remote/Taouch Screen, Austria). Cytokine concentrations was measured with a standard curve derived from known amounts of the relevant cytokine, provided by the manufacture. The values below the standard range were considered to be non-measurable values.
3.4. Statistical Analysis
A repeated measurement analysis was performed to find the significance of change in: IL-1β, TNF-α, CRP, IL-6, ICAM-1, IL-10 and ratios of TNF-α/IL-10 and IL-6/IL-10 during pre-exercise, post-exercise and at rest for the two exercise protocols. To determine significance using Post Hoc test (LSD) we compared the means of pre-exercise, post exercise and rest time. The Kolmogorov-Smirnov test was used to check the normality of the observations. Statistical significance was considered as P < 0.05. All data are presented as mean values ± standard error of the mean (SEM). The SPSS version 15.0 was used for analyzing the data and Excel was used to generate graphs.
4. Results
According to the characteristics of subjects, no significant difference was determined between the groups (age: 21.3 ± 1.6 years; body mass: 74.1 ± 8.3 kg; height: 180.1 ± 6.1 cm; FFM: 36.4 ± 0.8 Kg; FM%: 12.2 ± 2.4; and VO2max: 53 ± 4.2 mL/kg/min). Amongst the inflammatory biomarkers analyzed by repeated measurement analysis in the two exercise protocols, significant changes were observed in the first protocol that was submaximal (30 minutes of running at a speed of 65% of VO2max) for IL-1β (P = 0.003) and ICAM-1 (P = 0.008). Significant difference was also seen in the second protocol only for IL-6 (P < 0.00001) and IL-6/IL-10 ratios (P = 0.015). For the other markers, there was no significant difference between different sampling time points in the two protocols. The descriptive results are presented in Table 1.
Table 1. Cytokine Levels Pre-Exercise, Post-Exercise and at Rest in Response to Two Protocols in the Studied Samplesa.
| Variables | 65% VO2max | 85% VO2max | ||||
|---|---|---|---|---|---|---|
| Pre-exercise | Post-exercise | Rest | Pre-exercise | Post-exercise | Rest | |
| TNF-α, pg/ml | 1.87 ± 0.08 | 1.99 ± 0.13 | 1.91 ± 0.09 | 2.00 ± 0.11 | 2.04 ± 0.05 | 1.97 ± 0.07 |
| hs-CRP, ng/ml | 1.45 ± 0.35 | 1.56 ± 0.34 | 1.32 ± 0.41 | 1.35 ± 0.36 | 2.48 ± 0.68 | 1.57 ± 0.57 |
| IL-1β, pg/ml | 4.33 ± 0.28 | 4.32 ± 0.34 | 3.77 ± 0.28 | 4.32 ± 0.32 | 4.67 ± 0.26 | 4.49 ± 0.31 |
| IL-6, pg/ml | 2.48 ± 0.52 | 3.23 ± 0.76 | 2.26 ± 0.41 | 2.73 ± 0.58 | 6.55 ± 0.84 | 2.49 ± 0.45 |
| sICAM-1, ng/ml | 329.58 ± 20.82 | 302.70 ± 20.49 | 260.11 ± 15.64 | 314.13 ± 24.20 | 305.01 ± 22.24 | 286.10 ± 17.53 |
| IL-10, pg/ml | 2.19 ± 0.20 | 2.58 ± 0.45 | 3.08 ± 0.50 | 2.91 ± 0.77 | 3.38 ± 0.37 | 2.74 ± 0.28 |
| TNF-α/IL-10 | 0.88 ± 0.04 | 0.92 ± 0.14 | 0.73 ± 0.08 | 0.85 ± 0.09 | 0.65 ± 0.04 | 0.76 ± 0.06 |
| IL-6/IL-10 | 1.10 ± 0.14 | 1.34 ± 0.27 | 0.87 ± 0.16 | 1.16 ± 0.33 | 2.12 ±0.37 | 0.95 ± 0.19 |
Abbreviations: hs-CRP, High sensitive C-Reactive Protein; IL-6, Interleukin-6; IL-10, Interleukin-10; TNF-α, sICAM-1, soluble Intercellular Adhesion Molecule-1; Tumor Necrosis Factor-alpha.
aData are presented as mean ± standard error of the mean.
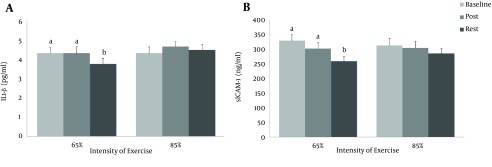
The results of Post Hoc test showed a decrease in IL-1β level at rest from baseline and immediately after exercise (P = 0.008 and P = 0.013, respectively) for the first protocol. The same response was observed for sICAM-1. There was a decrease in the level of sICAM-1 at rest from baseline and post exercise (P = 0.013 and P = 0.038, respectively). However, the differences between baseline and immediately after exercise were insignificant for IL-1β and sICAM-1 (P > 0.05) (Figure 1).
Figure 1. The Blood Concentrations of IL-1β and sICAM-1 in Response to Two Exercise Protocols.
Assessments were made at baseline (prior to exercise), immediately after exercise (post) and 24 hours after exercise (rest). The same letters (P > 0.05); and different letters (P < 0.05).
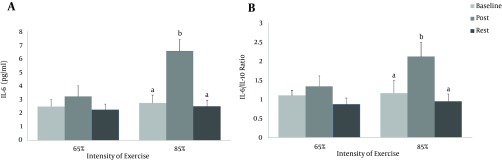
On the other hand, IL-6 and ratio of IL-6/IL-10 increased immediately after exercise from baseline and rest for the second protocol (P = 0.001 and P = 0.001, respectively for IL-6, and P = 0.047 and P = 0.024, respectively for IL-6/IL-10) (Figure 2).
Figure 2. The Blood Concentration of IL-6 and IL-6/IL-10 in Response to the Two Exercise Protocols (VO2max 65% and 85%).
Assessments were made at baseline (prior to exercise), immediately after exercise (post) and 24 hours after exercise (rest). The same letters (P > 0.05); and different letters (P < 0.05).
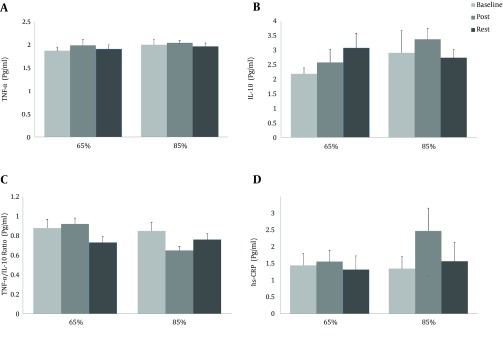
Figure 3. The Blood Concentrations of TNF-α, IL-10, TNF-α/IL-10 and IL-6/IL-10 ratios, and hs-CRP and IL-6 Response to the Two Exercise Protocols.
Assessments were made at baseline (prior to exercise), immediately after exercise (post) and 24 hours after exercise (rest), which were not significant (P > 0.05).
5. Discussion
Inflammation has a significant role in the pathogenesis of several chronic diseases including Cardiovascular disease CVD, type 2 diabetes mellitus, Alzheimer’s disease, osteoporosis, and certain cancers (1).
Although it is believed that a single bout of exercise induces immune activation, different studies have shown different results (1, 4). These differences may be due to factors, including differences in subject features, the type and intensity of the exercise, period of intervention, the time of taking blood samples and inherent variability in methods for quantifying the circulating level of cytokines. Nevertheless, extensive evidence suggests that both acute and chronic exercises can improve markers of systemic low-grade inflammation (7, 14, 15).
In this research, we investigated changes in certain inflammatory markers including IL-1β, TNF-α, CRP (hs-CRP), IL-6, sICAM-1, IL-10 and the ratios of TNF-α/IL-10 and IL-6/IL-10, and observed decreased serum levels of IL-1β and sICAM-1 in a single bout of the submaximal exercise (30 minutes of running at a speed of 65% of VO2max) that was less severe than the other exercise protocol (six periodic repetitions with three minutes at a speed of 85% of VO2max). These inflammatory biomarkers’ concentration was measured prior to exercise, and immediately after, as well as 24 hours after the exercise. It can be concluded that lower-intensity exercise has more anti-inflammatory effects than high-intensity exercise. In other words, one bout of aerobic exercise with lower intensity has better anti-inflammatory effects than exercises with high intensity.
While the recommendation to exercise for people with age-related chronic disorders is one of the treatment protocols, these exercise prescriptions might differ in terms of intensity. In other words, recommending lower-intensity (submaximal) exercise will be the best option for prevention and treatment of these diseases.
A single bout of exercise induces a transient increase in immune markers such as leukocyte numbers, and the release of chemical mediators such as cytokines and chemokines. These changes depend on the intensity and duration of the exercise period. The leukocyte and cytokine concentrations return to their resting level a few hours after the end of the exercise bout (7). A single exercise session can induce immune activation (8). Accordingly, it is expected for each exercise session (even during a low-intensity aerobic exercise) to slightly induce immune system activation. Although our results, in case of some markers, showed the above-mentioned claim, however, other markers showed immune modulation. It is important to note that in this study, participants were soccer players who exercised regularly and regular aerobic exercise, because of its anti-inflammatory effects, limits immune activation during workout (8).
Our findings about IL-6 in the first protocol support those of previously published studies, which reported that muscle IL-6 release is very low during moderate exercise. For example, a study performed on female subjects, during a 30-minute brisk walk on a treadmill, showed that plasma IL-6 concentration increased from 1.3 to 2 pg/m (9). During exercise, IL-6 cytokine is released by muscle fibers and induces an increase in the level of other anti-inflammatory cytokines such as IL-10. Furthermore, IL-6 also acts as a suppressor in the production of TNF-α, as a pro-inflammatory cytokine (16). It is expected that in the second protocol of our study, the increase in IL-6 might have stimulated IL-10 plasma level and suppressed the production of TNF-α, although the rise in IL-10 was not statistically significant.
The CRP is synthesized by the liver in response to inflammatory factors, primarily IL-6, and, to a lesser extent, IL-1 and TNF-α. Furthermore, CRP is used mainly as a marker of subclinical chronic vascular inflammation, and has a predictive value of future cardiovascular events. It mediates the expression of ICAM-1 and VCAM-1 (17).
While some previous studies have reported that regular exercise has a positive effect on circulating CAMs such as sICAM-1 (17, 18), we found that one session of low-intensity exercise decreases the level of sICAM-1 in individuals who exercise regularly. The IL-10, as an anti-inflammatory cytokine, can be increased by exercise training, and in turn decrease vascular wall inflammation (17). Our data showed that one session of exercise could not increase IL-10, a small increase in the level of IL-10 was observed after both protocols.
Some exercise interventions have shown that regular exercise decreases the level of IL-1β (19, 20); however like sICAM-1, we found that even after a single session of low-intensity exercise, IL-1β could be decreased in participants who exercise regularly. In addition, exercise interventions showed a 24-hour delay in the reduction on IL-1β and sICAM-1 levels (Figure 1).
Analysis of multiple biochemical markers and calculation of ratios between two or more of these markers have been frequently used to increase the diagnostic potency of cytokine measurement. A balanced ratio between pro- and anti-inflammatory cytokine levels is important for appropriate immune response (21) and the ratios of their expression levels were measured to represent the balance between two types of inflammatory cytokines (22). We selected the ratios of IL-6 and TNF-α as pro-inflammatory markers and IL-10 as an anti-inflammatory factor, because it has been suggested that imbalance between pro- and anti-inflammatory cytokines correlated with mechanisms for increased susceptibility to diseases related to chronic inflammation (23). Therefore, measurement of the ratio of pro- and anti-inflammatory factors show the degree of the net immunological status that the circulating inflammatory factors are likely to have on their targets, the overall immune bias of an individual’s physiological background, and of immune dysregulation (23, 24). Similarly, the TNF-α/IL-10 and IL-6/IL-10 ratios used to evaluate the balance between pro- and anti-inflammatory cytokines were significantly increased in some diseases and unhealthy status (24, 25) such as heart diseases (26, 27). In another study, moderate training for 60 minutes/day, three days per week for 24 weeks, decreased the ratio of TNF- /IL-10 in the elderly people (28). Although our data showed that one session of exercise cannot change TNF- /IL-10 and increase IL-6/IL-10 immediately after exercise from baseline and rest in the second protocol, further studies with longer interventions are needed in the future to find changes in these ratios in the inflammatory processes during exercise.
Based on our data the TNF-α/IL-10 ratio did not decrease significantly during the study period, yet IL-6/IL-10 showed an increase with the second protocol. In the best of our knowledge, this is one of the first few studies that have investigated these ratios. In our opinion, published data about the effect of exercise intervention on these ratios is insufficient. However, the potential for TNF-α/IL-10 and IL-6/IL-10 ratios serving as biomarkers for exercise intervention needs to be further tested and validated. It must also be acknowledged that insignificant changes in these ratios observed in this study are contrast with other studies and these ratios of inflammatory biomarkers may be sensitive to exercise with different intensities.
The present study was a semi-experimental study with a small population. Our subjects were soccer players therefore our data could not be generalized to other populations. More studies are needed to confirm our results. On the other hand, since football playing is not popular among females of our country, we used only male soccer players in this study. The strong point of our study was focusing on differences between aerobic exercises with difference intensities. On the other hand, we used the ratio of pro- and anti-inflammatory cytokines as a marker to represent the status of inflammation.
In summary, we found that IL-1β and sICAM-1 decreased significantly at rest (24 hours after 30 minutes of running at a speed of 65% of VO2max) and a significant increase was observed in IL-6 immediately after exercise from baseline and rest (24 hours after 30 minutes of running at a speed of 85% of VO2max) with the second protocol. Other inflammatory markers did not change significantly. In contrast, none of the inflammatory markers were unchanged after running at a speed of 85% VO2max. On the other hand, this paper showed that immune modulation and inflammatory status immediately and 24 hours after one exercise session differed in low-intensity (at 65% of VO2max) and high-intensity (at 85% VO2max) exercise. In simpler terms, exercise with lower intensity is more effective than with higher intensity in reducing inflammation. This observation may help patients with age-related chronic disorders and may also be useful for clinicians to make better choices about the type and intensity of exercise that they prescribe.
Acknowledgments
We would like to express our specific thanks to the deputy of research affairs of Ahvaz Jundishapur university of medical sciences for their financial support. This study was part of a project of the physiology research center (Grant No.PRC-58). The authors would like to thank all soccer players who participated in this research.
Footnotes
Authors’ Contribution:Mehri Ghafourian developed the original idea, the protocol and wrote the final report. Damoon Ashtary-Larky abstracted and prepared the manuscript. Rahim Chinipardaz consulted for the data analysis. Nahid Eskandary monitored the health of athletes. Mousa Mehavaran contributed to the development of the protocol.
Funding/Support:This project was supported by a grant from Ahvaz Jundishapur university of medical sciences.
References
- 1.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11-12):785–93. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kop WJ, Weinstein AA, Deuster PA, Whittaker KS, Tracy RP. Inflammatory markers and negative mood symptoms following exercise withdrawal. Brain Behav Immun. 2008;22(8):1190–6. doi: 10.1016/j.bbi.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Andersson J, Jansson JH, Hellsten G, Nilsson TK, Hallmans G, Boman K. Effects of heavy endurance physical exercise on inflammatory markers in non-athletes. Atherosclerosis. 2010;209(2):601–5. doi: 10.1016/j.atherosclerosis.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78(4):819–35. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JG, Kluger MJ. Endogenous pyrogen activity in human plasma after exercise. Science. 1983;220(4597):617–9. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- 6.Balderrama C, Ibarra G, De La Riva J, Lopez S. Evaluation of three methodologies to estimate the VO2max in people of different ages. Appl Ergon. 2010;42(1):162–8. doi: 10.1016/j.apergo.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 8.van de Weert-van Leeuwen PB, Arets HG, van der Ent CK, Beekman JM. Infection, inflammation and exercise in cystic fibrosis. Respir Res. 2013;14:32. doi: 10.1186/1465-9921-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieman DC, Henson DA, Austin MD, Brown VA. Immune response to a 30-minute walk. Med Sci Sports Exerc. 2005;37(1):57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13(1):56–62. doi: 10.1034/j.1600-0838.2003.20218.x. [DOI] [PubMed] [Google Scholar]
- 11.Gray SR, Clifford M, Lancaster R, Leggate M, Davies M, Nimmo MA. The response of circulating levels of the interleukin-6/interleukin-6 receptor complex to exercise in young men. Cytokine. 2009;47(2):98–102. doi: 10.1016/j.cyto.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546–62. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 13.Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147(11):3788–93. [PubMed] [Google Scholar]
- 14.Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci (Lond). 2007;112(11):543–55. doi: 10.1042/CS20060368. [DOI] [PubMed] [Google Scholar]
- 15.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev. 2009;15:6–41. [PubMed] [Google Scholar]
- 16.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro F, Alves AJ, Duarte JA, Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol. 2010;141(3):214–21. doi: 10.1016/j.ijcard.2009.09.548. [DOI] [PubMed] [Google Scholar]
- 18.Witkowska AM. Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine. 2005;31(2):127–34. doi: 10.1016/j.cyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20(8):608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–8. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 21.Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41(7):899–903. doi: 10.1515/CCLM.2003.136. [DOI] [PubMed] [Google Scholar]
- 22.You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, et al. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225(1):135–41. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W, et al. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain Behav Immun. 2010;24(3):350–7. doi: 10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43(11):962–9. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262–4. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Waehre T, Halvorsen B, Damas JK, Yndestad A, Brosstad F, Gullestad L, et al. Inflammatory imbalance between IL-10 and TNFalpha in unstable angina potential plaque stabilizing effects of IL-10. Eur J Clin Invest. 2002;32(11):803–10. doi: 10.1046/j.1365-2362.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 27.Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD. Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (Lond). 2003;105(1):45–50. doi: 10.1042/CS20020359. [DOI] [PubMed] [Google Scholar]
- 28.Santos RV, Viana VA, Boscolo RA, Marques VG, Santana MG, Lira FS, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60(3):731–5. doi: 10.1016/j.cyto.2012.07.028. [DOI] [PubMed] [Google Scholar]