Abstract
Anticoagulation therapy is essential for the effective treatment and secondary prevention of venous thromboembolism (VTE). For many years, anticoagulation for acute VTE was limited to the use of initial parenteral heparin, overlapping with and followed by a vitamin K antagonist. Although highly effective, this regimen has several limitations and is particularly challenging when given in an ambulatory setting. Current treatment pathways for most patients with deep-vein thrombosis typically involve initial hospital or community-based ambulatory care with subsequent follow-up in a secondary care setting. With the introduction of non-vitamin K antagonist oral anticoagulants (NOACs) into routine clinical practice, it is now possible for the initial acute management of patients with deep-vein thrombosis to be undertaken by primary care. As hospital admissions associated with VTE become shorter, primary care will play an increasingly important role in the long-term management of these patients. Although the NOACs can potentially simplify patient management and improve clinical outcomes, primary care physicians may be less familiar with these new treatments compared with traditional therapy. To assist primary care physicians in further understanding the role of the NOACs, this article outlines the main differences between NOACs and traditional anticoagulation therapy and discusses the benefit–risk profile of the different NOACs in the treatment and secondary prevention of recurrent VTE. Key considerations for the use of NOACs in the primary care setting are highlighted, including dose transition, risk assessment and follow-up, duration of anticoagulant therapy, how to minimize bleeding risks, and the importance of patient education and counseling.
Keywords: venous thromboembolism, oral anticoagulant, prevention, treatment, primary care, community
Introduction
Anticoagulation therapy is essential for the effective treatment and secondary prevention of venous thromboembolism (VTE), comprising deep-vein thrombosis (DVT) and pulmonary embolism (PE), but is associated with a risk of bleeding.1 Current treatment pathways for most patients with DVT typically involve initial hospital or community-based ambulatory care with subsequent follow-up in a secondary care setting. An increasing number of patients with low-risk PE are also being discharged early from hospital or treated entirely as outpatients. For many years, traditional anticoagulant treatment for acute VTE was limited to the use of initial parenteral heparin, overlapping with and followed by a vitamin K antagonist (VKA). This regimen is cumbersome for outpatients; VKA therapy necessitates routine coagulation monitoring of the international normalized ratio and frequent dose adjustment owing to a narrow therapeutic window and multiple drug and food interactions.2 Although prolonged VKA treatment further reduces the incidence of recurrent VTE compared with shorter treatment durations, it is also associated with increased risk of major bleeding.3 Consequently, the balance between the benefits and risks of continued anticoagulation remains a subject of debate, and many patients with VTE do not receive extended-duration anticoagulant therapy, despite the high long-term risk of recurrence and guideline recommendations supporting extended treatment.4–7
The non-VKA oral anticoagulants (NOACs; also known as novel oral anticoagulants) rivaroxaban, apixaban, dabigatran, and edoxaban have become available as alternative options for the management of several thromboembolic disorders, including the treatment of DVT/PE, secondary prevention of VTE, and stroke prevention in patients with non-valvular atrial fibrillation.8–11 Unlike VKAs, NOACs offer fixed dosing regimens without the need for routine coagulation monitoring, which makes the initial management of patients with DVT feasible in primary care settings, as well as facilitating easy transition from in-hospital to community care.
Primary care physicians play an increasingly important role in the long-term management of patients with VTE, but they may be far less familiar with newer treatment options (ie, NOACs) compared with traditional therapy. To assist primary care physicians in further understanding the role of the NOACs, this article outlines the main differences between NOACs and traditional anticoagulant therapy for the treatment and secondary prevention of recurrent VTE and discusses key considerations for their use in the primary care setting.
Differences between NOACs and traditional standard therapy for the treatment of VTE
Traditional anticoagulant therapy for patients with DVT (or PE in hemodynamically stable patients) utilizes a dual-drug approach consisting of a parenteral agent (most commonly low molecular weight heparin [LMWH] or fondaparinux) for ≥5 days, overlapping with a VKA until the international normalized ratio of VKA therapy is ≥2.0 for at least 24 hours, at which point VKA therapy alone is continued.12,13 This initial “bridging therapy” with a parenteral anticoagulant is required because of the slow onset of action of VKAs. In addition, VKAs require frequent coagulation monitoring and dose adjustment. By contrast, the predictable pharmacokinetic and pharmacodynamic properties of the NOACs allow for fixed dosing regimens without the need for routine coagulation monitoring. Furthermore, the NOACs have a fast onset of action, reaching their maximum plasma concentrations within a few hours of oral tablet intake (Table 1).14,15
Table 1.
Key pharmacological properties of NOACs and VKAs (eg, warfarin)
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Warfarin | |
|---|---|---|---|---|---|
| Drug class/mechanism of action | Direct thrombin inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor | VKA |
| Time to maximum plasma concentration (hours) | 0.5–2 | 2–4 | 3–4 | 1–2 | ∼90 minutes but requiring 5–6 days to achieve effective anticoagulation |
| Half-life (hours) | 12–14 | 5–13 | 8–13 | 10–14 | ∼40 hours for warfarin |
| Proportion of unchanged drug excreted renally (%) | ∼80 | ∼33 | ∼25 | 50 | Minor only |
| Predictable anticoagulant effect | Yes | Yes | Yes | Yes | No |
| Fixed dosing regimen | Yes | Yes | Yes | Yes | No |
| Routine coagulation monitoring required | No | No | No | No | Yes |
| Food effect | No interaction; taken with or without food | No interaction but 15 mg and 20 mg doses should be taken with food for optimal bioavailability | No interaction; taken with or without food | No interaction; taken with or without food | Affected by many common foods containing high levels of vitamin K |
| Relevant drug interactions | Strong P-gp inhibitors: ketoconazole, cyclosporin, itraconazole, and dronedarone are contraindicated. P-gp inducer rifampicin should be avoided | Strong inhibitors of both CYP3A4 and P-gp: azole antimycotics (eg, ketoconazole) and HIV protease inhibitors (eg, ritonavir) are not recommended | Strong inhibitors of CYP3A4 and P-gp: azole antimycotics (eg, ketoconazole) and HIV protease inhibitors (eg, ritonavir) are not recommended | Concomitant use of P-gp inhibitors cyclosporin, dronedarone, erythromycin, or ketoconazole requires edoxaban dose reduction to 30 mg oda | Interaction with numerous drugs |
Notes:
Concomitant use of edoxaban with quinidine, verapamil, or amiodarone does not require dose reduction based on clinical data. The use of edoxaban with other P-gp inhibitors, including HIV protease inhibitors, has not been studied.9 Data from studies.2,8–11,15
Abbreviations: CYP3A4, cytochrome P450 3A4; HIV, human immunodeficiency virus; NOAC, non-vitamin K antagonist oral anticoagulant; od, once daily; P-gp: P-glycoprotein; VKA, vitamin K antagonist.
Favorable benefit–risk profile of NOACs: evidence from Phase III clinical trials
In all the Phase III clinical trials for the treatment of acute VTE, the NOACs showed non-inferior efficacy compared with standard therapy, with significantly reduced rates of major bleeding in EINSTEIN PE (rivaroxaban) and AMPLIFY (apixaban; Table 2).16–21 The trials included patients with symptomatic VTE, but there is no reason to suspect that the NOACs would not be equally effective and safe in patients with asymptomatic VTE. It should be noted that the study design and patient populations differed between these studies, and that there are no head-to-head comparisons between the different NOACs.16–21 A pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies showed a significantly more favorable net clinical benefit for rivaroxaban in key subgroups vs standard therapy, eg, in fragile patients (defined as one or more of the following criteria: age >75 years; creatinine clearance [CrCl] <50 mL/min; or low body weight ≤50 kg), those with active cancer, and those with a previous VTE.22 Although these data are promising, it should be acknowledged that certain subgroups of patients, eg, those aged >75 years, with a body weight of <60 kg, with active cancer or thrombophilia, or taking dual antiplatelet therapy, were underrepresented in the Phase III clinical trials. Therefore, further research is needed to establish the benefit–risk profile of the NOACs in these specific patient groups.
Table 2.
Efficacy and safety outcomes of NOACs vs standard therapy in Phase III clinical trials for the treatment of acute VTE
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |||
|---|---|---|---|---|---|---|
|
| ||||||
| RE-COVER20 | RE-COVER II21 | EINSTEIN DVT16 | EINSTEIN PE17 | AMPLIFY18 | Hokusai-VTE19 | |
| Anticoagulant regimen | 150 mg bid after parenteral agent for 8–11 days | 150 mg bid after parenteral agent for 5–11 days | 15 mg bid for 21 days followed by 20 mg od | 15 mg bid for 21 days followed by 20 mg od | 10 mg bid for 7 days followed by 5 mg bid | 60 mg oda after heparin for a median of 7 days |
| Comparator arm | Parenteral agent/warfarin | Parenteral agent/warfarin | Enoxaparin/VKA | Enoxaparin/VKA | Enoxaparin/warfarin | Heparin/warfarin |
| Treatment duration (months) | 6 | 6 | 3, 6, or 12 | 3, 6, or 12 | 6 | 3–12 |
| Primary efficacy outcome | ||||||
| NOAC vs comparator (%) | 2.4 vs 2.1 | 2.3 vs 2.2 | 2.1 vs 3.0 | 2.1 vs 1.8 | 2.3 vs 2.7 | 3.2 vs 3.5 |
| HR/RR (95% CI) | HR 1.10 (0.65–1.84) | HR 1.08 (0.64–1.80) | HR 0.68 (0.44–1.04) | HR 1.12 (0.75–1.68) | RR 0.84 (0.60–1.18) | HR 0.89 (0.70–1.13) |
| P-value | P<0.001 for non-inferiority | P<0.001 for non-inferiority | P<0.001 for non-inferiority | P=0.003 for non-inferiority | P<0.001 for non-inferiority | P<0.001 for non-inferiority |
| Major bleeding | ||||||
| NOAC vs comparator (%) | 1.6 vs 1.9 | 1.2 vs 1.7 | 0.8 vs 1.2 | 1.1 vs 2.2 | 0.6 vs 1.8 | 1.4 vs 1.6 |
| HR/RR (95% CI) | HR 0.82 (0.45–1.48) | HR 0.69 (0.36–1.32) | HR 0.65 (0.33–1.30) | HR 0.49 (0.31–10.79) | RR 0.31 (0.17–0.55) | HR 0.84 (0.59–1.21) |
| P-value | NA | NA | P=0.21 | P=0.003 | P<0.001 | P=0.35 |
| Major or nonmajor clinically relevant bleeding | ||||||
| NOAC vs comparator (%) | 5.6 vs 8.8 | 5.0 vs 7.9 | 8.1 vs 8.1 | 10.3 vs 11.4 | 4.3 vs 9.7 | 8.5 vs 10.3 |
| HR/RR (95% CI) | HR 0.63 (0.47–0.84) | HR 0.62 (0.45–0.84) | HR 0.97 (0.76–1.22) | HR 0.90 (0.76–1.07) | RR 0.44 (0.36–0.55) | HR 0.81 (0.71–0.94) |
| P-value | P=0.002 | NA | P=0.77 | P=0.23 | P<0.001 | P=0.004 |
Notes:
30 mg od in patients with CrCl 30–50 mL/min, body weight ≤60 kg, or receiving concomitant treatment with a potent P-gp inhibitor.
Abbreviations: bid, twice daily; CI, confidence interval; CrCl, creatinine clearance; HR, hazard ratio; NA, not available; NOAC, non-vitamin K antagonist oral anticoagulant; od, once daily; P-gp, P-glycoprotein; RR, relative risk; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Long-term secondary prevention of recurrent VTE: NOACs or acetylsalicylic acid
The duration of anticoagulant treatment in patients with VTE has been a subject of debate. Although the extended VKA therapy is highly effective in preventing recurrent VTE, it is also associated with an increased risk of major bleeding.3 This, together with other limitations, discourages the extended use of VKA therapy in patients with VTE. In Phase III clinical trials, rivaroxaban (EINSTEIN EXT), apixaban (AMPLIFY EXT), and dabigatran (RE-SONATE) demonstrated superior efficacy compared with placebo for extended anticoagulation (6 months or 12 months) in patients who had already received anticoagulation therapy for 6–18 months.16–18,23 Compared with placebo, rivaroxaban (20 mg once daily) led to an 82% relative risk reduction (RRR) in the incidence of recurrent VTE, although there was a low incidence of nonfatal major bleeding events (0.7%).16 The RRR for recurrent VTE associated with apixaban was 81% for both doses (2.5 mg and 5 mg twice daily), without a significant increase in major bleeding events. Dabigatran significantly reduced the risk of recurrent VTE (RRR 92%) compared with placebo, but it was also associated with a significant increase in major and clinically relevant bleeding (principal safety end point).23
Acetylsalicylic acid (ASA) has also been investigated for long-term secondary prevention of recurrent VTE in patients with a first unprovoked VTE who have already had VKA therapy for 6–18 months. In the WARFASA study, which compared ASA 100 mg daily with placebo for 2 years, ASA treatment was associated with a 42% RRR in VTE recurrence, with no significant increase in the risk of bleeding.24 Using the same study design, the ASPIRE trial investigated continued treatment with ASA for up to 4 years. In this study, a significant decrease in VTE recurrence with ASA was not found compared with placebo, raising further doubts over the long-term efficacy of ASA in secondary prevention of recurrent VTE.25
Owing to concerns about bleeding, some physicians may prefer to prescribe ASA rather than oral anticoagulants for the secondary prevention of VTE. It should be noted that in the BAFTA study in an elderly (≥75 years) community population with atrial fibrillation, the risk of extracranial hemorrhage was similar for ASA and warfarin, but the incidence of primary end point events (fatal or disabling stroke, intracranial hemorrhage, or clinically significant arterial embolism) was significantly higher with ASA compared with warfarin.26 Similarly, although ASA treatment can reduce the rates of VTE recurrence, it is less effective than the NOACs (or VKAs). Current European Society of Cardiology guidelines specify that ASA should be considered for extended secondary prevention of VTE only if patients refuse or are unable to tolerate any form of oral anticoagulant.27
NOACs for the treatment of VTE: what do you need to know?
Contraindications for use
The NOACs are contraindicated in patients with active clinically significant bleeding, or in patients who have a lesion or a condition considered to be a significant risk for major bleeding. Concomitant treatment with any other anticoagulant is contraindicated, except under specific circumstances of switching anticoagulant therapy, or when unfractionated heparin is given at doses necessary to maintain an open central venous or arterial catheter.8–11 Apixaban, edoxaban, and rivaroxaban are contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk, and dabigatran is contraindicated in patients with hepatic impairment or liver disease that is expected to have any impact on survival.8–11 Dabigatran is also contraindicated in patients with severe renal impairment (CrCl <30 mL/min), and the other NOACs are not recommended in patients with CrCl <15 mL/min.8–11 Edoxaban and rivaroxaban are contraindicated during pregnancy, and all NOACs should be avoided during breastfeeding (edoxaban and rivaroxaban are contraindicated).8–11 Edoxaban is contraindicated in patients with uncontrolled severe hypertension, and rivaroxaban is not recommended in these patients9,10 Additional dabigatran contraindications are concomitant treatment with systemic ketoconazole, cyclosporin, itraconazole, and dronedarone, and patients with prosthetic valves who require anticoagulant treatment.8 Precautions for comedication use with other NOACs are discussed in the “Continued risk assessment and patient follow-up” section.
Dose regimens and managing dose transition
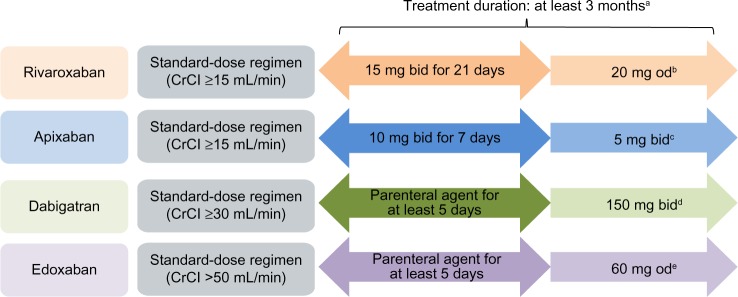
Of the NOACs currently licensed for VTE treatment, only rivaroxaban and apixaban offer the advantage of a single-drug approach for the total treatment duration, comprising an initial intensified treatment period without parenteral bridging. The approved NOAC dosing regimens for the treatment of VTE are shown in Figure 1.
Figure 1.
Approved dose regimens of the NOACs (based on the EU labels) for the treatment of VTE.
Notes: Rivaroxaban, dabigatran, apixaban, and edoxaban are approved in the EU. aThe duration of therapy should be individualized after careful assessment of the treatment benefit against the risk of bleeding. Short duration of therapy (at least 3 months) should be based on transient risk factors (eg, recent surgery, trauma, immobilization) and longer durations should be based on permanent risk factors or idiopathic DVT/PE. bA reduction in the dose from 20 mg od to 15 mg od should be considered if the patient’s assessed risk of bleeding outweighs the risk of recurrent DVT/PE. Rivaroxaban should be used with caution in patients with CrCl 15–29 mL/min and is not recommended in patients with CrCl <15 mL/min.10 cFor the prevention of recurrent DVT or PE following completion of 6 months of treatment for DVT or PE, 2.5 mg bid is recommended. Apixaban should be used with caution in patients with CrCl 15–29 mL/min and is not recommended in patients with CrCl <15 mL/min.11 dDabigatran 110 mg bid is recommended in patients aged ≥80 years and those who receive concomitant verapamil. In the following patient groups, the dose (150 mg bid or 110 mg bid) should be selected based on individual assessment of the thromboembolic and bleeding risks: patients aged 75–80 years; patients with moderate renal impairment; those with gastritis, esophagitis, or gastroesophageal reflux; and other patients at increased risk of bleeding. Dabigatran is contraindicated in patients with severe renal impairment (CrCl <30 mL/min).8 eEdoxaban 30 mg od is recommended in patients with one or more of the following clinical factors: moderate or severe renal impairment (CrCl 15–50 mL/min), low body weight (≤60 kg), or concomitant use of the following P-gp inhibitors: cyclosporin, dronedarone, erythromycin, or ketoconazole.9
Abbreviations: bid, twice daily; CrCl, creatinine clearance; DVT, deep-vein thrombosis; EU, European Union; od, once daily; NOAC, non-vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; P-gp, P-glycoprotein; VTE, venous thromboembolism.
In routine clinical practice, many patients with DVT (and/or low risk PE) may have been treated solely as outpatients, or transferred to outpatients or primary care after a short hospital stay (early discharge). Therefore, it is likely that some primary care physicians will manage dose transitions. Dose reductions may be considered in certain patient populations who have an increased risk of bleeding that outweighs the risk of recurrent DVT (eg, patients with renal impairment; Figure 1). Using the correct dosing regimen is essential to provide optimal protection for patients, and this is particularly important during the acute phase when patients are at higher risk of thrombus extension and embolization. After the initial acute treatment period, rivaroxaban is given as a once-daily regimen for long-term VTE treatment and secondary prevention of recurrent VTE, whereas both apixaban and dabigatran are administered twice daily. Once-daily dosing regimens (particularly as long-term therapy) have been shown to be associated with improved patient adherence,28 which may have positive effects on clinical outcomes. In the Phase III EINSTEIN DVT and EINSTEIN PE studies, patients receiving rivaroxaban reported higher treatment satisfaction than those receiving standard therapy with enoxaparin/VKA.29,30
Continued risk assessment and patient follow-up
Although routine coagulation or drug monitoring is not required for patients treated with NOACs, regular clinical follow-up is important for all patients receiving anticoagulants. This allows for ongoing assessment of the risks of recurrent VTE vs bleeding, as well as providing continued guidance and support to patients, particularly around drug adherence. Regular follow-up also offers opportunities to advise on anticoagulation management before invasive procedures (such as dental treatment). Risk factors for bleeding and VTE recurrence are listed in Table 3. Apart from recurrent DVT/PE, other consequences of VTE include post-thrombotic syndrome, a relatively frequent complication of DVT that may lead to long-term morbidity, and chronic thromboembolic pulmonary hypertension, a relatively rare but serious complication of PE.13
Table 3.
Risk factors associated with recurrent VTE and anticoagulant-related bleeding
| Recurrent VTE | Serious or fatal bleeding |
|---|---|
| Initial unprovoked VTE Initial proximal DVT or PE Residual venous thrombosis Cancer Elevated D-dimer concentrations when not receiving anticoagulation Male sex Post-thrombotic syndrome |
Low platelet count Previous gastrointestinal bleeding Recent major bleeding Previous stroke Increasing age Hepatic failure Severe renal impairment Diabetes Thrombocytopenia Poor anticoagulant control |
The timing and frequency of follow-up visits are influenced by a number of factors (such as the initial diagnosis and the individual patient’s bleeding risk) and are guided by local protocols. At each visit, patient medication compliance and use of comedications (including over-the-counter medications) should be reviewed. Moreover, patients should be assessed to ensure that VTE symptoms are resolving on anticoagulation therapy and asked whether they have experienced any bleeding events. Laboratory testing is not usually required in the early phase of follow-up, but assessment of renal function should be performed at least annually in patients treated with NOACs, using CrCl (Cockcroft–Gault method) rather than glomerular filtration rate. In patients with declining renal function or those receiving dabigatran (which is primarily eliminated renally), more frequent monitoring of CrCl is recommended. If potential drug accumulation or poor absorption is suspected, or if patients have impaired renal function and extreme body weight or require urgent surgery, there are assays available to measure NOAC plasma concentrations.31,32 A full blood count may be useful in patients with suspected bleeding.
Elderly patients often present with renal impairment, which is associated with increased risks of both thromboembolic and bleeding events. The NOACs are eliminated renally to differing extents (∼30%–80%; Table 1), and patients with severe renal impairment were excluded from the Phase III studies. A prespecified subgroup analysis of the EINSTEIN DVT and EINSTEIN PE studies showed that patients with symptomatic VTE and renal impairment are at increased risk of recurrent VTE, and that the rate of major bleeding increased in patients with renal impairment in the enoxaparin/VKA group but not in the rivaroxaban group.33 The Summary of Product Characteristics for rivaroxaban and apixaban states that these agents are not recommended for patients with CrCl <15 mL/min, and that caution should be taken when prescribing to patients with CrCl 15–29 mL/min.10,11 Dabigatran is contraindicated in patients with severe renal impairment (CrCl <30 mL/min).8 VKAs are currently the oral anticoagulants of choice in patients with the most severe renal dysfunction (CrCl <15 mL/min).34
How to minimize bleeding risks and manage bleeding events?
In all cases, the balance between the risk of bleeding and risk of recurrent VTE should be the primary consideration when prescribing anticoagulant therapy. There are many factors that are associated with increased bleeding risks, such as comorbidities and previous bleeding events (Table 3). Routine concomitant use of nonsteroidal anti-inflammatory drugs (including over-the-counter agents) and antiplatelet drugs (such as ASA or clopidogrel), which can lead to an increased risk of bleeding,35 should be avoided or used with caution. Although not commonly used in daily practice, coadministration of strong inhibitors of both cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp) with rivaroxaban or apixaban is not recommended, because these agents (mainly azole antimycotics and HIV protease inhibitors) could lead to increased drug exposure and bleeding risk (Table 1).10,11 In addition, coadministration of rivaroxaban or apixaban with rifampicin (a strong CYP3A4/P-gp inducer) may lead to an ∼50% decrease in rivaroxaban or apixaban exposure; therefore, coadministration of strong CYP3A4 inducers with rivaroxaban should be avoided unless the patient is closely observed for signs and symptoms of thrombosis.10 The product label for apixaban states that, for the treatment of DVT and PE in patients receiving concomitant systemic treatment with strong inducers of both CYP3A4 and P-gp, apixaban should not be used because the efficacy may be compromised.11 Rivaroxaban has no clinically relevant drug interactions with digoxin, atorvastatin, or omeprazole.10
Dabigatran is a substrate of P-gp; therefore, concomitant administration with strong P-gp inhibitors (ketoconazole, cyclosporin, itraconazole, and dronedarone) can lead to clinically relevant increases in dabigatran concentrations; thus, these agents are contraindicated.8 Edoxaban clearance is also affected by concomitant use of strong P-gp inhibitors (Table 1).9
If minor bleeding occurs in a patient taking an NOAC, it is often sufficient to delay or omit the next dose and to apply local hemostatic measures. For moderate–severe bleeding episodes, the NOAC should be temporarily stopped, and the patient should be directed to the hospital emergency department, where bleeding should be managed using established hemorrhage protocols, including mechanical compression, surgical hemostasis with bleeding control procedures, fluid replacement and hemodynamic support, and blood products or platelets. If bleeding cannot be controlled by these measures (or for life-threatening hemorrhage), administration of a specific procoagulant reversal agent can be considered.8–11
Long-term anticoagulation: when to review and when to stop
Current guidelines recommend 3 months of anticoagulation for provoked VTE and a minimum of 3-month anticoagulation for patients with unprovoked (or idiopathic) VTE.12,13,27 Previously anticoagulated patients remain at risk of recurrent VTE, and studies have shown that the benefits of extended anticoagulation treatment were not maintained after anticoagulation discontinuation, suggesting that indefinite treatment may be required for patients at a very high risk of recurrent VTE, such as those with more than one episode of unprovoked thrombosis, or those with active cancer.36,37
In general, the duration of therapy should be individualized following careful assessment of the treatment benefit (reduction in recurrent VTE) vs the risk of bleeding. A short duration of therapy (ie, 3 months) should be given if there are transient risk factors (eg, recent surgery, trauma, or immobilization), and longer durations (>3 months to indefinite) in the ase of permanent risk factors or unprovoked proximal DVT or unprovoked PE.8,10,11 Treatment should be reviewed at 3 months, and the decision on whether to stop or continue anticoagulant therapy should be based on a formal risk assessment and patient preference, preferably involving a practitioner with a sound knowledge of the evidence base of VTE management and anticoagulation therapy.
In patients with VTE and active cancer, the current guidelines recommend LMWH for the initial 3–6 months.13,27 It should be noted that a sub-analysis of the pooled EINSTEIN DVT and EINSTEIN PE data showed that, in patients with active cancer and VTE, rivaroxaban had similar efficacy and reduced the number of major bleeding events compared with enoxaparin/VKA.38 NOACs are contraindicated or not recommended during pregnancy, whereas LMWH can be used in pregnant women.8,10,11,39 As mentioned earlier, all of the NOACs should strictly be avoided during breastfeeding.8,11
Peri-procedural management
According to the relevant Summary of Product Characteristics, if a patient requires an invasive procedure or surgical intervention, edoxaban and rivaroxaban should be stopped at least 24 hours before; apixaban should be stopped at least 24 hours before a low-bleeding-risk procedure and at least 48 hours before a moderate- or high-bleeding-risk procedure.9–11 The European Heart Rhythm Association guidelines on the practical use of NOACs in patients with non-valvular atrial fibrillation also recommend that edoxaban and rivaroxaban are stopped at least 48 hours prior to a high-bleeding-risk procedure.40 For dabigatran, discontinuation timings are dependent on the renal function of the patient. In a patient with CrCl ≥80 mL/min, dabigatran should be stopped 48 hours (high risk of bleeding or major surgery) or 24 hours (standard risk) before the procedure. In patients with CrCl ≥50 mL/min to <80 mL/min, these timings are 2–3 days and 1–2 days, respectively, and in patients with CrCl ≥30 mL/min to <50 mL/min, they are 4 days and 2–3 days, respectively.8 For all of the NOACs, the increased risk of bleeding should be weighed against the urgency of the intervention. Anticoagulation with the NOACs should only be restarted once adequate hemostasis has been established.8–11
Patient education
NOACs offer convenient, fixed-dose regimens without the need for routine coagulation monitoring; however, strict adherence to the correct dosing regimen is critical for optimal outcomes given the relatively short half-lives of these drugs. Patients should be reminded about the increased risk of recurrent DVT/PE when stopping anticoagulant treatment. There is evidence suggesting that in patients with atrial fibrillation who require long-term anticoagulant therapy, patients’ fear of stroke outweighs their fear of bleeding.41 Similar evidence for patients with VTE is lacking, but patient education should further emphasize that noncompliance may lead to adverse outcomes. Missing a dose of their prescribed NOAC means that a patient is without adequate anticoagulation until the next dose (or until the missing dose is replaced). Patients who discontinue anticoagulant treatment should receive advice from their physician to increase their awareness of signs and symptoms of recurrent VTE. Patient counseling should include advice on contraception, invasive procedures, and long-distance travel, and when to seek advice on thromboprophylaxis at times of future increased risk.
Patients need to alert other health care professionals about their use of anticoagulants by carrying an anticoagulation alert card, eg, when undergoing dental procedures, seeing other doctors about other illnesses and visiting pharmacists for over-the-counter drugs, and especially if admitted to hospital via the emergency department. They should also be informed when to seek medical attention in case of adverse events or concerns regarding falls, minor trauma, or bleeding complications while receiving NOACs, particularly after head injury (even if it is minor). Clear guidance should be provided to patients on when or whether to stop their NOAC treatment before any elective surgery.8–11 In general, no bridging with a preoperative parenteral anticoagulant is needed, and in patients with normal renal function, stopping NOAC treatment 24 hours before minor surgical procedures and 48 hours before major procedures is sufficient.
Conclusion
With the introduction of NOACs into routine clinical practice, physicians and patients are no longer limited to VKAs for the treatment of VTE. All NOACs have proven to be at least as effective as the conventional standard therapy (eg, LMWH/VKA) in Phase III clinical trials, with favorable safety profiles. These newer agents offer more convenient treatment options without the need for routine anticoagulation monitoring. In addition, rivaroxaban and apixaban are given without the use of initial parenteral anticoagulation, which simplifies patient management. Primary care physicians play an important role in the treatment of patients with VTE. Continued risk assessment, regular follow-up, good adherence, and patient education are all essential for optimal clinical outcomes.
Acknowledgments
The author would like to acknowledge Yong-Ling Liu, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Footnotes
Disclosure
The author has received honoraria as a guest speaker at educational programs and advisory boards for Bayer. The author reports no other conflicts of interest in this work.
References
- 1.Cohen AT, Agnelli G, Anderson FA, et al. VTE Impact Assessment Group in Europe (VITAE) Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Ageno W, Gallus AS, Wittkowsky A, et al. American College of Chest Physicians Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutten BA, Prins MH. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2000;(3):CD001367. doi: 10.1002/14651858.CD001367. [DOI] [PubMed] [Google Scholar]
- 4.Caprini JA, Hyers TM. Compliance with antithrombotic guidelines: current practice, barriers, and strategies for improvement. Manag Care. 2006;15(9):49–66. [PubMed] [Google Scholar]
- 5.Merli G. Improving venous thromboembolism performance: a comprehensive guide for physicians and hospitalists. Hosp Pract (1995) 2010;38(3):7–16. doi: 10.3810/hp.2010.06.310. [DOI] [PubMed] [Google Scholar]
- 6.Vats V, Nutescu EA, Theobald JC, Wojtynek JE, Schumock GT. Survey of hospitals for guidelines, policies, and protocols for anticoagulants. Am J Health Syst Pharm. 2007;64(11):1203–1208. doi: 10.2146/ajhp060264. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82(4):423–428. [PubMed] [Google Scholar]
- 8.Pradaxa® (dabigatran etexilate) summary of product characteristics. Ingelheim am Rhein: Boehringer Ingelheim International GmbH; 2015. [Google Scholar]
- 9.Lixiana® (edoxaban) summary of product characteristics. Munich: Daiichi Sankyo Europe GmbH; 2015. [Google Scholar]
- 10.Xarelto® (rivaroxaban) summary of product characteristics. Berlin: Bayer Pharma AG; 2015. [Google Scholar]
- 11.Eliquis® (apixaban) summary of product characteristics. Uxbridge: Bristol-Myers Squibb, Pfizer; 2015. [Google Scholar]
- 12.National Institute for Health and Care Excellence [webpage on the Internet] Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. Clinical guidelines, CG144. 2012. [Accessed September 25, 2015]. Available from: http://guidance.nice.org.uk/CG144.
- 13.Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikelboom JW, Weitz JI. New anticoagulants. Circulation. 2010;121(13):1523–1532. doi: 10.1161/CIRCULATIONAHA.109.853119. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. doi: 10.1146/annurev-med-062209-095159. [DOI] [PubMed] [Google Scholar]
- 16.The EINSTEIN Investigators. Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 17.The EINSTEIN–PE Investigators. Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 18.Agnelli G, Buller HR, Cohen A, et al. AMPLIFY Investigators Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 19.The Hokusai-VTE Investigators. Büller HR, Décousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 20.Schulman S, Kearon C, Kakkar AK, et al. RE-COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Kakkar AK, Goldhaber SZ, et al. RE-COVER II Trial Investigators Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 22.Prins MH, Lensing AWA, Bauersachs R, et al. EINSTEIN Investigators Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulman S, Kearon C, Kakkar AK, et al. RE-MEDY Trial Investigators. RE-SONATE Trial Investigators Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 24.Becattini C, Agnelli G, Schenone A, et al. WARFASA Investigators Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 25.Brighton TA, Eikelboom JW, Mann K, et al. ASPIRE Investigators Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 26.Mant J, Hobbs FDR, Fletcher K, et al. BAFTA investigators. Midland Research Practices Network (MidReC) Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 28.Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28(5):669–680. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- 29.Bamber L, Wang MY, Prins MH, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thromb Haemost. 2013;110(4):732–741. doi: 10.1160/TH13-03-0243. [DOI] [PubMed] [Google Scholar]
- 30.Prins MH, Bamber L, Cano SJ, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135(2):281–288. doi: 10.1016/j.thromres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Baglin T, Keeling D, Kitchen S, British Committee for Standards in Haematology Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol. 2012;159(4):427–429. doi: 10.1111/bjh.12052. [DOI] [PubMed] [Google Scholar]
- 32.Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15(5):625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 33.Bauersachs RM, Lensing AWA, Prins MH, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J. 2014;12:25. doi: 10.1186/1477-9560-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125(21):2649–2661. doi: 10.1161/CIRCULATIONAHA.111.084996. [DOI] [PubMed] [Google Scholar]
- 35.Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti- inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947–953. doi: 10.1001/jamainternmed.2014.946. [DOI] [PubMed] [Google Scholar]
- 36.Bounameaux H, Perrier A. Duration of anticoagulation therapy for venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2008;1:252–258. doi: 10.1182/asheducation-2008.1.252. [DOI] [PubMed] [Google Scholar]
- 37.Eikelboom JW, Ginsberg JS, Hirsh J. Anticoagulation for venous thromboembolism. BMJ. 2007;334(7595):645. doi: 10.1136/bmj.39161.665579.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prins MH, Lensing AWA, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37–e46. doi: 10.1016/S2352-3026(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 39.Bates SM, Greer IA, Middeldorp S, et al. American College of Chest Physicians VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e691S–e736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 41.Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. Br Med J. 2001;323(7323):1218–1222. doi: 10.1136/bmj.323.7323.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torbicki A, Perrier A, Konstantinides S, et al. ESC Committee for Practice Guidelines (CPG) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29(18):2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 43.Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. Br Med J. 2011;342:d3036. doi: 10.1136/bmj.d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 45.Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) J Thromb Haemost. 2012;10(6):1019–1025. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 46.Nieto JA, Solano R, Ruiz-Ribó MD, et al. Riete Investigators Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2010;8(6):1216–1222. doi: 10.1111/j.1538-7836.2010.03852.x. [DOI] [PubMed] [Google Scholar]
- 47.Nijkeuter M, Söhne M, Tick LW, et al. Christopher Study Investigators The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007;131(2):517–523. doi: 10.1378/chest.05-2799. [DOI] [PubMed] [Google Scholar]