Abstract
The complexity of cell wall composition and structure determines the strength, flexibility, and function of the primary cell wall in plants. However, the contribution of the various components to cell wall integrity (CWI) and function remains unclear. Modifications of cell wall composition can induce plant responses known as CWI control. In this study, we used transgenic expression of the fungal feruloyl esterase AnFAE to examine the effect of post-synthetic modification of Arabidopsis and Brachypodium cell walls. Transgenic Arabidopsis plants expressing AnFAE showed a significant reduction of monomeric ferulic acid, decreased amounts of wall-associated extensins, and increased susceptibility to Botrytis cinerea, compared with wild type. Transgenic Brachypodium showed reductions in monomeric and dimeric ferulic acids and increased susceptibility to Bipolaris sorokiniana. Upon infection, transgenic Arabidopsis and Brachypodium plants also showed increased expression of several defense-related genes compared with wild type. These results demonstrate a role, in both monocot and dicot plants, of polysaccharide feruloylation in plant CWI, which contributes to plant resistance to necrotrophic pathogens.
Keywords: ferulic acid, cell wall integrity, Arabidopsis thaliana, Brachypodium distachyon, plant pathogen resistance
Introduction
In the heterogeneous, complex, and highly dynamic plant cell wall, structural polysaccharides form a cross-linked macromolecular network consisting of cellulose, hemicellulose, pectin, glycoproteins, and (in some specialized tissues) lignin (Albersheim et al., 2010). Cell wall constituents function in regulation of plant growth, development, signal transduction, and responses to environmental stresses (Somerville et al., 2004; Lionetti et al., 2012; Lionetti and Metraux, 2014; Tenhaken, 2015). In addition, plant biomass represents a valuable source of feed, fiber, and fuels, including a potential alternative to petroleum-based fuels. Cell wall lignification and the crosslinking of cell wall polysaccharides (Hatfield et al., 1999) hampers cell wall breakdown to simple sugars that can be used as an energy source by animals or be fermented to bioethanol (Himmel et al., 2007).
As plants are sessile, they have evolved a complex defense network to combat stresses, both biotic and abiotic, to survive in their environments. Many signaling pathways involved in plant stress responses and innate immunity have been identified and characterized (Jones and Dangl, 2006). Recent work has identified cell wall-related defense mechanisms, termed cell wall integrity (CWI) control (Ringli, 2010; Seifert and Blaukopf, 2010; Hamann and Denness, 2011; Pogorelko et al., 2013a; Bellincampi et al., 2014). As the first line of defense for plants, the cell wall must be rigid enough to maintain cell turgor and prevent pathogen invasion, but accommodate cell expansion, division, and organogenesis. Hence, the cell wall must respond rapidly to environmental changes without compromising its essential functions. Different cell wall components function in controlling cell expansion (Wolf et al., 2012) and defense responses (Nicaise et al., 2009; Tenhaken, 2015).
Certain phenolic compounds have key effects on cell wall structure, recalcitrance to degradation, and functions in defense (Buanafina et al., 2008; Pogorelko et al., 2011; Lionetti et al., 2015). For example, hydroxycinnamic acids, such as ferulic, sinapic, and coumaric acids, have been implicated in structural integrity, and some participate in crosslinking of cell wall constituents (Grabber et al., 1998), due to their ability to form homodimers, either photo-induced (Ralph et al., 1994) or peroxidase-assisted (Hartley et al., 1990). In particular, ferulate crosslinking has been broadly investigated, especially in monocot species (Ishii, 1997; Buanafina, 2009). As the predominant hydroxycinnamic component of grass cell walls, ferulate dimers crosslink hemicellulosic polymers via an ester linkage to the arabinose side chain of arabinoxylan or can be ether-linked to lignin (Ralph et al., 1995; Hatfield et al., 1999). In some dicots, by contrast, ferulic acid has been implicated in the crosslinking of pectic arabinans and galactans (Fry, 1982; Caffall and Mohnen, 2009). It has also been suggested that ferulic acid may crosslink pectins with wall-bound extensins, to negatively regulate cell expansion (Qi et al., 1995).
Ferulate can form homo- and heterodimers in numerous ways, such as 8-O-4, 8-8, and 4-O-5 linkages and ferulates can also bind in 8-5 and 5-5 conformations (Ralph et al., 2004). These dimers are considered an important structural aspect of the cell wall, but also hinder degradation for industrial purposes, such as biofuel production. Evidence indicates that ferulic acid (FA) affects plant-pathogen interactions, and that phenolic compounds are often induced in response to biotic stresses. FA is thought to play a role in fungal resistance and to be an important insect deterrent (Santiago and Malvar, 2010; Buanafina and Fescemyer, 2012). Apple leaves inoculated with the apple scab fungus Venturia inaequalis showed an increase in overall phenolic content, including FA (Mikulic Petkovšek et al., 2008). A negative correlation between FA concentration and disease severity was observed in maize exposed to Fusarium graminearum (Bily et al., 2003). Moreover, susceptibility to insect pathogens was negatively correlated with hydroxycinnamic acid content in maize (Bergvinson et al., 1994; García-Lara et al., 2004). Thus, these phenolic compounds may have key roles in cell wall structure and disease resistance.
Pathogens have evolved enzymatic mechanisms to target cell wall structure, including phenolic compounds; these enzymes have potential uses for improving digestibility of biomass. For example, some fungal species, such as Aspergillus nidulans, produce ferulic acid esterase (FAE), which hydrolyzes ester linkages between FA and cell wall polysaccharides of the host. Several studies investigated the effects of the ectopic expression of fungal FAE on plant cell wall composition and phenolic function. Vacuolar-targeted expression of a fungal FAE in Lolium multiflorum induced auto-digestion of hemicellulose after cell death (Buanafina et al., 2006). Targeted expression of Aspergillus niger FAE to the vacuole (Buanafina et al., 2008) or to the apoplast, endoplasmic reticulum, and Golgi (Buanafina et al., 2010) caused a reduction of cell wall feruloylation in Festuca arundinacea with a consequent improvement in cell wall digestibility. Aspergillus FAE, when added to fungal xylanase, improves the release of reducing sugars in oat hulls and in wheat bran (Faulds and Williamson, 1995; Yu et al., 2003). Arabinan-bound ferulic acid has been implicated in regulation of stomatal aperture of Commelina communis, and may confer flexibility in guard cells by preventing homogalacturonan (HGA) from binding calcium (Jones et al., 2003). Treatment with FAE, which hydrolyzes crosslinks between arabinans, allows HGA domains to associate through calcium crosslinked chain packing, thus reducing flexibility of the guard cell walls (Jones et al., 2003).
Arabidopsis plants expressing feruloyl esterase from A. nidulans have been previously generated and demonstrated to have reduced cell wall feruloylation and increased enzymatic saccharification of acid-pretreated plant biomass (Pogorelko et al., 2011). In this study, transgenic Brachypodium plants expressing the same microbial esterase were generated and characterized. Cell wall modifications in Arabidopsis and Brachypodium transgenic plants caused by expressed feruloyl esterase were characterized. The effect of these cell wall modifications on plant susceptibility to the necrotrophic fungi B. cinerea and B. sorokiniana was investigated and the results provide new insights into the relationship between the cell wall feruloylation, cell wall accessibility to degrading enzymes, and plant resistance to biotic stress. This study thus demonstrated that a reduction in cell wall cross-linking compromises plant resistance to pathogens.
Materials and Methods
Plant Growth Conditions
Arabidopsis seeds were planted in wet LC-1 potting soil mix (Sun Gro Horticulture, Agawam, MA, USA) and plants were grown in a growth chamber with controlled conditions: 16-h light/ 8-h dark at 21°C, with relative humidity of 65% and light intensity of 160 μmol s-1 m-2.
For the extensin quantification experiments, Arabidopsis seedlings were grown on plates in the same growth chamber as above. Seeds were sterilized with sequential treatments of 70% ethanol and 0.5% bleach, washed multiple times with sterile water and planted on -strength Murashige and Skoog medium (Murashige and Skoog, 1962) with 2% sucrose and 0.3% Gelrite (Research Products International, Mt. Prospect, IL, USA).
Brachypodium plants were grown in the same conditions as Arabidopsis. After a vernalization period of 14 days in the dark at 4°C to maximize germination rate, pots were transferred to a growth chamber maintained at 21°C, 65% relative humidity, and 160 μmol s-1 m-2 light intensity.
Transformation of Brachypodium Plants with AnFAE
Brachypodium transgenic plants were prepared as described previously (Pogorelko et al., 2013b). The A. nidulans AnFAE cDNA (AN5267.2) was amplified from Pichia pastoris recombinant strains (Bauer et al., 2006), which were obtained from the Fungal Genetics Stock Center1. The cloned AnFAE sequence was amplified by PCR with primers AnFAE-F and AnFAE-R containing restriction sites KpnI and HindIII, respectively. After restriction digest of AnFAE, the fragment was ligated into a cassette containing (5′ to 3′) sequences encoding: a Zea mays β-expansin signal peptide, AnFAE, and a green fluorescent marker (smGFP), fused to the C-terminus of AnFAE. This cassette was then ligated into a pMLBart binary vector backbone, as described in Fursova et al. (2012). A. tumefaciens-mediated transformation of Brachypodium callus was performed by the Plant Transformation Facility at Iowa State University2. Diagrams of the transgenic expression cassettes for Arabidopsis and Brachypodium plants are shown in Supplementary Figure S1.
Preparation of Apoplastic Fluid
Apoplast fluids were extracted from 6-week-old AnFAE and wild-type plants as described by Pogorelko et al. (2011). Briefly, aerial parts of the plants (0.5 g) were cut into 3–6 mm segments and placed vertically into a 10 mL syringe sealed with parafilm. Five mL of pre-cooled buffer (25 mM Tris-HCl, 50 mM EDTA, 150 mM Mg2Cl2, pH 7.4) was added and the syringe was placed under vacuum. After vacuum infiltration, the buffer was drained and the syringe was placed into a 15 mL centrifuge tube and centrifuged at 1000 g for 10 min. Apoplast fluid was collected from the bottom of the tube, transferred to a new tube, and frozen at -20°C.
Feruloyl Esterase Activity Assay
For activity assays, apoplastic total protein was quantified using Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA). Then, equal amounts of protein per sample were incubated with 2 mM methyl ferulate (Sigma–Aldrich, St. Louis, MO, USA) in sodium phosphate buffer (pH 7.4) for 24 h. The products of reaction were extracted with ethyl acetate, evaporated, dissolved in 100% methanol, and analyzed by reverse-phase HPLC on a Prevail C18 5 μ column (4.6 mm × 50 mm; Grace Davison Discovery Sciences, Deerfield, IL, USA) with UV detection at 290 and 320 nm. Product and substrate were separated using a gradient of 0.1% trifluoroacetic acid in water (pH 2.8) and acetonitrile at 1 mL min-1 under following conditions: 0–3 min—95% water; 3–10 min—85% water; 10–20 min—70% water; 20–25 min—5% water; 25–30 min—95% water.
Cell Wall Extraction
Cell walls were isolated from plants grown on soil as described in Zabotina et al. (2008). Whole aerial parts of plants were harvested and cut into 1-cm length segments. Tissue was frozen in liquid N2 and ground into a fine powder with a mortar and pestle. After homogenization, tissues were incubated in 80% ethanol at 80°C two times for 1 h, and further homogenized with a PolyTron (Kinematica, Inc., Bohemia, NY, USA) at 15,000 rpm for 5 min. The pellet was collected by centrifugation at 12,000 g and washed with 80% ethanol followed by several washes with 100% acetone until supernatant turned clear. The pellet was incubated in a solution of 20% SDS with 5 mM sodium metabisulfite at 4°C for 16 h and washed five times with distilled water. Finally, the pellet was incubated in 1:1 chloroform:methanol solution at room temperature for 20 min, washed three times with 100% acetone, and air-dried at 50°C.
Analysis of Cell Wall Phenolic Acids
Phenolic acids and other hydroxycinnamates were extracted from prepared cell walls and analyzed as described by Pogorelko et al. (2011). Briefly, each total cell wall sample was weighed, incubated twice for 24 h in 2 ml 2M NaOH, and supernatants were combined. The supernatant mixture was acidified and phenolics were extracted with ethyl acetate, which was then evaporated with a stream of N2. Phenolics were then dissolved in 100% methanol and analyzed by reverse-phase HPLC on a Prevail C18 5 μ column (4.6 mm × 250 mm; Grace Davison Discovery Sciences, Deerfield, IL, USA) with UV detection at 290 and 320 nm. Phenolic acids were separated using a gradient of 0.1% trifluoroacetic acid in water (pH 2.8) and acetonitrile at 1 mL min-1 under following conditions: 0–10 min—95% water; 10–30 min—85% water; 30–40 min—70% water; 40–47 min—5% water; 47–55 min—95% water. To determine response factors, standard curves were created using mixtures of standard p-coumaric acid and ferulic acid, (all from Sigma–Aldrich, St. Louis, MO, USA) at different concentrations.
Analysis of Cell Wall Sugars
To determine monosaccharide composition, 1 mg of dry, de-starched cell wall was hydrolyzed with 2 N trifluoroacetic acid at 120°C for 2 h. The hydrolysates were dried at 50°C, re-dissolved in water, and analyzed by high-performance anion-exchange chromatography with pulsed-amperometric detection using a CarboPac PA-20 column (3 mm× 150 mm; Dionex, Sunnyvale, CA, USA) as described earlier (Zabotina et al., 2008). Monosaccharides were separated using a gradient of 100mM NaOH in water at 0.5 mL min-1 under following conditions: 0–0.05 min—12 mM NaOH; 0.05–26 min—0.65 mM NaOH; 26–46 min—300 mM NaOH; 46–55 min—12 mM NaOH. Monosaccharide standards included L-Fuc, L-Rha, L-Ara, D-Gal, D-Glc, D-Xyl, D Man, D-GalA, and D-GlcA (all from Sigma–Aldrich, St. Louis, MO, USA). To determine response factors, standard curves were created using mixtures of all standard monosaccharides at different concentrations.
Reducing sugars were measured using the PAHBAH assay (Lever, 1972) with minor modifications. Briefly, 15 μL of supernatant was mixed with 135 mL of freshly prepared PAHBAH reagent (1 volume of 5% p-hydroxybenzoic acid hydrazide in 5% HCl mixed with 9 volumes of 1.25% trisodium citrate, 0.11% calcium chloride, and 2% sodium hydroxide) and heated at 95°C for exactly 6 min. Absorbance was measured at 410 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Calculations were done using a standard curve prepared using different concentrations of Glc.
Cell Wall Extensin Extraction and Quantification
Wild-type and transgenic Arabidopsis cell wall samples from 2-week-old whole plants (20–35 mg) were deglycosylated with anhydrous hydrogen fluoride (HF), as described previously (Sanger and Lamport, 1983). HF reactions were quenched, dialyzed (3.5 kDa molecular weight cut-off) against distilled deionized water, and then lyophilized. Lyophilized samples were re-suspended in water and fractionated into water-soluble (HF-soluble) and water-insoluble (HF-insoluble) fractions by centrifugation. HF-insoluble pellets were washed with water (~10–15 mL) to further separate HF-soluble components. Wash supernatants were pooled with HF-soluble fractions. Both HF-soluble and HF-insoluble fractions were lyophilized and weight recoveries were recorded on a microbalance. Untreated cell wall, HF-soluble, and HF-insoluble fractions were assayed for hydroxyproline after acid hydrolysis (constant boiling 6N HCl, 110°C, 24 h), as described earlier (Kivirikko and Liesmaa, 2009). Sample quantities permitting, analyses were performed in triplicate and error bars represent standard deviation.
Enzymatic Digestions of Cell Wall
Digestion of hemicelluloses was done using 10 mg of dry cell wall material incubated with a mixture of 50 units of endo-1,4-β-xylanase M6 (rumen microorganism; Megazyme International, Wicklow, Ireland) and 5 units of endo-1,4-β-xylanase M1 (Trichoderma viride; Megazyme) in a 0.3-mL total volume of sodium phosphate buffer (pH 6.0) for 24 h at 37°C. For the digestion of pectins, 10 mg of dry cell wall material was incubated with a mixture of 50 units of endo-polygalacturonase (Megazyme International, Wicklow, Ireland) and 15 units of PME (PROZOMIX LTD, Haltwhistle, UK) in a 0.3-mL total volume of sodium phosphate buffer (pH 6.0) for 24 h at 37°C.
Saccharification assays were performed as described in Pogorelko et al. (2011) with some modifications. Stems of 6-week-old plants were cut into pieces approximately 0.3 cm long (5 mg of fresh tissue) and incubated in 0.1 ml of citrate buffer (pH 4.9) containing 4 units of cellulase (from Trichoderma reesei, Sigma–Aldrich, C62730) and 1 unit of cellobiase (from A. niger, Sigma–Aldrich, C6105) on the shaker at 37°C. At each time point, the reaction was terminated by heating at 100°C for 15 min, supernatants were collected by centrifugation at 10,000 g, and the amount of reducing sugars released was analyzed by p-hydroxybenzoic acid hydrazide (PAHBAH) assay.
Inoculation of Arabidopsis with Botrytis cinerea Conidia
Botrytis cinerea strain SF1 (Lionetti et al., 2007) was grown for 15 days on potato dextrose agar (PDA) at 39 g liter-1 at 23°C with a 12-h photoperiod before spore collection. The spores were harvested by washing the culture surface of the agar and suspended in 5 mL of sterile distilled water. Spore suspensions were filtered through glass wool to remove residual mycelia and the concentration was determined using a Thoma chamber. Conidia, at a concentration of 5 × 105 conidia mL-1, were germinated in potato dextrose broth (PDB) at 24 g L-1 at room temperature for 3 h. Fully developed leaves were detached from four 6-week-old Arabidopsis plants (three leaves/plant), grown in a growth chamber maintained at 22°C and 70% relative humidity, with a 12 h/12 h light-dark photoperiod. The detached leaves were placed in square petri dishes with petioles embedded in 0.8% agar. Six droplets of spore suspension (5 μL each) were placed on the surface of each leaf. Mock inoculation was performed using PDB. Leaves were incubated at 24°C with a 12-h photoperiod and lesion diameter was measured 48 h post inoculation. Lesion sizes were measured using IMAGE-J software (Abràmoff et al., 2004).
Infection of Brachypodium with Bipolaris sorokiniana
Macroconidia of B. sorokiniana strain DSMZ 62608 (kindly provided by Prof. F. Favaron, University of Padua) were produced by culturing the fungus on PDA before spore collection. The conidia were collected by washing the culture surface with 3 mL of sterile water, and conidia concentrations were estimated using a Thoma chamber. The infection of Brachypodium leaves was performed as described previously (Pogorelko et al., 2013b). Briefly, fully expanded leaves were detached from 60-d-old Brachypodium plants grown under a 16 h/8 h light/dark cycle in a climate-controlled chamber at 22°C with a relative humidity of 70%. The leaves were cut to 5-cm lengths and placed in square petri dishes containing 0.8% agar. Four droplets (10 μL) of conidia suspension (1 × 106 conidia mL-1), with 0.05% Tween 20, were deposited onto each leaf at a distance of about 2 cm from each other. Mock inoculation was performed using sterile distilled water with 0.05% Tween 20. The plates were incubated at 22°C under a 16-h/8-h light/dark cycle. Disease symptoms were recorded at 48 h and lesion sizes were measured using ImageJ (Abràmoff et al., 2004).
RNA Extraction, cDNA Synthesis, and Real-Time qPCR
Total RNA was extracted from the uninfected, infected, and mock-inoculated leaves of 6-week-old plants using the SV Total RNA Isolation kit (Promega Corp, Madison, WI, USA), and cDNA synthesis was performed with the SuperScript III First Strand Synthesis system (Invitrogen Corp, Carlsbad, CA, USA) following the manufacturer’s recommendations. The Maxima SYBR Green qPCR Master Mix (2X; Thermo Scientific, Waltham, MA, USA) with appropriate primers (Supplementary Table S1) and the CFX-96 Thermal cycler (Bio-Rad) were used to determine relative expression of the genes. Relative expression levels were calculated in comparison with an appropriate control gene (for primer sequences, see Supplementary Table S1) from wild-type plants, and the ACTIN2 gene (At3g18780; for Arabidopsis) whose expression level was not affected, was used as reference gene. Relative expression for Brachypodium samples was calculated by comparison with GAPDH. Gene expression levels in transgenic plants are presented relative to the expression of the same gene detected in wild-type plants (for which gene expression was set to 1). The comparative threshold cycle method (Schmittgen and Livak, 2008) was used for determining differences between transcript copy numbers in wild-type and transgenic plants. For calculation of relative expression levels for B. cinerea genes, a fungal house-keeping gene ACTIN (for primer sequences, see Supplementary Table S1) was used as a reference gene.
Chlorophyll Extraction and Quantification
Chlorophyll extraction was performed as described in Lolle et al. (1997). Whole rosette leaves were isolated from 4-week-old Arabidopsis plants, then carefully weighed and placed in a 24-well plate. Leaves were immersed in dimethyl sulfoxide at room temperature, covered in foil, and placed on a shaker for incubation. Aliquots of supernatant were taken from solution every 10 min for 80 min. Absorption spectra at 664 and 647 nm were then measured. Chlorophyll was quantified as micromoles per mg tissue using the equation: chlorophyll (micromoles) = 7.93(A664) + 19.53(A647).
Peroxide Staining of Arabidopsis Leaves
Arabidopsis leaves were assayed for hydrogen peroxide accumulation according to the protocol outlined by Daudi and O’Brien (2012). An aqueous solution of 1 mg/mL 3,3′-diaminobenzidine (DAB)(Acros Organics, New Jersey, USA) was prepared and adjusted to pH 3.0 with HCl. Tween-20 and Na2HPO4 were added to the solution to final concentrations of 0.05% and 10 mM, respectively. Rosette leaves from different plants were harvested and placed in a 24-well plate, then immersed in DAB solution. Samples were then vacuum infiltrated twice to ensure DAB solution penetrated the tissues. The 24-well plate was covered and placed on a shaker for 24 h.
At the 24 h time-point, leaf samples were placed in a bleaching solution of 3:1:1 ethanol: acetic acid: glycerol and heated to 95°C for 15 min. After boiling, fresh bleaching solution replaced the boiled solution and leaves were stored at 4°C until ready for imaging.
Results
Generation and Characterization of Transgenic Brachypodium Plants Expressing Aspergillus nidulans Feruloyl Esterase
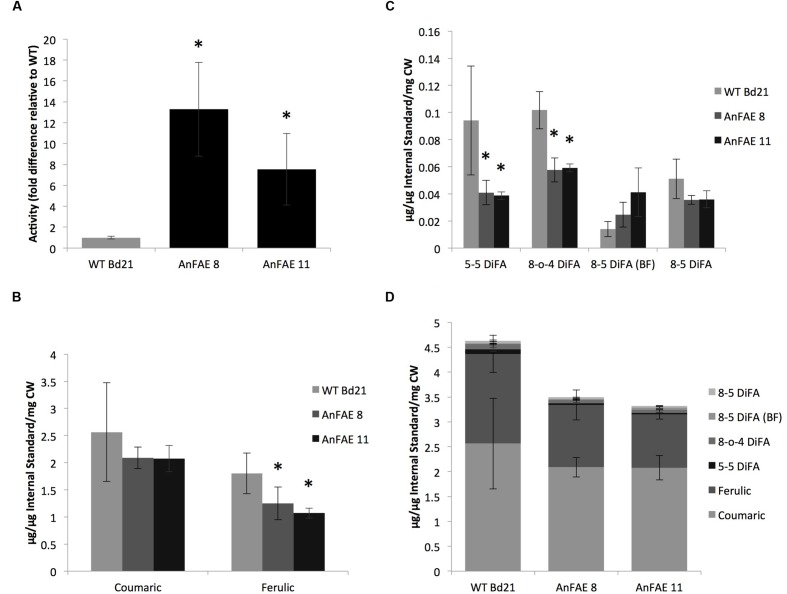
To introduce A. nidulans feruloyl esterase (AnFAE), which we previously used to reduce the amount of ferulic acids in Arabidopsis plants (Pogorelko et al., 2011), to Brachypodium plants, a construct harboring AnFAE was prepared using the binary vector described by Fursova et al. (2012). Two selected independent transgenic Brachypodium lines were tested for production of AnFAE in the extracellular matrix by assaying enzymatic activity in their apoplastic fluids, with methyl ferulate as a substrate. All apoplastic fluids extracted from transgenic plants showed significantly higher feruloyl esterase activity in comparison with wild-type plants (Figure 1A).
FIGURE 1.
Analysis of AnFAE activity and quantification of hydroxycinnamates in Brachypodium plants. (A) Enzyme activity assay from Brachypodium apoplast. Quantification was done by measuring quantity and calculating the ratio of product (ferulic acid) to substrate (methyl ferulate). This ratio was then normalized to the average wild-type (WT) ratio to calculate fold difference. (B,C), Quantification of hydroxycinnamate monomers and dimers in the Brachypodium cell wall. Compounds were identified under UV, then quantified based on peak area and normalized using an internal standard (cinnamic acid). Micrograms of each sample were determined using standard curves of known compounds. (D) Quantification of total hydroxycinnamates in Brachypodium cell wall. All peaks from quantitative analyses were added to determine overall reduction in total cell wall phenolics. Asterisks (A–C) indicate data sets significantly differently between AnFAE and wild-type plants, according to a Student’s t-test (p < 0.05).
To demonstrate that AnFAE expressed in Brachypodium apoplast reduces the amount of ferulic acids (FA), we quantified the total contents of FA, di-FA, and p-coumaric acid in cell walls extracted from aerial parts of transgenic and wild-type plants. Amount of both hydroxycinnamic acids present in cell wall extracted from transgenic plants varied among different plants; however, both lines had significantly less FA in comparison with wild-type plants (Figure 1B). In addition, cell walls from transgenic plants had lower amount of two types of di-FA (5–5 and 8-O-4 di-FA), whereas the content of two other di-FAs detected in Brachypodium cell wall (8–5 and 8–5 benzofuran di-FA) did not significantly change (Figure 1C). The total content of mono- and di-FA was also significantly lower in transgenic plants in comparison with wild-type plants (Figure 1D).
To investigate the effect of cell wall de-feruloylation on the polysaccharide composition, monosaccharide analyses of cell walls extracted from transgenic and wild-type plants were performed. For these analyses, Brachypodium transgenic lines AnFAE 8 and AnFAE 11, which showed strong reduction of FA in their cell walls, were also analyzed separately. A significant increase of xylose and reduction of glucose was observed in both transgenic lines in comparison with wild-type cell walls (Table 1A), indicating an increase in xylan content and a decrease in 1,3;1,4-mixed glucans in cell walls of Brachypodium transgenic plants, most likely in response to a reduction of polysaccharide cross-linking.
Table 1.
Monosaccharide composition (mol %) of cell walls from wild-type (WT) and AnFAE-expressing Brachypodium and Arabidopsis plants.
| (A) | Fuc | Rha | Ara | Gal | Glc | Xyl | GalA | GlcA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| WT Bd21 | 0.9 ± 0.3 | 0.6 ± 0.4 | 12.7 ± 2.8 | 3.4 ± 1.1 | 31.2 ± 9.5 | 49.6 ± 6.5 | 0.8 ± 0.7 | 0.7 ± 0.7 | ||
| AnFAE 8 | 0.7 ± 0.3 | 0.5 ± 0.6 | 14.8 ± 0.7 | 3.8 ± 0.1 | 13.4 ± 1.1 | 64.3 ± 1.6 | 1.9 ± 0.6 | 0.7 ± 0.8 | ||
| AnFAE 11 | 0.6 ± 0.3 | 0.7 ± 0.6 | 14.5 ± 1.1 | 3.5 ± 0.4 | 13.1 ± 2.4 | 65.6 ± 2.9 | 1.2 ± 0.5 | 0.7 ± 0.6 | ||
| (B) | Fuc | Rha | Ara | Gal | Glc | Xyl | Man | GalA | GlcA | |
| WT Col-0 | 1.2 ± 0.3 | 4.6 ± 0.4 | 5.3 ± 1.7 | 8.8 ± 2.5 | 9.0 ± 0.1 | 55.2 ± 3.2 | 6.9 ± 0.9 | 7.6 ± 1.7 | 1.5 ± 0.8 | |
| AnFAE | 1.5 ± 0.5 | 5.3 ± 2.1 | 4.5 ± 1.2 | 8.7 ± 2.2 | 8.7 ± 0.4 | 56.0 ± 5.6 | 6.5 ± 0.3 | 7.1 ± 1.4 | 1.6 ± 0.6 | |
(A) Cell wall composition of Brachypodium non-cellulosic polysaccharides from WT and AnFAE plants. (B) Cell wall composition of Arabidopsis non-cellulosic polysaccharides from WT and AnFAE plants. Numbers in bold represent statistical significance (Student’s t-test, p < 0.05, n = 3–6).
In addition, monosaccharide analysis was performed for cell walls extracted from one previously characterized line of transgenic Arabidopsis plants expressing the same fungal enzyme (AnFAE). Arabidopsis transgenic plants showed a reduction in mono-FA content (~50%), but no di-FA was detected in their cell walls (Pogorelko et al., 2011). In contrast to Brachypodium, Arabidopsis transgenic plants did not display any alteration in monosaccharide composition (Table 1B).
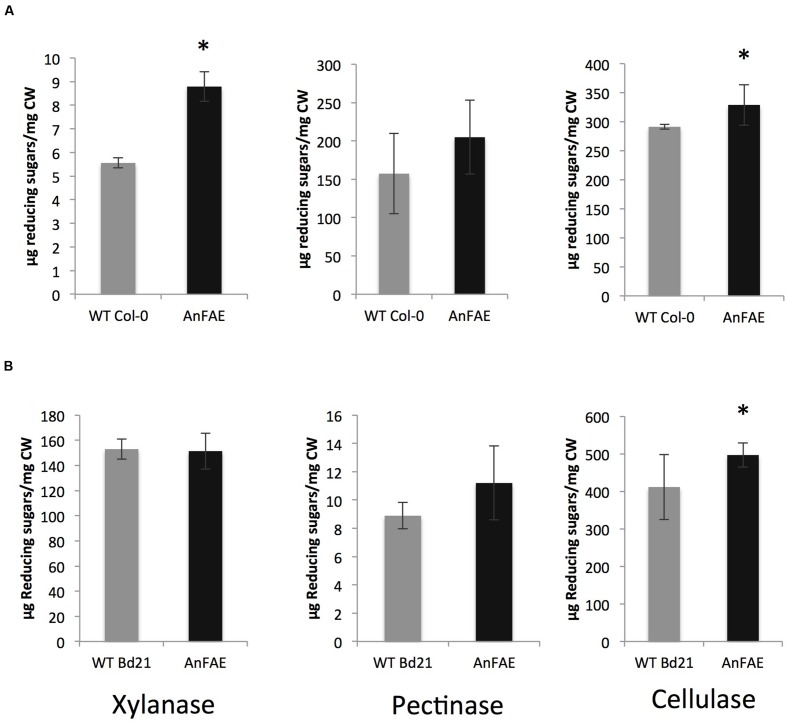
AnFAE in the Apoplast Affects Arabidopsis and Brachypodium Cell Wall Digestibility
To determine whether reduction of cell wall feruloylation alters cell wall digestibility, we measured the amount of reducing sugars released during treatment of cell walls extracted from Arabidopsis and Brachypodium transgenic and wild-type plants with either xylanase, pectin methyesterase plus polyglacturonase (PME+PG) or a cellulase cocktail. Treatment of cell walls from Arabidopsis transgenic plants with xylanases and cellulases released significantly more reducing sugars in comparison with wild-type cell wall, whereas amount of sugars released by PME+PG treatment was not different (Figure 2A). Cell walls extracted from Brachypodium transgenic plants released higher amount of reducing sugars only after treatment with cellulases, but not after xylanase or PME+PG treatment (Figure 2B).
FIGURE 2.
Amount of reducing sugars released after incubation of cell wall material from 8-week old plants with wall-degrading enzymes. (A) Arabidopsis cell wall treated with xylanase, pectinase, and cellulase. (B) Brachypodium cell wall treated with xylanase, pectinase, and cellulase. Asterisks indicate significant differences according to Student’s t-test (p < 0.05, n = 3–6).
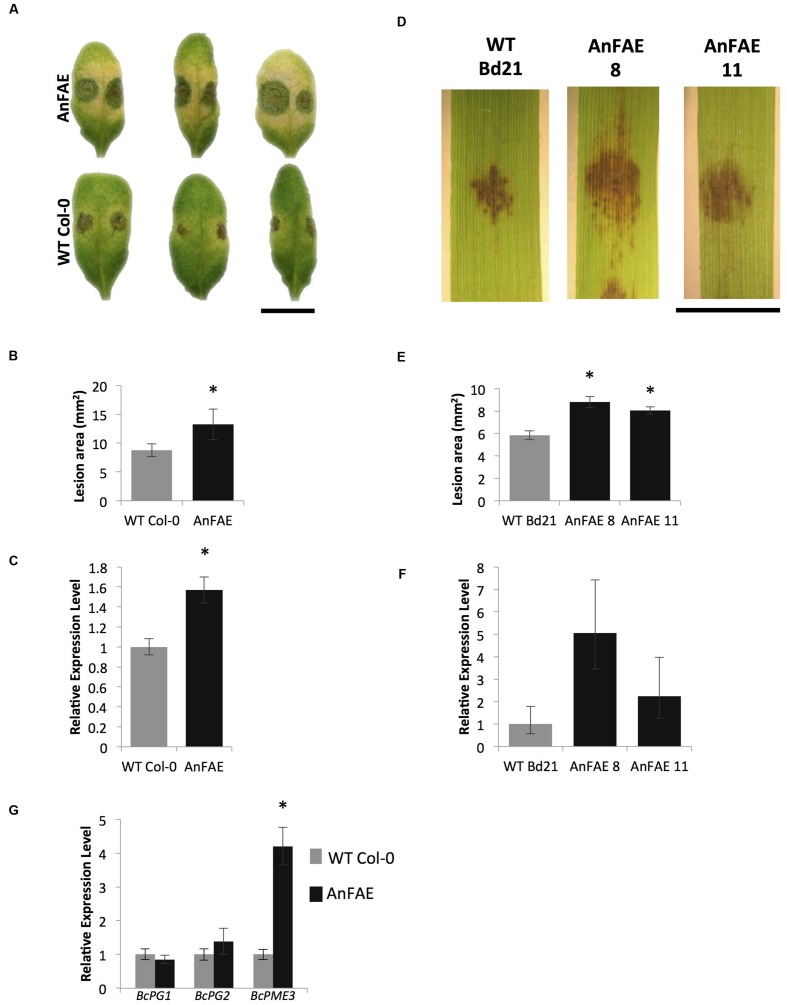
AnFAE-expressing Plants Have Higher Susceptibility to Necrotrophic Fungal Pathogens
Since modification of cell wall composition, and specifically reduction of FA, may affect plant-pathogen interactions (Venkata et al., 2013), we next used pathogen infection to challenge the transgenic Arabidopsis generated previously (Pogorelko et al., 2011) and the Brachypodium plants generated in this study. We measured the susceptibility of Arabidopsis plants expressing AnFAE and wild-type Col-0 plants by inoculating leaves with B. cinerea conidia and evaluating symptoms 48 h post inoculation (hpi). Similarly, leaves of two Brachypodium transgenic lines and wild-type plants were infected with B. sorokiniana and symptoms were evaluated at 48 hpi. Both Arabidopsis and Brachypodium transgenic plants showed a significant increase in the areas of lesions produced by the pathogens in transgenic plants compared with wild-type plants (Figures 3A,B,D,E).
FIGURE 3.
Analysis of Arabidopsis and Brachypodium transgenic plants susceptibility to fungal necrotrophs. (A,D), B. cinerea and B. sorokiniana symptoms on Arabidopsis and Brachypodium transgenic AnFAE and wild-type leaves. (B,E), Measurements of lesion areas on Arabidopsis and Brachypodium leaves 48 h post inoculation (HPI). (C,F), Relative expression levels of B. cinerea-Actin in Arabidopsis and of B. sorokiniana GAPDH in Brachypodium AnFAE and wild-type leaves 48 HPI. (G) Expression of B. cinerea virulence-related genes in Arabidopsis AnFAE and Col-0 plants. Asterisks indicate statistical significance according to Student’s t-test (p < 0.05, n = 3). Scale bars = 1 cm.
In Arabidopsis, higher accumulation of B. cinerea Actin transcripts was detected in infected transgenic leaves in comparison with infected wild-type leaves. In Brachypodium, a slightly higher, though not statistically significant, amount of B. sorokiniana GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE-LIKE (GADPH) was detected in infected transgenic leaves in comparison with infected wild-type leaves (Figures 3C,F).
Botrytis cinerea expresses a multitude of wall-degrading enzymes during pathogenesis and we also measured transcript levels of BcPG1, BcPG2, and BcPME3 during infection to investigate possible relationships between plant susceptibility, cell wall structural integrity, and fungal virulence. While the expression of PG genes did not change in transgenic plants, up-regulation of BcPME3 was observed in infected Arabidopsis transgenic leaves (Figure 3G). These results indicate that the expression of AnFAE in Arabidopsis and Brachypodium can increase susceptibility to fungal necrotrophs.
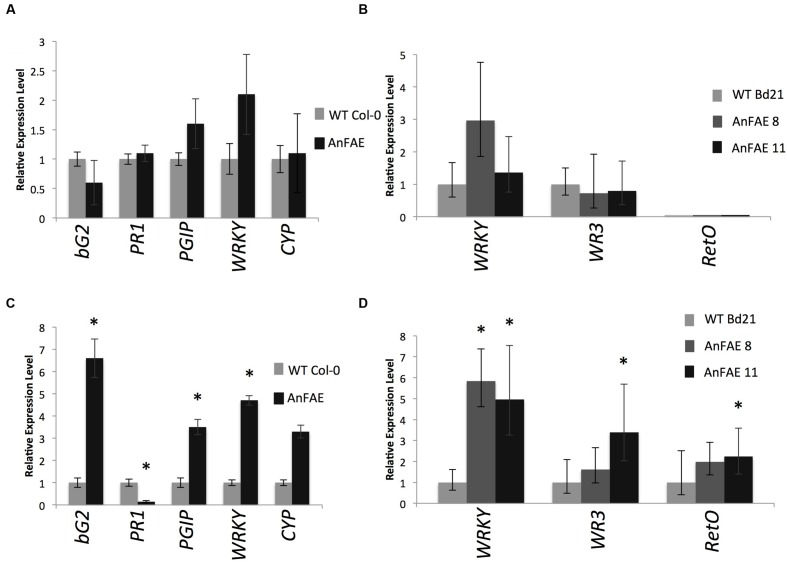
De-feruloylation of Cell Walls Alters the Expression of Pathogen-responsive Genes during Pathogenesis
Increased susceptibility to pathogens can be caused by the release and accumulation of ferulate in the apoplastic fluid of transgenic plants or by compromised CWI, which may induce plant-specific responses. Measurement of FA contents in apoplastic fluids of transgenic Arabidopsis plants did not reveal the presence of detectable free FA (Supplementary Figure S2).
To investigate whether the expression of fungal AnFAE and the consequent decrease of FA content in the cell wall affect the pathways involved in plant response to infection, we used RT-qPCR to measure the transcript levels of several known defense genes in infected and un-infected Arabidopsis and Brachypodium transgenic and wild-type plants. In healthy, un-infected transgenic plants, expression of these genes did not differ from their expression in wild-type plants (Figures 4A,B). However, several defense-related genes were significantly up-regulated in transgenic plants in comparison with wild-type plants upon infection (Figures 4C,D). Thus, in Arabidopsis transgenic plants, expression of PGIP1, bG2, and WRKY40 was significantly higher than in wild-type plants, whereas the level of PR1 transcript was significantly lower (Figure 4C). Some increase of CYP81F2 was also observed in infected Arabidopsis plants but the difference was not statistically significant. In Brachypodium, expression of WRKY40, WR3, and RetOX was higher in comparison with infected wild-type plants (Figure 4D). A full list of genes assayed, including those not significantly different from wild-type and not amplified in Brachypodium, is included in Supplementary Figure S3.
FIGURE 4.
Real-time qPCR analysis of selected pathogen-inducible defense genes in mock-infected (A-Arabidopsis, B-Brachypodium) and 48 HPI (C-Arabidopsis, D-Brachypodium) plants. Gene expression levels in transgenic plants normalized to the expression of the same gene detected in wild-type plants (for which gene expression was set to 1), ACTIN2 was used as reference gene for Arabidopsis and GAPDH for Brachypodium expression analysis. The comparative threshold cycle method was used for determining differences between transcript copy numbers in wild-type and transgenic plants. Data represent average obtained for three independent transgenic lines. Asterisks indicate significant differences between transgenic plants and wild-type plants (Student’s t-test, P < 0.05; n = 3).
Reduction of Cell Wall Ferulic Acid Content Affects Extensin Extractability in Transgenic Arabidopsis Plants
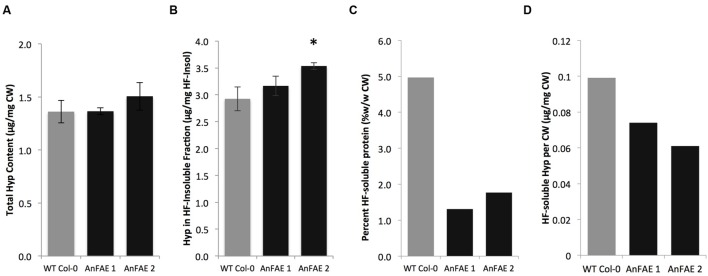
Previous work indicated that in dicots, FA may be involved in cross-linking pectins with extensins (Qi et al., 1995). Extensins are also induced during defense responses of Arabidopsis plants (Lamport et al., 2011). To investigate whether Arabidopsis plants expressing AnFAE have altered amounts of extensin in their cell walls, HF was used to deglycosylate cell walls. The HF-soluble fraction represents the pool of wall-associated extensins, while the HF-insoluble fraction represents the pool of crosslinked extensins (Mort and Lamport, 1977). The amount of hydroxyproline was quantified as an indicator of extensin contents. We found that the total extensin content was unchanged in transgenic and wild-type cell walls (Figure 5A). The amount of crosslinked extensin was slightly elevated in walls from AnFAE transgenic plants (Figure 5B). However, the proportion of HF-soluble protein per mg cell wall was significantly reduced in cell walls from AnFAE transgenic plants (Figure 5C). In the HF-soluble fraction, AnFAE cell wall samples contained lower amounts of hydroxyproline (Figure 5D). This indicates that reduction of cell wall feruloylation affects extensin self-cross-linking.
FIGURE 5.
Hydroxyproline content in cell walls of wild-type and two AnFAE Arabidopsis lines. (A) Total hydroxyproline content in cell walls as determined by absorbance at 560 nm. (B) Hydroxyproline content in the HF-insoluble cell wall fraction (μg Hyp/mg HF-Insoluble). (C) Percent of HF-soluble protein per mg cell wall (mg/mg CW) (D) Hydroxyproline content in HF-soluble protein (μg Hyp/mg CW). Asterisks indicate statistical significance (Student’s t-test, p < 0.05, n = 3).
Discussion
Increasing evidence demonstrates that changes in cell wall composition can stimulate plant responses similar to stress responses (Hamann, 2012; Pogorelko et al., 2013a). For example, Arabidopsis and tobacco plants expressing an attenuated version of endopolygalacturonase II from A. niger had improved resistance to fungal and bacterial pathogens and increased cell wall digestibility, but exhibited a dwarf phenotype (Capodicasa et al., 2004; Ferrari et al., 2007). Overexpression of a fruit-specific pectin methylesterase (PME) in wild strawberry induced defense-related gene expression and increased plant resistance to B. cinerea (Osorio et al., 2011). Arabidopsis and Brachypodium plants expressing either acetylxylan esterase or rhamnogalacturonan acetylesterase from A. nidulans exhibited higher resistance to fungal necrotrophs and had increased transcript levels for some defense-related genes (Pogorelko et al., 2013b). Expression of a thermostable xylanase in rice induced upregulation of plant defense-related genes such as SOD, CAT, and the xylanase inhibitor RIXI (Weng et al., 2013). Cell wall structures can also serve as a mechanical barrier to microbial penetration and thus influence plant resistance. Increased pectin methylesterification caused by overexpression of PME inhibitors (PMEI) decreased susceptibility to fungal and bacterial pathogens and viruses (Lionetti et al., 2007, 2012, 2014). Arabidopsis and wheat plants overexpressing PMEI had increased biomass and improved tissue saccharification (Lionetti et al., 2010; Volpi et al., 2011).
By contrast, modifications that compromise cell wall mechanical properties can potentially have the opposite effect, reducing plant resistance to pathogens and other stresses. For example, reduction in lignin content in soybean Rpp2 plants was correlated with their loss of resistance to the soybean rust fungus Phakopsora pachyrhizi (Pandey et al., 2011). Therefore, such cell wall modifications require detailed characterization of plant fitness to be effective in applied crop biotechnology.
Earlier, we reported that Arabidopsis plants expressing the A. nidulans feruloyl esterase AnFAE exhibited significant reduction of cell wall feruloylation and increased biomass saccharification by fungal cellulases after acid pretreatment (Pogorelko et al., 2011). In this study, we additionally generated the Brachypodium transgenic plants expressing the same AnFAE protein and confirmed reduction of monomeric and dimeric FA in their cell walls, which increased Brachypodium cell wall degradability by fungal cellulases. Using the Arabidopsis transgenic plants created earlier and the Brachypodium transgenic plants created in this study, we investigated the effect of cell wall deferuloylation on plant susceptibility to fungal necrotrophic pathogens and obtained new insights into the potential role of FA in plant CWI.
The increased degradability of cell walls observed in the plants expressing AnFAE indicates that reduction of FA affects the accessibility of polysaccharides in both type I (dicots) and type II (grasses) cell walls. While the effect of monomeric and dimeric FA reduction on Brachypodium cell wall digestibility demonstrated in this study was not surprising and conforms to similar observations in other grasses (Buanafina et al., 2010; Harholt et al., 2010), to the best of our knowledge, there are no reports about the effect of FA on the digestibility of the cell wall in dicots. Our results indicate that most likely, in the Arabidopsis cell wall, FA is esterified to fatty acids in the cutin (Rautengarten et al., 2012; Fich et al., 2016) as well as to the polysaccharides in the cell wall. It is possible that monomeric FA forms cross-links between those molecules creating a connection between the cell wall and cutin, affecting the accessibility of cell wall degrading enzymes or the integrity of the cutin. However, these possible changes in cutin-cell wall connections do not seem to affect cutin permeability/integrity, since our experiments studying chemical leaching of chlorophyll showed no difference between AnFAE and wild-type plants (Supplementary Figure S4). It cannot be excluded that the reduction of FA occurs exclusively on polysaccharides within the cell wall.
In dicots, FA may cross-link polysaccharides with extensins, as previously proposed (Qi et al., 1995), in response to pathogen attack (Deepak et al., 2010). While in AnFAE-expressing plants, total extensin content remained unchanged in comparison with wild-type Arabidopsis plants (Figure 5A), the amount of loosely associated HF-soluble extensin significantly decreased (Figure 5D) due to the increased proportion of self-cross-linked extensin that became insoluble. This shift from loosely associated extensins, toward self-cross-linked extensins may occur in response to reduction of polysaccharide-extensin interconnections. It is plausible to propose that crossed-linked extensins contribute to recalcitrance of the type I cell wall in dicots somewhat similarly to diFA in monocot type II cell walls. Hence, the reduction of FA content in transgenic Arabidopsis plants expressing AnFAE can induce higher self-cross-linking of extensins as a compensatory response by the plants to fortify their cell walls.
Reduction of cell wall crosslinking and increase of their degradability in AnFAE plants negatively affects the plant resistance to necrotrophic fungal pathogens. Both the Arabidopsis and Brachypodium transgenic plants showed significantly higher susceptibility to B. cinerea and B. sorokiniana, respectively, consistent with higher digestibility of their cell walls. It is plausible to expect that mechanical strength of the wall with reduced di-FA cross-linking in Brachypodium plants is compromised, which makes it more accessible to the cell wall degrading enzymes secreted by fungus. Most likely, in Arabidopsis transgenic plants, the strength of cell walls is reduced as well, though perhaps due to reduction of polysaccharide-extensin connections. This notion will need further investigation in the future.
Transcript analysis of the known plant defense-related genes PR1, bG2, PR5, PAD3, WR3, JR1, WRKY40, PGIP1, CYP81F2, and RetOx (Galletti et al., 2008; Raiola et al., 2011), as well as susceptibility genes PME3 (Raiola et al., 2011), PMR4 (Nishimura and Somerville, 2002), PMR5 (Vogel et al., 2004), PMR6 (Vogel et al., 2002), and MYB46 (Ramírez et al., 2011), demonstrated that none of the investigated genes were constitutively up- or down-regulated in AnFAE plants. Also, peroxide staining did not show an alteration in the accumulation of hydrogen peroxide in the leaves of transgenic plants with respect to wild-type plants. (Supplementary Figure S5). These results indicate that a perturbation of FA-mediated crosslinking in the cell wall does not induce defense responses in the absence of biotic stress, in contrast to what was observed in the case of post-synthetic cell wall de-acetylation (Pogorelko et al., 2013b) or pectin modification (Ferrari et al., 2008). However, Arabidopsis plants expressing AnFAE showed higher expression of bG2 (PR2), PGIP1, and WRKY40, and repression of PR1 upon B. cinerea infection in comparison with wild-type plants. By contrast, Brachypodium plants expressing AnFAE showed higher expression of genes homologous to Arabidopsis WRKY40, WR3, and RetOx, in comparison with wild-type plants, upon infection with B. sorokiniana. The pattern of affected defense-related genes in AnFAE plants was somewhat different from those that were differentially expressed in the plants with reduced cell wall acetylation or reduced homogalacturonan contents (Ferrari et al., 2008; Pogorelko et al., 2013b). This could suggest that different alterations of cell wall structure initiate different CWI signaling pathways, which activate discrete defense pathways in response to pathogen infection.
The bG2 gene encodes a β-glucanase, which is up-regulated during the Arabidopsis response to biotic stress (Dong et al., 1991). This gene was also constitutively up-regulated in Arabidopsis plants expressing rhamnogalacturonan acetylesterase in response to reduced acetylation of pectin and xyloglucan. Moreover, basal β-1,3-glucanase activity was significantly higher in tobacco and Arabidopsis lines expressing high levels of A. nidulans polygalacturonase (Ferrari et al., 2008). This indicates that despite the presence of specific responses, the activation of β-glucanase is a common response to the loss of CWI, regardless of the polysaccharide type in the cell wall being perturbed.
The accumulation of the cell wall associated polygalacturonase inhibiting proteins (PGIPs) is considered to contribute to resistance against polygalacturonase-producing pathogenic fungi (De Lorenzo et al., 2001). A higher level of PGIP expression was observed in Arabidopsis AnFAE-expressing plants in response to fungal treatment. It is possible that although AnFAE plants try to defend themselves, the integrity of their cell walls is altered to the extent that it cannot be compensated by the activation of these defense genes.
The Arabidopsis WOUND RESPONSIVE 3 (WR3) encodes a high-affinity nitrate transporter, which was proposed to be involved in jasmonic acid-independent wound signal transduction (Titarenko et al., 1997) and was shown to be activated by treatment with fungal-derived oligosaccharide elicitor (Rojo et al., 1999). WR3 was proposed to be involved in hypersensitive-like cell death induction (Kim et al., 2008). Higher expression of the homologous gene in Brachypodium AnFAE-expressing plants upon infection might suggest that reduction of FA-mediated cross-linking in their cell walls increases the response of the plant upon wounding associated with the action of necrotrophic microorganisms.
After fungal inoculation, Arabidopsis AnFAE-expressing plants showed lower expression of the salicylic acid (SA)-dependent PR1 gene, which was consistent with upregulation of the transcription factor gene WRKY40, considered to be a repressor of SA signaling (Xu et al., 2006; Zheng et al., 2006). This suggests that the weakening of cell walls due to reduction of FA-mediated crosslinks might affect either the SA metabolic pathway or activation of SA-dependent pathways. We did not observe higher accumulation of FA or any other phenolic acids in the apoplast of AnFAE plants in comparison with wild-type plants; therefore, we can eliminate the possibility that increased free FA in the apoplast contributes to repression of PR1.
The B. cinerea genome contains at least six endopolygalacturonase genes (BcPGs) (Wubben et al., 1999), of which BcPG1 and BcPG2 are required for virulence (Kars et al., 2005). At the same time, most pectinolytic enzymes cannot degrade highly methylated pectin. Therefore, the action of pectin methylesterase (PME), which de-methylesterifies pectin to pectate, is required. Although neither BcPME1 nor BcPME2 are required for fungal virulence, a possible involvement of BcPME3 was not excluded (Kars et al., 2005). Thus, we measured expression of fungal BcPG1, BcPG2, and BcPME3 during infection to investigate possible relations between plant susceptibility, cell wall structural integrity and fungal virulence. Since different sets of cell wall degrading enzymes are secreted at different stages of fungal infection (Laluk and Mengiste, 2010), the higher expression of BcPME3 but not of BcPG1 and BcPG2 during growth of B. cinerea on AnFAE plants, in comparison with wild-type plants might reflect the faster development of fungal colonies at the stage that requires contribution of BcPME3.
Based on the results of this study, we propose that some improvements of feedstock biomass digestibility can negatively affect plant stress resistance, so a better understanding of this trade-off can help in finding optimal directions in plant biotechnology.
Conclusion
In this study, we demonstrated that alteration of plant cell wall feruloylation has a negative effect on plant fitness. While improving cell wall digestibility, reduction of ferulic acid content negatively affects plant resistance to biotic stress, most likely due to compromised CWI. Thus, in Brachypodium, reduction of FA-mediated cross-linking reduces cell wall strength, which leads to lower resistance against microbial penetration. Similarly, the cell wall in Arabidopsis becomes weaker, most likely due to changes in the polysaccharide-extensin network, but further detailed analyses are necessary to confirm this.
Author Contributions
NR designed and conducted experiments for cell wall and expression analysis, enzyme activity assays, and wrote the paper. GP designed and conducted experiments for cell wall and expression analysis, and wrote the paper. VL designed experiments and conducted experiments in pathogen response. LC assisted with conducting experiments for cell wall analysis. MH designed and conducted experiments for extensin analysis. DB designed experiments for pathogen response. OAZ designed experiments, supervised all activity, and wrote the paper. All authors conducted revisions of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by Roy J. Carver Charitable Trust (#09-3384; 2010-2013 to OAZ), NSF-MCB (#1121163; 2011–2016 to OAZ), and by Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN Grant #2010T7247Z, http://www.istruzione.it/ to DB).
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00630
References
- Abràmoff M. D., Magalhães P. J., Ram S. J. (2004). Image processing with image. J. Biophotonics Int. 11 36–41. 10.1117/1.3589100 [DOI] [Google Scholar]
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. (2010). Plant Cell Walls. New York, NY: Garland Science, Taylor & Francis Group LLC. [Google Scholar]
- Bauer S., Vasu P., Persson S., Mort A. J., Somerville C. R. (2006). Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc. Natl. Acad. Sci. U.S.A. 103 11417–11422. 10.1073/pnas.0604632103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D., Cervone F., Lionetti V. (2014). Plant cell wall dynamics and wall-related susceptibility in plant – pathogen interactions. Front. Plant Sci. 5:228 10.3389/fpls.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergvinson D. J., Arnason J. T., Pietrzak L. N. (1994). Localization and quantification of cell wall phenolics in European corn borer resistant and susceptible maize inbreds. Can. J. Bot. 72 1243–1249. 10.1139/b94-152 [DOI] [Google Scholar]
- Bily A. C., Reid L. M., Taylor J. H., Johnston D., Malouin C., Burt A. J., et al. (2003). Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: resistance factors to Fusarium graminearum. Phytopathology 93 712–719. 10.1094/PHYTO.2003.93.6.712 [DOI] [PubMed] [Google Scholar]
- Buanafina M. M. D. O. (2009). Feruloylation in Grasses: current and future perspectives. Mol. Plant 2 861–872. 10.1093/mp/ssp067 [DOI] [PubMed] [Google Scholar]
- Buanafina M. M. D. O., Fescemyer H. W. (2012). Modification of esterified cell wall phenolics increases vulnerability of tall fescue to herbivory by the fall armyworm. Planta 236 513–523. 10.1007/s00425-012-1625-y [DOI] [PubMed] [Google Scholar]
- Buanafina M. M. D. O., Langdon T., Hauck B., Dalton S., Morris P. (2006). Manipulating the phenolic acid content and digestibility of italian ryegrass (Lolium multiflorum) by vacuolar-targeted expression of a fungal ferulic acid esterase. Appl. Biochem. Biotechnol. 129 416–426. [DOI] [PubMed] [Google Scholar]
- Buanafina M. M. D. O., Langdon T., Hauck B., Dalton S., Morris P. (2008). Expression of a fungal ferulic acid esterase increases cell wall digestibility of tall fescue (Festuca arundinacea). Plant Biotechnol. J. 6 264–280. 10.1111/j.1467-7652.2007.00317.x [DOI] [PubMed] [Google Scholar]
- Buanafina M. M. D. O., Langdon T., Hauck B., Dalton S., Timms-taravella E., Morris P. (2010). Targeting expression of a fungal ferulic acid esterase to the apoplast, endoplasmic reticulum or golgi can disrupt feruloylation of the growing cell wall and increase the biodegradability of tall fescue (Festuca arundinacea). Plant Biotechnol. J. 2030 316–331. 10.1111/j.1467-7652.2009.00485.x [DOI] [PubMed] [Google Scholar]
- Caffall K. H., Mohnen D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344 1879–1900. 10.1016/j.carres.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Capodicasa C., Vairo D., Zabotina O., McCartney L., Caprari C., Mattei B., et al. (2004). Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol. 135 1294–1304. 10.1104/pp.104.042788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A., O’Brien J. A. (2012). Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio Protocol. 2:e263. [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo G., D’Ovidio R., Cervone F. (2001). The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu. Rev. Phytopathol. 39 313–335. 10.1146/annurev.phyto.39.1.313 [DOI] [PubMed] [Google Scholar]
- Deepak S., Shailasree S., Kini R. K., Muck A., Mithöfer A., Shetty S. H. (2010). Hydroxyproline-rich glycoproteins and plant defence. J. Phytopathol. 158 585–593. 10.1111/j.1439-0434.2010.01669.x [DOI] [Google Scholar]
- Dong X., Mindrinos M., Davis K. R., Ausubel F. M. (1991). Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3 61–72. 10.1105/tpc.3.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds C. B., Williamson G. (1995). Release of ferulic acid from wheat bran by a ferulic acid esterase (FAE-III) from Aspergillus niger. Appl. Microbiol. Biotechnol. 43 1082–1087. 10.1007/BF00166929 [DOI] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Denoux C., De Lorenzo G., Ausubel F. M., Dewdney J. (2007). Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 144 367–379. 10.1104/pp.107.095596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Pontiggia D., Manfredini C., Lionetti V., Bellincampi D., et al. (2008). Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol. 146 669–681. 10.1104/pp.107.109686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fich E. A., Segerson N. A., Rose J. K. C. (2016). The plant polyester cutin: biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 67 annurev–arlant–043015–111929. 10.1146/annurev-arplant-043015-111929 [DOI] [PubMed] [Google Scholar]
- Fry S. C. (1982). Phenolic components of the primary cell wall. Biochem. J. 203 493–504. 10.1042/bj2030493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fursova O., Pogorelko G., Zabotina O. A. (2012). An efficient method for transient gene expression in monocots applied to modify the Brachypodium distachyon cell wall. Ann. Bot. 110 47–56. 10.1093/aob/mcs103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R., Denoux C., Gambetta S., Dewdney J., Ausubel F. M., De Lorenzo G., et al. (2008). The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 148 1695–1706. 10.1104/pp.108.127845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lara S., Bergvinson D. J., Burt A. J., Ramputh A. I., Díaz-Pontones D. M., Arnason J. T. (2004). The role of pericarp cell wall components in maize weevil resistance. Crop Sci. 44 1546–1552. 10.2135/cropsci2004.1546 [DOI] [Google Scholar]
- Grabber J. H., Hatfield R. D., Ralph J. (1998). Diferulate cross-links impede the enzymatic degradation of non-lignified maize walls. J. Sci. Food Agric. 193 193–200. 10.1002/(SICI)1097-0010(199806)77 [DOI] [Google Scholar]
- Hamann T. (2012). Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 3:77 10.3389/fpls.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T., Denness L. (2011). Cell wall integrity maintenance in plants: lessons to be learned from yeast? Plant Signal. Behav. 6 1706–1709. 10.4161/psb.6.11.17782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Bach I. C., Lind-Bouquin S., Nunan K. J., Madrid S. M., Brinch-Pedersen H., et al. (2010). Generation of transgenic wheat (Triticum aestivum L.) accumulating heterologous endo-xylanase or ferulic acid esterase in the endosperm. Plant Biotechnol. J. 8 351–362. 10.1111/j.1467-7652.2009.00490.x [DOI] [PubMed] [Google Scholar]
- Hartley R. D., Morrison W. H., Himmelsbach D. S., Borneman W. S. (1990). Cross-linking of cell wall phenolic arabinoxylans in graminaceous plants. Phytochemistry 29 3705–3709. 10.1016/0031-9422(90)85317-9 [DOI] [Google Scholar]
- Hatfield R. D., Ralph J., Grabber J. H. (1999). Cell wall cross-linking in grasses by ferulates and diferulates. Lignin Lignan Biosynth. 697 209–236. 10.1021/bk-1998-0697.ch016 [DOI] [Google Scholar]
- Himmel M. E., Ding S.-Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., et al. (2007). Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315 804–807. 10.1126/science.1137016 [DOI] [PubMed] [Google Scholar]
- Ishii T. (1997). Structure and functions of feruloylated polysaccharides. Plant Sci. 127 111–127. 10.1007/BF00393644 [DOI] [Google Scholar]
- Jones J. D. G., Dangl J. L. (2006). The plant immune system. Nature 444 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Jones L., Milne J. L., Ashford D., Mcqueen-mason S. J. (2003). Cell wall arabinan is essential for guard cell function. Proc. Natl. Acad. Sci. U.S.A. 100 11783–11788. 10.1073/pnas.1832434100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars I., Krooshof G. H., Wagemakers L., Joosten R., Benen J. A. E., Van Kan J. A. L. (2005). Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 43 213–225. 10.1111/j.1365-313X.2005.02436.x [DOI] [PubMed] [Google Scholar]
- Kim C. Y., Bove J., Assmann S. M. (2008). Overexpression of wound-responsive RNA-binding proteins induces leaf senescence and hypersensitive-like cell death. New Phytol. 180 57–70. 10.1111/j.1469-8137.2008.02557.x [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Liesmaa M. (2009). A colorimetric method for determination of hydroxyproline in tissue hydrolysates. Scand. J. Clin. Lab. Invest. 11 128–133. 10.3109/00365515909060420 [DOI] [Google Scholar]
- Laluk K., Mengiste T. (2010). Necrotroph attacks on plants: wanton destruction or covert extortion? Arab. B. 8:e0136 10.1199/tab.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. A., Kieliszewski M. J., Chen Y., Cannon M. C. (2011). Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 156 11–19. 10.1104/pp.110.169011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. (1972). A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47 273–279. 10.1016/0003-2697(72)90301-6 [DOI] [PubMed] [Google Scholar]
- Lionetti V., Cervone F., Bellincampi D. (2012). Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 169 1623–1630. 10.1016/j.jplph.2012.05.006 [DOI] [PubMed] [Google Scholar]
- Lionetti V., Francocci F., Ferrari S., Volpi C., Bellincampi D., Galletti R., et al. (2010). Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc. Natl. Acad. Sci. U.S.A. 107 616–621. 10.1073/pnas.0907549107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Giancaspro A., Fabri E., Giove S. L., Reem N., Zabotina O. A., et al. (2015). Cell wall traits as potential resources to improve resistance of durum wheat against Fusarium graminearum. BMC Plant Biol. 15:361 10.1186/s12870-014-0369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Metraux J.-P. (2014). Plant cell wall in pathogenesis, parasitism and symbiosis. Front. Plant Sci. 5:612 10.3389/fpls.2014.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Camardella L., Giovane A., Obel N., Pauly M., et al. (2007). Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 143 1871–1880. 10.1104/pp.106.090803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Cervone F., Bellincampi D. (2014). Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol. Plant Pathol. 15 265–274. 10.1111/mpp.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle S. J., Berlyn G. P., Engstrom E. M., Krolikowski K. A., Reiter W. D., Pruitt R. E. (1997). Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle. Dev. Biol. 189 311–321. 10.1006/dbio.1997.8671 [DOI] [PubMed] [Google Scholar]
- Mikulic Petkovšek M., Stampar F., Veberic R. (2008). Increased phenolic content in apple leaves infected with the apple scab pathogen. J. Plant Pathol. 90 49–55. [Google Scholar]
- Mort A. J., Lamport D. T. A. (1977). Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal. Biochem. 82 289–309. 10.1016/0003-2697(77)90165-8 [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 15 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Nicaise V., Roux M., Zipfel C. (2009). Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol. 150 1638–1647. 10.1104/pp.109.139709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Somerville S. (2002). Resisting attack. Science 295 2032–2033. 10.1126/science.1070443 [DOI] [PubMed] [Google Scholar]
- Osorio S., Bombarely A., Giavalisco P., Usadel B., Stephens C., Aragez I., et al. (2011). Demethylation of oligogalacturonides by FaPE1 in the fruits of the wild strawberry Fragaria vesca triggers metabolic and transcriptional changes associated with defence and development of the fruit. J. Exp. Bot. 62 2855–2873. 10.1093/jxb/erq465 [DOI] [PubMed] [Google Scholar]
- Pandey A. K., Yang C., Zhang C., Graham M. A., Horstman H. D., Lee Y., et al. (2011). Functional analysis of the Asian soybean rust resistance pathway mediated by Rpp2. Mol. Plant. Microbe. Interact 24 194–206. 10.1094/MPMI-08-10-0187 [DOI] [PubMed] [Google Scholar]
- Pogorelko G., Fursova O., Lin M., Pyle E., Jass J., Zabotina O. A. (2011). Post-synthetic modification of plant cell walls by expression of microbial hydrolases in the apoplast. Plant Mol. Biol. 77 433–445. 10.1007/s11103-011-9822-9 [DOI] [PubMed] [Google Scholar]
- Pogorelko G., Lionetti V., Bellincampi D., Zabotina O. A. (2013a). Targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens Cell wall integrity. Plant Signal. Behav. 8:e25435 10.4161/psb.25435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelko G., Lionetti V., Fursova O., Sundaram R. M., Qi M., Whitham S. A., et al. (2013b). Arabidopsis and Brachypodium distachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol. 162 9–23. 10.1104/pp.113.214460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Behrens B. X., West P. R., Mort A. J. (1995). Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures. Evidence for a covalent cross-link between extensin and pectin. Plant Physiol. 108 1691–1701. 10.1104/pp.108.4.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiola A., Lionetti V., Elmaghraby I., Immerzeel P., Mellerowicz E. J., Salvi G., et al. (2011). Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant. Microbe. Interact 24 432–440. 10.1094/MPMI-07-10-0157 [DOI] [PubMed] [Google Scholar]
- Ralph J., Bunzel M., Marita J. M., Hatfield R. D., Lu F., Schatz P. F., et al. (2004). Peroxidase-dependent cross-linking reactions of p -hydroxycinnamates in plant cell walls. Phytochem. Rev. 158 2013–2027. [Google Scholar]
- Ralph J., Grabber J. H., Hatfield R. D. (1995). Lignin-ferulate cross-links in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr. Res. 275 167–178. 10.1016/0008-6215(95)00237-N [DOI] [Google Scholar]
- Ralph J., Quideau S., Grabber J. H., Hatfieida R. D. (1994). ldentification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. 602 3485–3498. [Google Scholar]
- Ramírez V., Agorio A., Coego A., García-Andrade J., Hernández M. J., Balaguer B., et al. (2011). MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis. Plant Physiol. 155 1920–1935. 10.1104/pp.110.171843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C., Ebert B., Ouellet M., Nafisi M., Baidoo E. E. K., Benke P., et al. (2012). Arabidopsis deficient in cutin ferulate encodes a transferase required for feruloylation of -hydroxy fatty acids in cutin polyester. Plant Physiol. 158 654–665. 10.1104/pp.111.187187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C. (2010). Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 153 1445–1452. 10.1104/pp.110.154518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E., León J., Sánchez-Serrano J. J. (1999). Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 20 135–142. 10.1046/j.1365-313x.1999.00570.x [DOI] [PubMed] [Google Scholar]
- Sanger M. P., Lamport D. T. A. (1983). A microapparatus for liquid hydrogen fluoride solvolysis: sugar and amino sugar composition of Erysiphe graminis and Triticum aestivum cell walls. Anal. Biochem. 128 66–70. 10.1016/0003-2697(83)90345-7 [DOI] [PubMed] [Google Scholar]
- Santiago R., Malvar R. A. (2010). Role of dehydrodiferulates in maize resistance to pests and diseases. Int. J. Mol. Sci. 11 691–703. 10.3390/ijms11020691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Seifert G. J., Blaukopf C. (2010). Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 153 467–478. 10.1104/pp.110.153940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J., et al. (2004). Toward a systems approach to. Science 306 2206–2211. 10.1126/science.1102765 [DOI] [PubMed] [Google Scholar]
- Tenhaken R. (2015). Cell wall remodeling under abiotic stress. Front. Plant Sci. 5:771 10.3389/fpls.2014.00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titarenko E., Rojo E., León J., Sánchez-Serrano J. J. (1997). Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 115 817–826. 10.1104/pp.115.2.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkata B. P., Lauter N., Li X., Chapple C., Krupke C., Johal G., et al. (2013). crw1 - a novel maize mutant highly susceptible to foliar damage by the western corn rootworm beetle. PLoS ONE 8:e71296 10.1371/journal.pone.0071296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Raab T. K., Schiff C., Somerville S. C. (2002). PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14 2095–2106. 10.1105/tpc.003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Raab T. K., Somerville C. R., Somerville S. C. (2004). Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 40 968–978. 10.1111/j.1365-313X.2004.02264.x [DOI] [PubMed] [Google Scholar]
- Volpi C., Janni M., Lionetti V., Bellincampi D., Favaron F., D’Ovidio R. (2011). The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol. Plant. Microbe. Interact 24 1012–1019. 10.1094/MPMI-01-11-0021 [DOI] [PubMed] [Google Scholar]
- Weng X., Huang Y., Hou C., Jiang D. (2013). Effects of an exogenous xylanase gene expression on the growth of transgenic rice and the expression level of endogenous xylanase inhibitor gene RIXI. J. Sci. Food Agric. 93 173–179. 10.1002/jsfa.5746 [DOI] [PubMed] [Google Scholar]
- Wolf S., Hematy K., Hofte H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63 381–407. 10.1146/annurev-arplant-042811-105449 [DOI] [PubMed] [Google Scholar]
- Wubben J. P., Mulder W., Have A., Kan J. A. L., Visser J. (1999). Cloning and partial characterization of endopolygalacturonase genes from botrytis cinerea these include?: cloning and partial characterization of endopolygalacturonase. Genes Botrytis Cin. 65 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen C., Fan B., Chen Z. (2006). Physical and functional interactions between and WRKY60 transcription factors. Plant Cell 18 1310–1326. 10.1105/tpc.105.037523.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., McKinnon J. J., Maenz D. D., Olkowski A. A., Racz V. J., Christensen D. A. (2003). Enzymic release of reducing sugars from oat hulls by cellulase, as influenced by Aspergillus ferulic acid esterase and Trichoderma xylanase. J. Agric. Food Chem. 51 218–223. 10.1021/jf020476x [DOI] [PubMed] [Google Scholar]
- Zabotina O., Malm E., Drakakaki G., Bulone V., Raikhel N. (2008). Identification and preliminary characterization of a new chemical affecting glucosyltransferase activities involved in plant cell wall biosynthesis. Mol. Plant 1 977–989. 10.1093/mp/ssn055 [DOI] [PubMed] [Google Scholar]
- Zheng Z., Qamar S. A., Chen Z., Mengiste T. (2006). Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48 592–605. 10.1111/j.1365-313X.2006.02901.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.