Abstract
Oncolytic viral (OV) therapy has been considered as a promising treatment modality for brain tumors. Vasculostatin, the fragment of brain-specific angiogenesis inhibitor-1, shows anti-angiogenic activity against malignant gliomas. Previously, a vasculostatin-expressing oncolytic HSV-1, Rapid Antiangiogenesis Mediated By Oncolytic virus (RAMBO), was reported to have a potent antitumor effect. Here, we investigated the therapeutic efficacy of RAMBO and cilengitide, an integrin inhibitor, combination therapy for malignant glioma. In vitro, tube formation was significantly decreased in RAMBO and cilengitide combination treatment compared to RAMBO or cilengitide monotherapy. Moreover, combination treatment induced a synergistic suppressive effect on endothelial cell migration compared to the control virus. RAMBO, combined with cilengitide, induced synergistic cytotoxicity on glioma cells. In the caspase-8 and -9 assays, the relative absorption of U87ΔEGFR cell clusters treated with cilengitide and with RAMBO was significantly higher than of those treated with control. In addition, the activity of caspase 3/7 was significantly increased with combination therapy. In vivo, there was a significant increase in the survival of mice treated with combination therapy compared to RAMBO or cilengitide monotherapy. These results indicate that cilengitide enhanced vasculostatin-expressing OV therapy for malignant glioma and provide a rationale for designing future clinical trials combining these two agents.
Keywords: oncolytic viral therapy, cilengitide, glioma
Introduction
Glioblastoma multiforme (GBM) is the most frequent primary malignant brain tumor in adults. Pathologically, GBM is characterized by features of malignancy including marked neovascularity, rapid cell proliferation, infiltrative cell migration, and extensive necrosis. The median survival of patients remains at 14–16 months and the 5-year survival rate is less than 3%1, even if the most aggressive treatment is attempted. Given the poor prognosis of this disease, there is a desperate need for novel methods of intervention including oncolytic therapy and molecular targeted therapy2.
Oncolytic viral (OV) therapy is an emerging biological treatment modality that exploits the tumor-specific properties of some viruses3. Oncolytic herpes simplex viruses (HSVs) are genetically engineered to replicate selectively in and lyse tumor cells, but cause minimal damage to normal tissue. Vasculostatin (Vstat120) is the extracellular fragment of brain-specific angiogenesis inhibitor 1, and has been shown to be a potent antiangiogenic and antitumorigenic factor4. We have created an armed oncolytic HSV-1 that expresses Vstat120 under the control of an immediate early IE4/5 HSV promoter5. On the basis of its potent and rapid induction of antiangiogenic Vstat120, we named this virus Rapid Antiangiogenesis Mediated By Oncolytic virus (RAMBO). Treatment of mice bearing intracranial and subcutaneous gliomas revealed a significant increase in the antitumor efficacy of RAMBO compared to the control virus.
Integrins control the attachment of cells to the extracellular matrix (ECM) and participate in cellular defense against genotoxic assaults6. Integrins are expressed in tumor cells and tumor endothelial cells7–9, and they play important roles in angiogenesis and invasion in glioma10–12. The integrins αvβ3 and αvβ5 regulate cell adhesion13, 14, and inhibitors of these integrins suppress tumor growth in certain pre-clinical models15. Therefore, integrins have attracted attention as potential therapeutic targets in glioma. Several integrin-targeted drugs are in clinical trials as potential compounds for the treatment of cancer. Among them, cilengitide (EMD121974) is a novel integrin antagonist for the treatment of glioblastoma16. Cilengitide is currently being assessed in phase III trials for glioblastoma patients and phase II trials for other types of cancers, with promising therapeutic outcomes reported to date. Several preclinical studies have shown an enhanced antitumor effect of cilengitide when administered in combinatorial therapeutic regimens17–20.
Previously, we presented an in vivo study of OV therapy – induced changes in tumor blood vessels and the impact of modulating tumor vasculature on the efficacy of OV therapy in a rat glioma model21. OV treatment of rat gliomas increased the permeability of the tumor vasculature, tumor inflammation, and leukocyte infiltration22, 23. Pretreatment of gliomas with cilengitide reduced inflammation, vascular hyperpermeability, and leukocyte infiltration of tumor tissue following treatment with an oncolytic virus and enhanced the anticancer efficacy of OV treatment by increasing viral propagation in tumors. In this study, we demonstrate that RAMBO and cilengitide combination treatment of glioma. An evaluation of the cytotoxic mechanism revealed the increased activation of multiple caspase molecules in cells treated with cilengitide and RAMBO and cilengitide combined. We also demonstrate that cilengitide auguments the therapeutic effect of the vasculostatin-expressing oncolytic virus for malignant glioma by systemic injection.
Materials & Methods
Cell lines, viruses, and reagents
U87ΔEGFR, Gli36, U251, human glioma cells, and Vero cells were prepared and maintained as described previously24. Glioma cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2.
HSVQ is an HSV-1-derived recombinant oncolytic virus with a disrupted UL39 locus and deletions in both copies of the γ34.5 gene25. The HSV-1-derived oncolytic virus named RAMBO was used to incorporate the cDNA encoding for human vasculostatin, driven by the HSV-1 IE4/5 promoter.
Cilengitide was generously provided by Merck KgaA (Darmstadt, Germany) and the Cancer Therapy Evaluation Program, National Cancer Institute, National Institutes of Health.
Immunocytological analysis
U251, Gli36, and U87ΔEGFR glioma cells were seeded onto 4-Chamber Polystyrene Vessel Tissue Culture Treated Glass Slides (BD Falcon, Franklin Lakes, NJ, USA) and incubated overnight. For immunofluorescence, the cells were fixed in 4% paraformaldehyde in PBS for 15 min. After the cells were fixed, they were rinsed three times with PBS. Nonspecific binding was blocked by incubation in a blocking buffer containing 2% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The cells were incubated overnight at 4°C with a mouse monoclonal anti-human integrin αvβ3 antibody (CHEMICON MAV1976) or mouse monoclonal anti-human integrin αvβ5 antibody (Abcam, Cambridge, MA, USA), which were diluted 1:100 and 1:500 in blocking buffer, respectively.
The cells were washed in blocking buffer 3 times for 5 min before incubation with a secondary anti-mouse CY3-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) diluted 1:300 in blocking buffer for 2 h at room temperature in the dark. After 3 washes in PBS, the cells were counterstained with 4′, 6-diamino-2-phenylindole (1:500) (Invitrogen, Carlsbad, CA, USA) (100 ng/mL) for 20 min at room temperature. The slides were washed three times in PBS and mounted.
Tube formation assay
An Angiogenesis Assay Kit (Kurabo, Osaka, Japan) was used according to the manufacturer’s instructions. Briefly, HUVECs, co-cultured with neonatal normal human dermal fibroblasts26, were treated with cilengitide (0.1 μM), RAMBO (5.0 × 102 pfu), and VEGF (10 ng/mL). Suramin (50 μM) was used as a positive anti-angiogenic control. The medium was changed every 3 days. After 10 days, the dishes were washed with PBS and fixed with 70% ethanol at 4°C. After the fixed cells were rinsed 3 times with PBS, the cells were incubated with mouse anti-human CD31 in PBS containing 1% BSA for 60 min. After washing 3 times with 1% BSA-PBS, the cells were incubated with a goat anti-mouse IgG AlkP conjugate. Metal-enhanced 3, 3′-diamino-benzidine-tetrahydrochloride was used as the substrate, the reaction yielding a dark reddish-brown insoluble end-product. Finally, the cells were washed 5 times with PBS and viewed under a microscope (BZ-8000; Keyence, Osaka, Japan). Tube formation was counted under high magnification (×20), and the average tube area in 3 hot spots was taken as the tube formation per view field. Tube formation was counted in at least 3 dishes.
Endothelial cell migration assay (scratch wound assay)
U251 glioma cells were infected with RAMBO or HSVQ (MOI: 2), and after 14 h, the CM was harvested, cellular debris was removed by centrifugation, and free floating viral particles were removed by further centrifugation at approximately 28,000 × g for 1 h. For the endothelial cell migration assays, early passage HMVEC-d (passage 3) were purchased from Lonza (Basel, Switzerland). The cells were used between passages 4 and 6, and they were maintained in endothelial growth media. HMVEC-d were plated onto a 24-well dish and allowed to grow to confluence. A thin line was made with a sterile 1000 μL pipette tip in the middle of the well and they were incubated with CM derived from U251 glioma cells (prepared in 2% FBS DMEM as described previously) and the indicated concentration of cilengitide was added. HMVEC-d were incubated for 12 h at 37°C to allow cellular migration into the denuded area, and they were assessed by phase contrast microscopy.
Virus yield assay
To assess virus yield, U87ΔEGFR glioma cells were seeded on 6-well plates at 3.0 × 105 cells per well. The cells were infected with RAMBO at an MOI of 0.1, and after 3 h of infection, we added either PBS or cilengitide (10 μM). At 36 h after infection, the glioma cells were harvested by scraping, and lysed by freezing and thawing three times. Cellular debris was removed by centrifugation, and the number of infectious viral particles (pfu) in the cells was assessed using a standard viral titration assay.
Cytotoxicity assays
The cytotoxicity of U251 Gli36, and U87ΔEGFR glioma cells was analyzed by the WST-1 quantitative colorimetric assay for cell survival according to the manufacturer’s instructions (Roche Molecular Biochemicals, Mannheim, Germany). This assay detects living, but not dead, cells, and the generated signal depends on the degree of cell activation. We seeded 1.0 × 104 cells/well in 96-well plates and they were left to attach at 37°C. Subsequently, the cells were treated with saline, cilengitide, RAMBO, or RAMBO and cilengitide combined. The cell lines were treated with the indicated concentration of cilengitide or the indicated MOI. Cell survival was measured at the indicated time points by adding the reaction solution (10 μL) to 100 μL of culture medium per well. The samples were then incubated for an additional 60–150 min at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The absorption of the samples was measured with a Thermo Scientific Multiskan® FC Microplate Photometer (Thermo Fisher Scientific, Inc., Mississauga, Ontario, Canada) at 450 nm wavelength using a 620 nm reference filter. The amount of formazan dye formed directly correlates to the number of metabolically active, viable cells. After subtraction of the background absorption, the mean value of the untreated control cells was set as 100%.
Caspase assays
Activity assay for caspase-8 and -9
Caspase-8 and -9 levels were measured using a FLICE/Caspase-8 Colorimetric Assay Kit and Caspase-9 Colorimetric Assay Kit (BioVision, Mountain View, CA, USA), respectively, according to the manufacturer’s instructions. Briefly, U87ΔEGFR glioma cells were seeded in five 10-cm dishes (1.0 × 106 cells per dish), and after confirmation of their attachment, they were treated with culture medium containing cilengitide (10 μM), RAMBO (MOI 0.1), and with a combination of RAMBO (same as above) and cilengitide in triplicate. At 16 h after adding the reagents and oncolytic virus, the cells were harvested and lysed using a cell lysis buffer. We centrifuged the cell lysate and harvested the supernatant, and the caspase solutions were then added. After incubation with these substrates for 1–2 h, the absorption of each group was measured by using a microplate reader.
Activity assay for caspase-3/7
Caspase-3/7 activity levels were measured using the CellEvent™ Caspase-3/7 Green Detection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Briefly, U87ΔEGFR glioma cells were seeded in 96-well plates (10,000 cells per well), and after confirmation of their attachment, they were treated with culture medium containing cilengitide (10 μM), RAMBO (MOI 0.1), and with a combination of RAMBO (same as above) and cilengitide in triplicate. The caspase solution was added at 16 h after adding the reagents and oncolytic virus. Before and after incubation with these substrates, the fluorescence intensity of each well was measured using a microplate reader. We determined the caspase activity by measuring the fluorescence intensity and subtracted the background fluorescence of each well.
Animal surgery and treatment
All animal experiments were performed according to the guidelines of the Subcommittee on Research Animal Care of the Okayama University and were approved by the Institutional Review Board. Athymic nude mice (6–8-week-old balb/c-nu/nu; CLEA Japan, Inc., Tokyo, Japan) were used for all studies. For intracranial tumor studies, the mice were anesthetized with ketamine and somnopentyl and fixed in a stereotactic apparatus (Narishige, Tokyo, Japan) with their skull exposed. A burr hole was made at 1 mm posterior and 3 mm lateral from the bregma, and a Hamilton syringe (Hamilton, Reno, NV, USA) was then inserted to a depth of 3 mm below the brain surface. Then, 2 μL of 2.0 × 105 U87ΔEGFR glioma cells were slowly injected into the brain at a rate of 1 μL per min. The syringe was kept in place for 5 min after the injection and then slowly retracted to minimize backflow. We cleaned the skull, sealed the hole with bone wax, and sutured the incision. Seven days after the implantation of the U87ΔEGFR cells (105 cells), anesthetized mice were stereotaxically inoculated with 5.0 × 104 pfu of RAMBO at the same location. Nine days after the implantation of the U87ΔEGFR cells, the mice were treated with either cilengitide (200 μg/100 μL PBS) or PBS intraperitoneally 3 times a week. The animals were observed daily and were sacrificed when they showed signs of morbidity.
Statistical analysis
The Mann-Whitney U test was used to analyze changes in cell death, HMVEC-d migration assay, and caspase assay data. Data are presented as the means ± standard error. P values ≤ less than 0.05 were considered statistically significant in the Mann-Whitney U test. Kaplan-Meier curves were compared using the log-rank test. All statistical analysis was performed using SPSS statistical software (version 20; SPSS, Inc., Chicago, IL, USA).
Results
Antiangiogenic effect of combination treatment in the tube formation assay
To investigate the antiangiogenic effect of RAMBO and cilengitide, the tube formation assay was performed using human umbilical vein endothelial cells (HUVECs) co-cultured with fibroblasts. When HUVECs were cultured in the presence of vascular endothelial growth factor (VEGF), efficient tube formation was observed. Conversely, an anti-VEGF drug (suramin: 50 μM) efficiently inhibited tube formation. Both RAMBO and cilengitide inhibited tube formation in HUVECs. Moreover, RAMBO and cilengitide combination treatment significantly inhibited tube formation compared to RAMBO or cilengitide monotherapy. Quantitative analysis of tube area confirmed that RAMBO and cilengitide efficiently inhibited in vitro angiogenesis, and combination treatment reduced angiogenesis more intensively than monotherapy (RAMBO and cilengitide combination: 8.4 × 103 ± 2.1 × 102 pixels; vs. RAMBO; 1.3 × 103 ± 1.3 × 102 pixels, P = 0.009: vs cilengitide: 1.4 × 103 ± 3.5 × 102 pixels, P = 0.028, respectively) (Figure 1).
Figure 1.
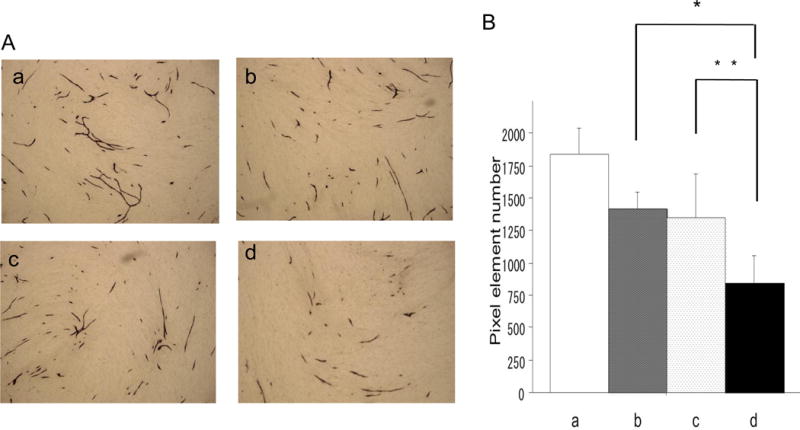
Inhibition of endothelial cell tube formation. HUVECs were treated with medium containing VEGF-A, RAMBO, and cilengitide, and then assayed by the KURABO Angiogenesis Kit. A: The representative images show tube formation of HUVECs. a: VEGF-A (10 ng/mL), b: VEGF-A + RAMBO (5×102 pfu), c: VEGF-A + cilengitide (0.1 μM), and d: VEGF-A+RAMBO (5.0 ×102 pfu) + cilengitide (0.1 μM).
B: Significant reduction in tube formation of HUVECs treated with RAMBO and cilengitide combined compared to RAMBO or cilengitide monotherapy (*: P = 0.0090 and **: P = 0.028, respectively).
Inhibition of endothelial cell migration with combination treatment
HMVEC-d were assayed for movement after 12 h of exposure to the conditioned medium (CM) of cells treated with HSVQ or RAMBO plus cilengitide. Wound closure was significantly reduced by treatment with RAMBO and cilengitide CM. The rate of wound closure was decreased by cilengitide in a dose-dependent manner. Especially, combining cilengitide with the CM of RAMBO-treated cells significantly decreased wound closure compared to combining cilengitide (1, 5, and 10 μM) with the CM of HSVQ-treated cells (P = 0.0102, P = 0.0019, and P = 0.0002, respectively). This assay indicated that treatment with RAMBO and cilengitide combined induced a synergistic suppressive effect on endothelial cell migration (Figure 2).
Figure 2.
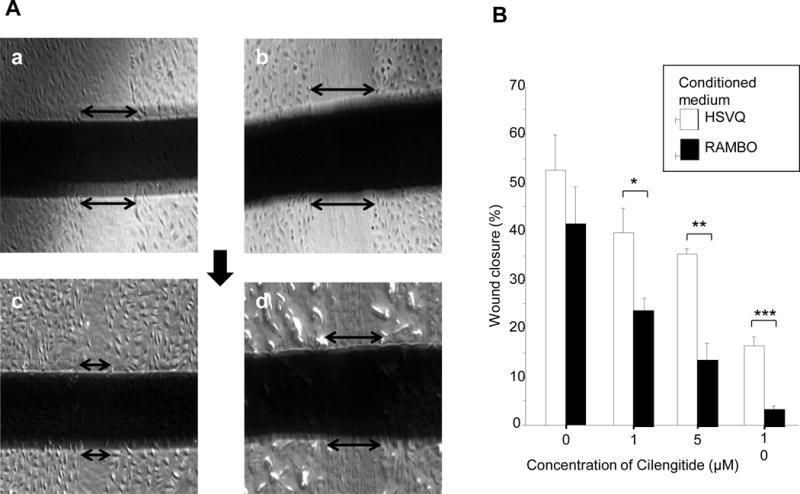
Inhibition of endothelial cell migration. HMVEC-d were incubated with CM derived from U251 glioma cells treated with HSVQ or RAMBO. Additionally, they were treated with the indicated concentration of cilengitide. A: Representative images from the scratch wound assay. HMVEC-d were assayed for movement after 12 h of exposure to CM with HSVQ or RAMBO plus cilengitide. B: Higher concentrations of cilengitide decreased the rate of wound closure. Especially, wound closure was significantly decreased in cells treated with CM from RAMBO-treated cells compared with CM from HSVQ-treated cells (*: P = 0.010, **: P = 0.0019, and ***: P = 0.00020, respectively).
Effect of cilengitide on oncolytic virus replication in vitro
The difference in the replication capability of RAMBO was evaluated between glioma cells treated with RAMBO monotherapy and those treated with RAMBO and cilengitide combined. The viral yield at 36 h after infection of U87ΔEGFR glioma cells was determined at a multiplicity of infection (MOI) of 0.1. Virus (RAMBO)-infected glioma cells treated with cilengitide had significantly higher titers of infectious virus compared with those treated with phosphate-buffered saline (PBS) (viral titer for RAMBO and cilengitide combined versus RAMBO monotherapy = 2.8 × 105 ± 1.6 × 105 plaque forming units (pfu) versus 6.5 × 104 ± 2.1 × 104 pfu, respectively, and the mean fold increase in GFP expression for RAMBO and cilengitide combined versus RAMBO monotherapy = 4.3, P = 0.0273) (Figure 3).
Figure 3.
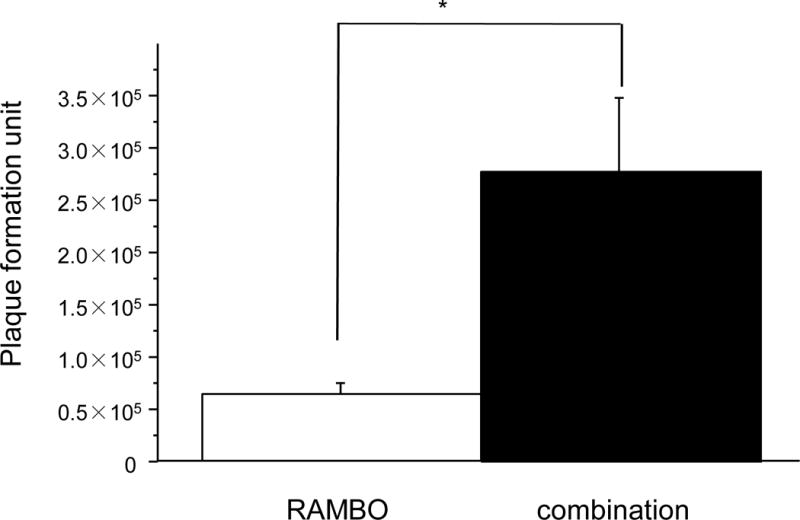
Enhancement of viral replication in glioma cells. U87ΔEGFR glioma cells were plated on a 6-well plate and infected with RAMBO, and then either PBS or cilengitide was added to them. At 36 h after infection, the number of infectious viral particles in the cells was assessed using a standard viral titration assay. There was a significant increase in the titer of cells treated with RAMBO and cilengitide combined compared to those treated with PBS (mean fold increase in the expression of GFP for the RAMBO and cilengitide combination treatment versus RAMBO monotherapy = 4.3, P = 0.0273).
Immunocytochemical analysis of αvβ3 and αvβ5 integrin expression in glioma cell lines
Immunofluorescence assays were conducted to determine the expression of αvβ3 and αvβ5 integrins in U251, Gli36, and U87ΔEGFR glioma cell lines. Most of these cultured cell lines were immunopositive for αvβ3 and αvβ5. The percentage of cells staining for αvβ3 and αvβ5 was as follows: U251: αvβ3 95.3 ± 1.3%, αvβ5 93.4 ± 6.7%; Gli36: αvβ3 98.3 ± 2.9%, αvβ5 97.0 ± 5.2%; U87dEGFR: αvβ3 95.1 ± 4.3% αvβ5 87.1 ± 6.0% (Supplementary figure 1).
Cytotoxic effect of combination treatment
The cytotoxic effect of RAMBO and cilengitide on glioma cells was investigated in vitro. Figure 4 shows the results of the water-soluble tetrazolium (WST)-1 assay, which demonstrate the cytotoxicity effect of RAMBO and cilengitide. Glioma cell lines were incubated with the indicated concentration of cilengitide or the indicated MOI. After 36 h, cell viability was assessed by the WST-1 proliferation/viability assay. Cell survival after a 36 h incubation was decreased more by treatment with a high MOI than with cilengitide. Moreover, the addition of cilengitide enhanced the reduction of cell viability. This result indicated that the combination of RAMBO with cilengitide induced additive or synergistic cytotoxicity.
Figure 4.
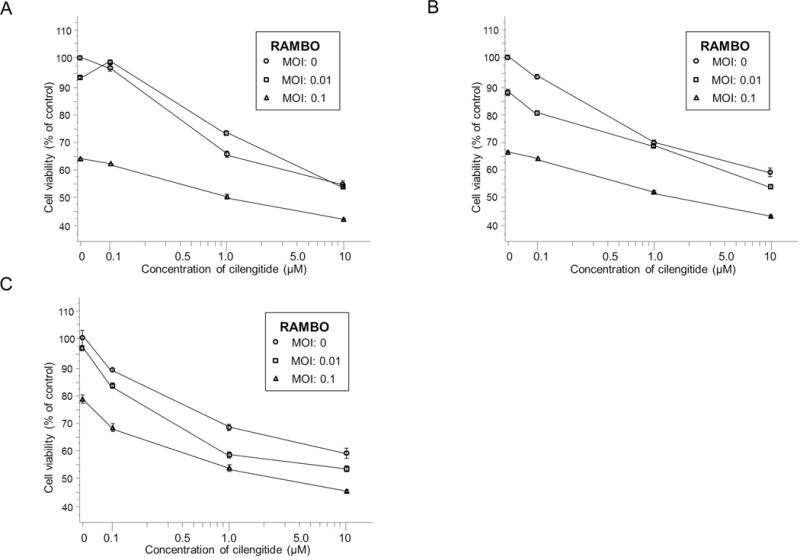
Cytotoxicity effect of RAMBO, cilengitide, and RAMBO and cilengitide combined on glioma cell lines. Glioma cells grown in 96-well plates were treated for 36 h with various MOIs of RAMBO and for 33 h with various concentrations of cilengitide. Cell viability was determined using the WST-1 assay. For the most part, the higher was the MOI, the greater the reduction in cell survival. Moreover, the addition of cilengitide enhanced the reduction of cell viability. U251 glioma cells (A): Gli36 glioma cells (B): U87ΔEGFR glioma cells(C).
Apoptotic effect of RAMBO and cilengitide combination treatment
U87ΔEGFR glioma cells were treated with cilengitide (10 μM), with RAMBO (MOI 0.1), and with RAMBO and cilengitide combined (same as above). At 16 h after infection with RAMBO and 13 h after adding cilengitide or PBS, glioma cells were harvested and the activity of caspase-8,-9, and -3/7 was evaluated. In the caspase-8 assay, the relative absorption (RA) of U87ΔEGFR cell clusters treated with cilengitide was significantly higher than of those treated with control (caspase-8: 0.33 ± 0.065 RA vs. 0.27 ± 0.054 RA, P = 0.043) (Figure 5A). In the caspase -9 assay, the (RA) of U87ΔEGFR cell clusters treated with RAMBO was significantly higher than of those treated with control (0.33 ± 0.027 vs. 0.029 ± 0.029, P = 0.043) (Figure 5B).
Figure 5.
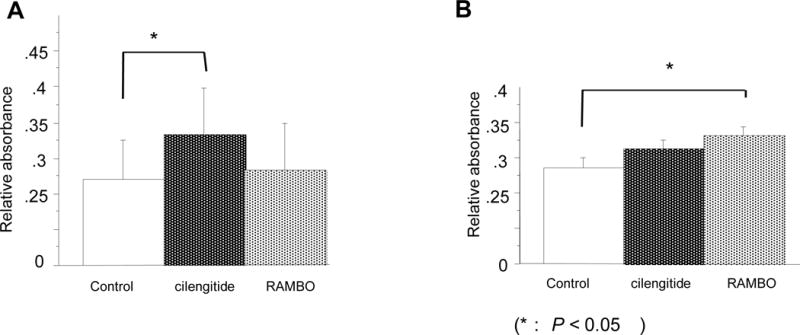
Induction of Caspase-8 and -9 by treatment with RAMBO, cilengitide, and RAMBO and cilengitide combined in vitro. A: Caspase-8 activity assay. U87ΔEGFR glioma cells were treated with cilengitide (10 µM), with RAMBO (MOI 0.1), and with RAMBO and cilengitide combined (same as above). At 16 h after infection with RAMBO and 13 h after adding cilengitide or PBS, caspase activity was evaluated in the cells using a FLICE/Caspase-8 Colorimetric Assay Kit. There was a significant increase in caspase-8 activity in glioma cells treated with cilengitide compared to those of control. The symbol (*) show a significant increase in caspase-8 activity. B: Assay of caspase-9 activity. U87ΔEGFR glioma cells were treated as mentioned, and evaluated using a Caspase-9 Colorimetric Assay Kit. There was a significant increase in caspase-9 activity in glioma cells treated with RAMBO compared to those of (A: *: P = 0.043, B: *: P = 0.043, respectively).
The activity of caspase-3/7 was also increased in treated cells; especially, a significant increase of caspase-3/7 activity was observed in cells treated with cilengitide and with RAMBO and cilengitide combined. Moreover, there was a significant increase in caspase-3/7 activity in cells treated with combination therapy compared to RAMBO monotherapy, cilengitide monotherapy, and untreated cells (P = 0.0008, P = 0.0117, and P = 0.0008, respectively) (Figure 6).
Figure 6.
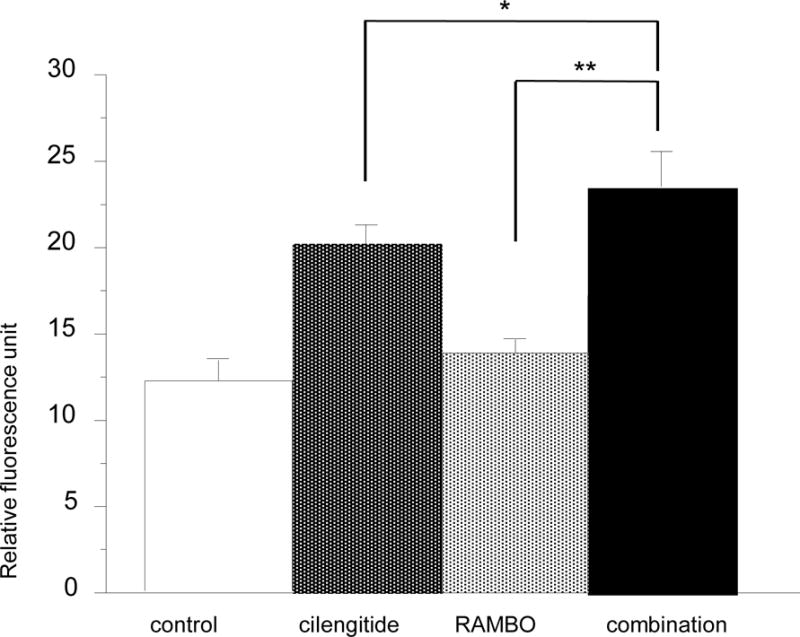
Induction of caspase-3/7 following treatment with RAMBO, cilengitide, and RAMBO and cilengitide combined in vitro. Assay of caspase-3/7 activity. U87ΔEGFR glioma cells were treated with cilengitide, RAMBO, and RAMBO and cilengitide combined (same as above). At 16 h after infection with RAMBO and 13 h after adding cilengitide or PBS, caspase activity was evaluated in the cells using the CellEvent™ Caspase-3/7 Green Detection Reagent. Caspase-3/7 activity was increased in the treated cells; especially, a significant increase of caspase-3/7 activity was observed in the cells treated with cilengitide and RAMBO and cilengitide combined. There was a significant increase in the activity of caspase-3/7 in the cells treated with the combination therapy compared to RAMBO monotherapy, cilengitide monotherapy, and untreated cells (P = 0.0008, P = 0.0117, and P = 0.0008, respectively).
Effect of cilengitide treatment on the therapeutic efficacy of RAMBO in a xenograft mouse model
The antitumor effect of RAMBO, cilengitide, and RAMBO and cilengitide combined was tested in mice bearing intracerebral glioma (U87ΔEGFR). Kaplan-Meier survival curves were used to assess the survival time of the U87ΔEGFR mouse glioma model treated with PBS, cilengitide, RAMBO, and RAMBO and cilengitide combined. There was a significant increase in the survival of mice treated with combination therapy compared to RAMBO monotherapy, cilengitide monotherapy, or untreated mice (median survival = 38.5, 29, 19, and 19 days, respectively) (P < 0.005, P < 0.001, and P < 0.001, respectively) (Figure 7).
Figure 7.
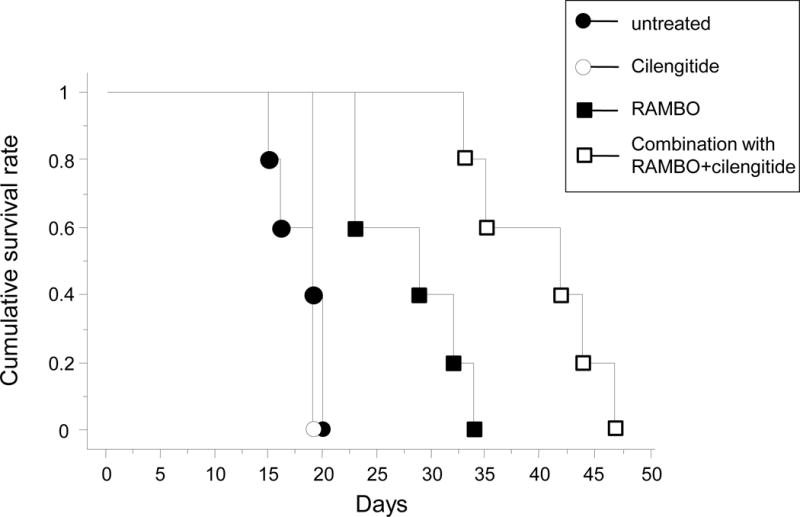
Kaplan–Meier survival curves of mice implanted with intracranial U87ΔEGFR glioma cells treated with PBS, cilengitide, RAMBO, or RAMBO and cilengitide combined. Athymic nude mice bearing intracranial U87ΔEGFR gliomas were treated with 0.5 × 105 pfu RAMBO at 7 days after tumor cell implantation. At 9 days after tumor cell implantation, cilengitide was administered 3 times per week intraperitoneally at 200 μg/100 μL PBS. The mice were then closely monitored for survival. There was a significant increase in the survival of mice treated with combination therapy compared to RAMBO or cilengitide monotherapy (median survival = 38.5, 29, and 19 days, respectively) (P < 0.005 and P < 0.001, respectively).
Discussion
Our results indicate that the combination of RAMBO and cilengitide significantly inhibited tube formation compared to RAMBO or cilengitide monotherapy. Moreover, RAMBO and cilengitide combination treatment induced a synergistic suppressive effect on endothelial cell migration. The addition of cilengitide enhanced the reduction of cell viability and a significant increase of caspase-8, -9, and -3/7 activity was observed in cells treated with cilengitide and with RAMBO and cilengitide combined. Furthermore, the survival time of mice bearing intracerebral glioma treated with combination therapy was significantly increased compared to monotherapy.
1) The advantage of the RAMBO virus on OV therapy
We have shown that the reduction of tumoral blood vessel density by a single dose of cilengitide treatment prior to oncolytic HSV-1 treatment reduced oncolytic virus-induced hyperpermeability and tumor inflammation, and prolonged tumoral viral propagation, thereby enhancing the antitumor efficacy of the oncolytic virus21. In addition, we showed that RAMBO had a significantly greater antitumor effect compared to OV treatment alone5. We suggested that the increased antitumor efficacy of RAMBO might be attributed to its ability to counter oncolytic virus-induced angiogenic changes in the tumor microenvironment. Certainly, in our experiments, we could have the antiangiogenic effect and apoptotic effect following treatment with RAMBO as single treatment.
2) The advantage of combination therapy with cilengitide
Several preclinical studies have shown an enhanced antitumor effect of cilengitide when administered in combinatorial therapeutic regimens17–20. Mikkelsen et al. demonstrated that cilengitide dramatically amplified the efficacy of radiation therapy in an animal glioma model27. We demonstrated the enhanced therapeutic efficacy of an oncolytic virus on experimental glioma following pretreatment with cilengitide21. OV treatment of experimental rat gliomas results in vascular hyperpermeability of the tumor. In this study, we reported the effects of a novel oncolytic HSV virus and the systemic injection of cilengitide. In addition, we also revealed that there was synergistic effect between cilengitide and viral injection. Especially, the combination of RAMBO with cilengitide induced a synergistic suppressive effect on endothelial cell migration compared to combining cilengitide with the CM of HSVQ-treated cells. Future studies will elucidate the relationship between Vstat120 expression and cilengitide.
3) A role for apoptosis in combination treatment with oncolytic virus and cilengitide
Previously, the cytotoxic effect of cilengitide was clearly shown. Recently, similar changes in HUVECs have been reported for S 36578-2, a novel RGD mimetic that selectively activates the αvβ3 and αvβ5 integrins28. This compound induces cell detachment and apoptosis by the direct activation of caspase-829. In our study, cilengitide inhibited integrin binding and activated caspase-8. This caspase-8 activation effect of cilengitide would enhance the effect of other cytotoxic therapies. On the other hand, a previous study showed the induction of increased mitochondrial membrane permeability, a surrogate marker for apoptosis, in an oncolytic virus derived from HSV-130. Prominent and early caspase-9 processing is compatible with the mitochondrial triggering of the caspase cascade, as reported previously [37]. In our data, especially, a significant increase of caspase -3/7 activity was observed in cells treated with both RAMBO and cilengitide. This combination between the mitochondria-dependent pathway with caspase-9 activation and the death-receptor pathway, including caspase-8 activation, might be more efficient for inducing apoptosis than a single pathway (Figure 8).
Figure 8.
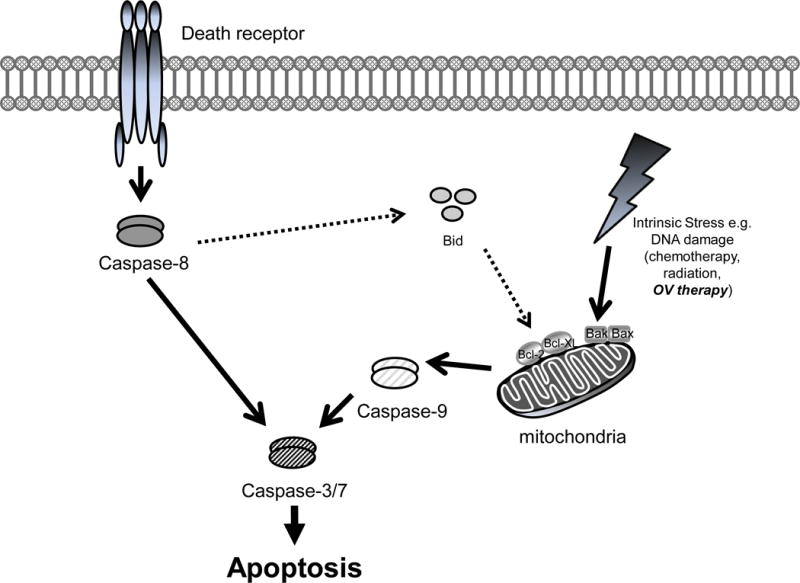
Apoptotic pathways whereby RAMBO and cilengitide combination exerts this cytotoxic effect: the mitochondrial pathway and the death receptor pathway
4) Future directions
Cilengitide monotherapy or combination treatment with radiation and/or temozolomide is well tolerated and exhibits modest antitumor activity20, 31, 32. The CENTRIC controlled phase III study was launched in 2008, with primary outcome measures due in September 2012. The results of this and other clinical studies are expected with great hope and interest for combination therapy with an oncolytic virus. In addition, cysteine-rich 61 (CYR61) is known to interact with integrins, such as αvβ3, α6β1, αvβ5, and αIIβ3, which leads to a wide range of biological activity, including cell adhesion, migration, and invasion33. Previously, we showed that the induction of CYR61 after oncolysis results in the production of a proangiogenic glioma ECM that plays a key role in the neovascularization of a residual tumor. Moreover, exogenous CYR61 in glioma ECM is reported to orchestrate a cellular antiviral response that reduces viral replication and limits the efficacy of the oncolytic virus34. Future studies will investigate the mechanism of the CYR61-induced antitumor effect of RAMBO and cilengitide combination therapy. Recently, Yoo et al. described the construction and testing of a novel HSV-1-derived oncolytic virus, 34.5ENVE (viral ICP34.5 Expressed by Nestin promoter and Vstat120 Expressing), for the treatment of cancer35. We also intend to use this new virus in combination with cilengitide.
In conclusion, our results indicated that cilengitide enhaced vasculostatin-expressing OV therapy for malignant glioma and unveiled a rationale for designing future clinical trials combining these two agents.
Supplementary Material
Supplementary figure 1: Expression of αvβ3 and αvβ5 integrins on the surface of HMVEC-d. The expression of αvβ3 and αvβ5 was recognized on the U251 (A), Gli36 (B), and U87ΔEGFR (C) cells.
Acknowledgments
We thank H. Wakimoto, M. Arao, and A. Ishikawa for their technical assistance. This study was supported by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology to K.K. (No. 20890133; No. 21791364), and T.I. (No. 19591675; No. 22591611).
Footnotes
Conflict of interest
We declare that we have no conflict of interest.
References
- 1.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 2.Haseley A, Alvarez-Breckenridge C, Chaudhury AR, Kaur B. Advances in oncolytic virus therapy for glioma. Recent Pat CNS Drug Discov. 2009;4(1):1–13. doi: 10.2174/157488909787002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A, et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16(8):1382–91. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur B, Cork SM, Sandberg EM, Devi NS, Zhang Z, Klenotic PA, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res. 2009;69(3):1212–20. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardcastle J, Kurozumi K, Dmitrieva N, Sayers MP, Ahmad S, Waterman P, et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther. 2010;18(2):285–94. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8(5):724–30. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 8.Varner JA, Cheresh DA. Tumor angiogenesis and the role of vascular cell integrin alphavbeta3. Important Adv Oncol. 1996:69–87. [PubMed] [Google Scholar]
- 9.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6(6):725–40. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270(5241):1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 11.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 12.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79(7):1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314(1):131–44. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 14.Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117(5):1101–7. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48(1):151–7. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294(5541):339–45. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62(15):4263–72. [PubMed] [Google Scholar]
- 18.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, et al. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11(17):6270–9. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 19.Tentori L, Dorio AS, Muzi A, Lacal PM, Ruffini F, Navarra P, et al. The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol Rep. 2008;19(4):1039–43. [PubMed] [Google Scholar]
- 20.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–7. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 21.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99(23):1768–81. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 22.Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67(19):9398–406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103(34):12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65(7):2832–9. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 25.Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13(8):705–14. doi: 10.1038/sj.gt.3302717. [DOI] [PubMed] [Google Scholar]
- 26.Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3(4):335–44. doi: 10.1023/a:1026546219962. [DOI] [PubMed] [Google Scholar]
- 27.Mikkelsen T, Brodie C, Finniss S, Berens ME, Rennert JL, Nelson K, et al. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int J Cancer. 2009;124(11):2719–27. doi: 10.1002/ijc.24240. [DOI] [PubMed] [Google Scholar]
- 28.Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, et al. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108(9):3035–44. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 29.Onishi M, Kurozumi K, Ichikawa T, Kurozumi K, Fujii Kentaro, Inoue Satoshi, Chiocca E Antonio, Kaur Balveen, Date Isao. Omics analysis cilengitide treatment of malignant glioma. Congress of Neurosurgery. 2012 submitted. [Google Scholar]
- 30.Spear MA, Sun F, Eling DJ, Gilpin E, Kipps TJ, Chiocca EA, et al. Cytotoxicity, apoptosis, and viral replication in tumor cells treated with oncolytic ribonucleotide reductase-defective herpes simplex type 1 virus (hrR3) combined with ionizing radiation. Cancer Gene Ther. 2000;7(7):1051–9. doi: 10.1038/sj.cgt.7700208. [DOI] [PubMed] [Google Scholar]
- 31.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17(8):1225–35. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabors LB, Mikkelsen T, Hegi ME, Ye X, Batchelor T, Lesser G, et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306) Cancer. 2012 doi: 10.1002/cncr.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh CT, Radeff-Huang J, Matteo R, Hsiao A, Subramaniam S, Stupack D, et al. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(11):4011–21. doi: 10.1096/fj.08-113266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haseley A, Boone S, Wojton J, Yu L, Yoo JY, Yu J, et al. Extracellular matrix protein CCN1 limits oncolytic efficacy in glioma. Cancer Res. 2012;72(6):1353–62. doi: 10.1158/0008-5472.CAN-11-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo JY, Haseley A, Bratasz A, Chiocca EA, Zhang J, Powell K, et al. Antitumor efficacy of 34.5ENVE: a transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Mol Ther. 2012;20(2):287–97. doi: 10.1038/mt.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Expression of αvβ3 and αvβ5 integrins on the surface of HMVEC-d. The expression of αvβ3 and αvβ5 was recognized on the U251 (A), Gli36 (B), and U87ΔEGFR (C) cells.


