Abstract
An unsolved mystery in cell biology is how unusually large secretory cargoes are exported from the endoplasmic reticulum. In this issue, Santos et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201603072) report the function of a Mia2/cTAGE5 transcript fusion, named TALI, in the endoplasmic reticulum export of chylomicrons and very low-density lipoproteins, but not collagen XII.
COP-II vesicles transport regular-sized cargoes from the ER to the Golgi and are typically 60–90 nm in diameter (Jensen and Schekman, 2011; D’Arcangelo et al., 2013). This apparent size restriction poses a challenge for intestinal cells, which appear to use COP-II vesicles to export triglyceride-rich chylomicron particles that can be >250 nm in diameter (Mansbach and Siddiqi, 2010). Previous work has identified the proteins TANGO1 and cTAGE5 as key players in the export of large collagen oligomers from the ER (Malhotra and Erlmann, 2015). In this issue, Santos et al. describe a specific role for a TANGO-related “TALI” protein in large chylomicron and very low-density lipoprotein (VLDL) secretion from cultured cells.
In the liver, the 4,563-residue apolipoprotein B100 (ApoB100) protein packages triglycerides for secretion as 30–80-nm-diameter VLDL particles, each containing a single copy of ApoB100. In contrast, ApoB RNA undergoes an unusual editing step in intestinal enterocytes that generates a protein representing the first 48% of ApoB100, named ApoB48. In intestinal enterocytes, dietary fats are reesterified to form triacylglycerol, which is combined with phospholipids, cholesterol esters, and ApoB48 to form triglyceride-rich chylomicrons. Because ApoB secretion is closely coupled with lipoprotein assembly, it can be used as a proxy for monitoring lipoprotein secretion from liver and intestinal cells.
Our assumption that chylomicron secretion uses the COP-II machinery comes in part from the analysis of chylomicron retention disease that is caused by mutations in the Sar1B GTPase (Jones et al., 2003). Sar1 activation by Sec12 protein triggers recruitment of the Sec23/Sec24 and Sec13/Sec31 subunits to form the COP-II transport vesicle coat. Thus, liver and intestinal cells must somehow modify the COP-II vesicle formation process on the cytoplasmic surface of the ER, in relation to the presence of larger cargoes within the ER lumen, to enable COP-II vesicles to accommodate larger VLDL and chylomicron cargoes.
In a clever, genome-wide RNAi screen for cells that could not secrete horseradish peroxidase, Bard et al. (2006) discovered a protein they named TANGO1. This large, multi-domain protein is required for the secretion of collagens I, II, III, IV, VII, and IX from chondrocytes, fibroblasts, endothelial cells, and mural cells (Saito et al., 2009; Wilson et al., 2011; Santos et al., 2015). TANGO1 is a transmembrane ER resident that uses its lumenal sequences to interact with collagen; on the surface of the ER, TANGO1 binds COP-II coat proteins as well as another TANGO1-related protein called cTAGE5 (Saito et al., 2011). Unlike TANGO1, cTAGE5 does not contain a luminal cargo binding domain. cTAGE5 was recently shown to be important for the localization of Sar1’s guanine nucleotide exchange factor, Sec12, to so-called ER exit sites and cTAGE5 also cooperates with TANGO1 in collagen secretion (Saito et al., 2014).
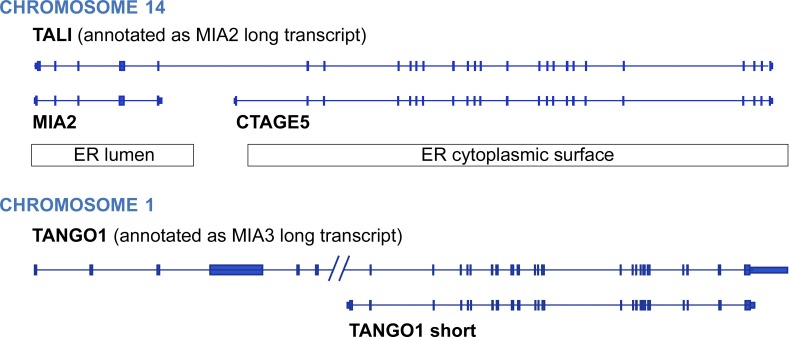
Fig. 1 (top row) shows the genomic organization and corresponding transcripts derived from the portion of human chromosome 14 that encodes cTAGE5, just downstream of the ER-to-Golgi SEC23A and TRAPPC6B genes. Three predominant transcripts are generated from this region. A short transcript (labeled MIA2) includes six exons and encodes a 654-residue secreted SH3 domain. This SH3 domain is related to sequences within the N terminus of TANGO1 (Fig. 1, bottom; encoded on chromosome 1). cTAGE5 is transcribed from sequences just downstream of the short MIA2 transcript on chromosome 14 (Fig. 1), and many splicing variants of cTAGE5 have been detected. Transcriptional read-through and splicing of MIA2’s exons 1–4 and part of exon 6 with the adjacent exons 2–24 of cTAGE5 generate a fusion protein in mice and humans (Pitman et al., 2011). Santos et al. (2016) have uncovered a related, cTAGE5 fusion protein variant in human colon carcinoma Caco2 cells that includes MIA2’s exon 5; they have named this TANGO-related fusion protein TALI (for TANGO1-like). TALI and TANGO are both expressed in the small intestine and liver, which produce chymomicrons and VLDLs, respectively. A possible role for TALI in lipoprotein secretion was hinted at from the observation that mice harboring mutations in the MIA2 SH3 domain had reduced cholesterol and triglycerides (Pitman et al., 2011). These previous experiments are slightly complicated to interpret, as mutations within the region of the gene encoding the MIA2 transcript may compromise the functions of the products of both the short and long variant transcripts (MIA2 and TALI).
Figure 1.
TALI, TANGO1, and related human transcripts. Transcript map derived from human chromosome 14 (39,233,916–39,253,655; top row) and chromosome 1 (222,618,086–222,668,012; bottom row) using Gencode version 22 comprehensive transcript set, redrawn from the University of California Santa Cruz Genome Browser on human (Dec. 2013 [GRCh38/hg38] assembly). Exons are shown as bars. Regions of these genes that reside in the ER lumen or on the ER cytoplasmic surface are indicated.
Santos et al. (2016) used CRISPR to target Exon 5 in the 5′ region of TALI, which leaves cTAGE5 expression intact. Loss of TALI in Caco2 cells blocked ApoB secretion by 82%; loss of TANGO1 also blocked ApoB secretion but to a lesser extent (44%). This suggests that TANGO1 and TALI may cooperate for some cargoes and work independently for others. Indeed, collagen XII accumulated significantly in the ER of TANGO-depleted cells but not in TALI-depleted Caco2 cells. Santos et al. (2016) used the same CRISPR strategy to deplete TALI and TANGO1 in the hepatocyte cell line HepG2 and demonstrate that TALI is also required for the efficient secretion of ApoB-containing lipoproteins from those cells. Like TANGO1 and cTAGE5, immunofluorescence experiments reveal that TALI colocalizes with ApoB at ER exit sites and, when ER exit is blocked, TALI is associated with distinctive, arc-shaped membrane structures that may represent the early stages of chymomicron or VLDL formation. Preliminary immunoprecipitations suggest that each of these proteins interacts with ApoB, even if only indirectly (Santos et al., 2016). This work highlights the importance of the TANGO1 and TALI protein luminal domains in cargo recognition.
The finding by Santos et al. (2016) that the TALI fusion protein is required for the secretion of lipoproteins raises several questions. How many different types of TANGO1 and TALI complexes exist in a given cell type? cTAGE5 is expressed ubiquitously and is identical with the C terminus of TALI. Are there TANGO1/cTAGE complexes that exclude TALI and specialize in collagen versus lipoprotein vesicle formation? Note that on chromosome 1, the TANGO1 gene includes a cTAGE5-like, orthologous transcript (Fig. 1, TANGO1 short). The TANGO1 short product is predicted to interact with TANGO1, cTAGE5, and/or TALI. Moreover, the MIA2 product could interfere with the ability of TALI to recognize cargo. How and in which cell types these putative complexes form and how they are regulated will be important to determine. In addition, very little is known to date regarding the precise mechanisms by which these proteins engage specific cargoes.
Recently, Zanetti et al. (2013) used cryoelectron tomography to determine the structure of a complete, in vitro membrane-assembled COP-II coat. They showed that COP-II subunits assemble to form inner and outer layers that can coat both spherical and tubular membranes, thereby having the capacity to accommodate large cargoes. TANGO1 and Sedlin (Venditti et al., 2012) have been proposed to modulate Sar1 GTPase activity, to delay outer coat recruitment and vesicle scission, and to permit the formation of a larger, COP-II decorated carrier. Zanetti et al. (2013) suggest that stabilization of the inner coat by TANGO1 or Sedlin could promote growth of larger carriers not only by delaying scission but also by promoting tubular membrane morphology. Reconstitution of the COP-II coats in the presence of soluble cTAGE5, Sedlin, and the TANGO1 (Mia3) cytoplasmic domain may yield important clues to the mechanisms by which these proteins potentially influence COP-II coat structure to facilitate large cargo packaging.
Finally, within the ER lumen, an intestine- and liver-specific ER chaperone protein, microsomal triglyceride transfer protein (MTTP), facilitates the assembly of both chylomicrons and VLDL particles by catalyzing triglyceride assembly onto ApoB proteins (Hussain et al., 2012). MTTP exists as a dimer with protein disulfide isomerase. MTTP might also interact with TALI and TANGO1 lumenal sequences to coordinate lipoprotein assembly with COPII vesicle packaging. Future experiments will be needed to test this possibility and to further explore the cargo selectivity of TALI and TANGO1 proteins.
Acknowledgments
The author’s scholarly activities are supported in part by funds from the National Institutes of Health (DK37332).
The author declares no competing financial conflicts.
References
- Bard F., Casano L., Mallabiabarrena A., Wallace E., Saito K., Kitayama H., Guizzunti G., Hu Y., Wendler F., Dasgupta R., et al. 2006. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 439:604–607. 10.1038/nature04377 [DOI] [PubMed] [Google Scholar]
- D’Arcangelo J.G., Stahmer K.R., and Miller E.A.. 2013. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim. Biophys. Acta. 1833:2464–2472. 10.1016/j.bbamcr.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M.M., Rava P., Walsh M., Rana M., and Iqbal J.. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond.). 9:14 10.1186/1743-7075-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D., and Schekman R.. 2011. COPII-mediated vesicle formation at a glance. J. Cell Sci. 124:1–4. 10.1242/jcs.069773 [DOI] [PubMed] [Google Scholar]
- Jones B., Jones E.L., Bonney S.A., Patel H.N., Mensenkamp A.R., Eichenbaum-Voline S., Rudling M., Myrdal U., Annesi G., Naik S., et al. 2003. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 34:29–31. 10.1038/ng1145 [DOI] [PubMed] [Google Scholar]
- Malhotra V., and Erlmann P.. 2015. The pathway of collagen secretion. Annu. Rev. Cell Dev. Biol. 31:109–124. 10.1146/annurev-cellbio-100913-013002 [DOI] [PubMed] [Google Scholar]
- Mansbach C.M., and Siddiqi S.A.. 2010. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72:315–333. 10.1146/annurev-physiol-021909-135801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman J.L., Bonnet D.J., Curtiss L.K., and Gekakis N.. 2011. Reduced cholesterol and triglycerides in mice with a mutation in Mia2, a liver protein that localizes to ER exit sites. J. Lipid Res. 52:1775–1786. 10.1194/jlr.M017277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Chen M., Bard F., Chen S., Zhou H., Woodley D., Polischuk R., Schekman R., and Malhotra V.. 2009. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 136:891–902. 10.1016/j.cell.2008.12.025 [DOI] [PubMed] [Google Scholar]
- Saito K., Yamashiro K., Ichikawa Y., Erlmann P., Kontani K., Malhotra V., and Katada T.. 2011. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol. Biol. Cell. 22:2301–2308. 10.1091/mbc.E11-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Yamashiro K., Shimazu N., Tanabe T., Kontani K., and Katada T.. 2014. Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J. Cell Biol. 206:751–762. 10.1083/jcb.201312062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A.J., Raote I., Scarpa M., Brouwers N., and Malhotra V.. 2015. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. eLife. 4:e10982 10.7554/eLife.10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A.J.M., Nogueira C., Ortega M., and Malhotra V.. 2016. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J. Cell Biol. 10.1083/jcb.201603072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R., Scanu T., Santoro M., Di Tullio G., Spaar A., Gaibisso R., Beznoussenko G.V., Mironov A.A., Mironov A. Jr., Zelante L., et al. 2012. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 337:1668–1672. 10.1126/science.1224947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.G., Phamluong K., Li L., Sun M., Cao T.C., Liu P.S., Modrusan Z., Sandoval W.N., Rangell L., Carano R.A., et al. 2011. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J. Cell Biol. 193:935–951. (published erratum appears in J. Cell Biol. 194:347) 10.1083/jcb.2010071621942c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G., Prinz S., Daum S., Meister A., Schekman R., Bacia K., and Briggs J.A.. 2013. The structure of the COPII transport-vesicle coat assembled on membranes. eLife. 2:e00951 10.7554/eLife.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]