Abstract
Hirschsprung Disease (HSCR) is a potentially deadly birth defect characterized by the absence of the enteric nervous system (ENS) in distal bowel. Although HSCR has clear genetic causes, no HSCR-associated mutation is 100% penetrant, suggesting gene-gene and gene-environment interactions determine HSCR occurrence. To test the hypothesis that certain medicines might alter HSCR risk we treated zebrafish with medications commonly used during early human pregnancy and discovered that ibuprofen caused HSCR-like absence of enteric neurons in distal bowel. Using fetal CF-1 mouse gut slice cultures, we found that ibuprofen treated enteric neural crest-derived cells (ENCDC) had reduced migration, fewer lamellipodia and lower levels of active RAC1/CDC42. Additionally, inhibiting ROCK, a RHOA effector and known RAC1 antagonist, reversed ibuprofen effects on migrating mouse ENCDC in culture. Ibuprofen also inhibited colonization of Ret+/− mouse bowel by ENCDC in vivo and dramatically reduced bowel colonization by chick ENCDC in culture. Interestingly, ibuprofen did not affect ENCDC migration until after at least three hours of exposure. Furthermore, mice deficient in Ptgs1 (COX 1) and Ptgs2 (COX 2) had normal bowel colonization by ENCDC and normal ENCDC migration in vitro suggesting COX-independent effects. Consistent with selective and strain specific effects on ENCDC, ibuprofen did not affect migration of gut mesenchymal cells, NIH3T3, or WT C57BL/6 ENCDC, and did not affect dorsal root ganglion cell precursor migration in zebrafish. Thus, ibuprofen inhibits ENCDC migration in vitro and bowel colonization by ENCDC in vivo in zebrafish, mouse and chick, but there are cell type and strain specific responses. These data raise concern that ibuprofen may increase Hirschsprung disease risk in some genetically susceptible children.
Keywords: Enteric nervous system development, gene-environment interactions, migration
Introduction
The enteric nervous system (ENS) is an intrinsic neuronal and glial network in the bowel that controls intestinal function (Furness, 2012; Sasselli et al., 2012). Most ENCDC that give rise to the ENS begin to delaminate from vagal neural tube by E8.5 in mice and prior to week four in humans (Fu et al., 2004; Kapur et al., 1992). Sacral ENCDC also contribute to distal bowel ENS (Kapur, 2000; Wang et al., 2011). Vagal ENCDC migrate to the foregut and then begin coordinated proliferation, rostro-caudal migration, and differentiation, a process that normally results in interconnected neurons and glia throughout the bowel by E13.5 in mice and by week 7 in humans. This migratory route is one of the longest traversed by any cell during development. In one in 5000 human infants, ENCDC do not colonize the bowel completely, causing Hirschsprung disease (HSCR), a condition defined by the absence of distal bowel enteric neurons (Heuckeroth, 2013; Hirschsprung, 1888; McKeown et al., 2013; Skinner, 1996).
HSCR is potentially fatal because aganglionic bowel (i.e., bowel without neurons) tonically contracts, causing constipation, bilious vomiting, abdominal distension, enterocolitis, and sepsis (Dasgupta and Langer, 2004; Heuckeroth, 2013; Skinner, 1996). Most children with HSCR (80%) have only a short region of aganglionic bowel (Amiel et al., 2008; Suita et al., 2005) and even modestly increased ENCDC bowel colonization (e.g. 5–10% increase) might have prevented HSCR. For children with extensive aganglionosis, having only slightly greater bowel colonization by ENCDC could prevent the need for intravenous nutrition and associated life-threatening infections. Therefore, it is valuable to identify non-genetic potentially avoidable factors that influence ENCDC migration.
Because ENS development depends on many signaling pathways (Anderson et al., 2006; Goldstein et al., 2013; Lake and Heuckeroth, 2013; Laranjeira and Pachnis, 2009), many medicines may increase HSCR risk. One critical pathway includes RET receptor tyrosine kinase (Pachnis et al., 1993; Schuchardt et al., 1994), the ligand glial cell line-derived neurotrophic factor (GDNF), the co-receptor GFRα1 (Airaksinen and Saarma, 2002) and the small RhoGTPases RAC1 and CDC42 that induce actin polymerization and reorganization at the leading edge of migrating cells (de Curtis, 2008; Fukata et al., 2003; Goto et al., 2013; Ridley, 2006; Stewart et al., 2007; Vohra et al., 2007a). Among other roles, RAC1 induces lamellipodia, promotes ENCDC migration in vitro and ex vivo and inhibits RHOA, a small GTPase (Stewart et al., 2007; Wu et al., 2009). RHOA also inhibits RAC1 via its effector ROCK (Guilluy et al., 2011; Nakayama et al., 2008). The complex signaling pathways needed for normal development increase the vulnerability of ENS precursors to medicines that impact these and many other signaling pathways.
By testing medicines commonly used during early human pregnancy, we discovered that ibuprofen dramatically reduced bowel colonization by ENCDC in zebrafish and chick. Furthermore, ibuprofen delayed Ret+/− mouse bowel colonization by ENCDC in vivo, but effects were not observed in WT mice. Consistent with these observations, ibuprofen reduced migration speed on 2-dimensional surfaces, reduced lamellipodia and reduced filamentous actin in murine ENCDC. These changes correlated with reduced active RAC1/CDC42. Furthermore, ibuprofen’s effects on migration could be prevented using a ROCK inhibitor. In contrast, ibuprofen did not slow migration or reduce lamellipodia in mouse gut mesenchymal or NIH3T3 cells. Surprisingly, mice with mutations in Ptgs (i.e., cyclooxygenase, COX), ibuprofen’s main therapeutic target, had normal ENCDC bowel colonization and normal migration in vitro. Additionally, live imaging experiments demonstrated that ENCDC required at least three hours of exposure to ibuprofen before ENCDC migration speed was impacted, a much slower time course than would be expected for a solely COX dependent effect. Collectively these studies suggest ibuprofen use during early pregnancy could increase HSCR risk in some genetically susceptible children. This may be important since 23.5 % of women in the United State take ibuprofen during early pregnancy (Thorpe et al., 2013).
Materials and Methods
Zebrafish
Wild type AB zebrafish, fertilized in vitro, were exposed to drugs from 34 to 96 hours post fertilization (hpf) in E3 media with 1% DMSO (Murphey and Zon, 2006). At 96 hpf, zebrafish were stained with HuC/D antibody to visualize neurons. Uncolonized gut was measured from most distal HuC/D+ cell to end of bowel. Drugs (from Sigma, St. Louis) tested, number of fish evaluated and drug concentrations are in Table 1.
Table 1.
Medicines tested for an effect on zebrafish ENS development
Ibuprofen and acetylsalicylic acid inhibit bowel colonization by ENCDC in zebrafish at doses within the human therapeutic range.
| Medicine | Human therapeutic blood concentration (μM) | Lowest concentration that affects ENCDC* (μM) | Concentration range tested (μM) [Actual concentrations tested (number evaluated at each concentration)] | Reference |
|---|---|---|---|---|
| Acetaminophen | 65–130 | 2,315 | 331–3,308 [331 (9), 662 (8), 1650 (8), 2315 (7) 3308 (7)] | (Ritschel and Kerns, 2009) |
| Acetylsalicylic Acid | 110–1,700 | 333 | 56–666 [56 (21), 111(22), 222 (22), 333 (24), 666 (7)] | (Ritschel and Kerns, 2009) |
| Caffeine | 10–50 | 257 | 5–1,030 [5 (21), 51 (15), 257 (17), 514 (18), 1030 (16)] | (Baselt, 1982) |
| Chlorpheniramine | 0.013–0.025 | 256# | 13–1,279 [13 (6), 26 (9), 128 (10), 256 (5), 1279 (6)] | (Ritschel and Kerns, 2009) |
| Clomiphene | 0.050** | 83# | 17–836 [(17 (9) 83 (≥5), 167 (≥5) 836 (≥5)] | (Ghobadi et al., 2009) |
| Dextromethorphan | 0.74–1.3 | 368 | 18–1,840 [18 (9), 37 (9) 184 (7) 368 (7), 1840 (≥5)] | (Ritschel and Kerns, 2009) |
| Diphenhydramine | 0.034–0.34 | 103# | 17–343 [17 (9) 34 (9) 103 (≥5), 172 (≥5), 343 (≥5)] | (Ritschel and Kerns, 2009) |
| Doxylamine | 0.3** | 1,287# | 129–1,287 [129 (8), 257 (8) 1287 (6)] | (Ritschel and Kerns, 2009) |
| Erythromycin | 0.68–3.4 | >1,363 | 14–1363 [14 (7), 68 (9), 136 (9), 681 (8), 1363 (9)] | (Ritschel and Kerns, 2009) |
| Guaifenesin | 7.6** | >3,31 | 252–3,531 2522 (7), 3531 (8)] [252 (5), 504 (9), 1009 (9), | (Aluri and Stavchansky, 1993) |
| Ibuprofen | 25–240 | 25 | 2.5–50 [2.5 (21) 5 (24), 12.5 (24), 25 (24), 50 (20)] | (Ritschel and Kerns, 2009) |
| Loratadine | 0.13** | >1,306 | 65–1,306 [65 (12), 131 (12), 261 (9) 652 (9) 1306 (10)] | (Hilbert et al., 1988) |
| Sulfamethoxazole | 200–790 | 1974 | 400–7,896 [395 (10), 987 (11), 1974 (12), 3948 (11), 7896 (8)] | (Ritschel and Kerns, 2009) |
Data in this column indicate the lowest drug concentration that causes a statistically significant increase in the length of distal zebrafish bowel that lacks ENCDC (compared to 1% DMSO vehicle) after treatment with drug from 34–96 hpf.
Indicates medicines that caused severe malformation or death at concentrations listed (i.e., defects were not ENS selective).
Based on the available literature we indicate peak human therapeutic blood concentration instead of therapeutic blood ranges.
Avian intestine organ culture
Fertilized White Leghorn chicken eggs from commercial breeders were maintained in a 37°C humidified incubator. E6 midgut plus hindgut from umbilicus to cloaca was pinned to Sylgard, cultured in DMEM, 100 U/mL penicillin-G, 0.1 mg/mL streptomycin (Sigma), DMSO (5 μM), +/− ibuprofen (250 μM) for 48 hours and then processed for immunocytochemistry. 5-ethynyl-2′-deoxyuridine (EdU) (10 μM, Invitrogen) was added 3 hours before fixation.
Mice
Mice used include WT CF-1 (Charles River Laboratories), WT C57BL/6J (The Jackson Laboratory), Ptgs1tm1Unc (called Ptgs1 or Cox1, RRID:MGI_4366280), Ptgs2tm1Unc (called Ptgs2 or Cox2, RRID:MGI_4366244) (C57BL/6J background) (Langenbach et al., 1995; Morham et al., 1995), Sox10tm1Weg (called Sox10+/− or Sox10 LacZ, RRID:MGI_4437131) (C3H background) (Britsch et al., 2001) (from M. Wegner (Friedrich Alexander University Erlangen Nuremberg, Erlangen, Germany) and M. Southard-Smith (Vanderbilt University, Nashville, Tennessee, USA)), and Rettm1Jmi (Ret (tau-EGFP-myc), Ret-TGM, RRID:MGI_3623107), called Ret+/−, C57/B6J background)(Enomoto et al., 2001). The day of the vaginal plug was called E0.5. Mice were genotyped by PCR as described in the references above.
Midgut Slice Explant Culture
E12.5 small bowel (midgut) was cut into 300–500 micron slices. These slices were cultured on fibronectin-coated (250 μg/mL; Life Technologies) plastic or glass Lab-Tek Permanox chamber slides (ThermoFisher) in OptiMem (Life Technologies), 2 mM L-glutamine (Life Technologies), 100 IU/mL penicillin, 100 μg/mL streptomycin (Life Technologies). Ibuprofen stock (1 g/L) was prepared fresh in media daily. Unless noted, ibuprofen was added when slices were plated. Four hours after plating, GDNF (100 ng/mL final concentration) was added to cultures, which were then maintained for an additional 16 hours (37°C, 5% CO2) after GDNF addition. When needed, 10 μM bromodeoxyuridine (BrdU) was added four hours before fixation. Y-27632 (5 μM, Sigma, St. Louis MO) in DMSO was added four hours after plating slices. 16, 16-dimethyl prostaglandin E2 and 16, 16-dimethyl prostaglandin F2 (Cayman Chemicals, Ann Arbor, MI) dissolved in ethanol were added (final concentration 1 μM) at the same time as GDNF (for 16, 16-dimethyl prostaglandin E2) or at plating (for 16, 16-dimethyl prostaglandin F2). Control cultures received equivalent diluent volumes.
Immunoselected ENCDC Culture
E12.5 CF-1 small and large bowel were treated with collagenase (0.2 mg/mL) and dispase (0.2 mg/mL), triturated, resuspended in media (Neurobasal® (Life Technologies), B27® (50x, Life Technologies), 2 mM L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin), and then exposed to rabbit anti-p75NTR (anti-nerve growth factor receptor, P75, EMD Millipore, 1:1000, one hour, 4°C), followed by goat anti-rabbit coupled paramagnetic beads (anti-rabbit IgG MicroBeads1:50, Miltenyi Biotec, GmbH) (30 min, 4°C). ENCDC (p75NTR positive) were isolated with MACS columns (Miltenyi Biotec, GmbH), before culturing on poly-d-lysine (100 μg/mL, Sigma, St. Louis) and laminin (20 μg/mL, BD Biosciences) coated glass chamber slides (Sato and Heuckeroth, 2008). Cells were treated with GDNF (50 ng/mL) with or without ibuprofen (250 μM) at plating and then grown 48 hours before fixation.
In Vivo Mouse Ibuprofen Treatment
Pregnant mice were fed Lab Diet 5053 (Test Diet, Richmond, IN) with or without 375 parts per million ibuprofen from E8.5 to until analysis.
Embryo Sections
10 μm sagittal E12.5 CF-1 mouse sections were prepared from immersion fixed (4% paraformaldehyde overnight, rotating, 4°C), sucrose treated (30% overnight), frozen (Optimal Cutting Temperature Compound, Tissue-Tek) mice.
Immunohistochemistry
Fixed zebrafish (2% paraformaldehyde, two hours, 25°C) were washed in PBS, stained with mouse anti-HuC/HuD (16A11) antibody (overnight, 4°C) and examined after Alexa Fluor® anti-mouse 594 secondary (1:250, Life Technologies) (two hours, 25°C) treatment.
Mouse cells were fixed (4% paraformaldehyde, 25°C) for 20 minutes (slice and dissociated culture) or 30 minutes (whole bowel)), washed (PBS or Tris-buffered saline plus 0.1% Triton-X (TBST)), and blocked (4% normal donkey serum/TBST, 1 hour, 25°C or overnight 4°C) before primary antibodies (Table 2) were applied in TBST (4°C, overnight). TuJ1 and Phalloidin were applied only two hours at 25°C. Samples were washed in PBS or TBST before incubating with secondary antibodies (Alexa Fluor® Donkey anti-Rabbit, Donkey anti-goat, Donkey anti-mouse 488, 594, or 647nm, Invitrogen, 1:400) and DAPI (100 ng/mL, 4′,6–diamidine–2–phenylidole–dihydrochloride, Vector Labs) in TBST (1 hour, 25°C) and then washing again in PBS or TBST before mounting (50% glycerol).
Table 2.
Primary and secondary antibodies for immunohistochemistry or immunoselection
| Antibody | Concentration | Catalog # | Source |
|---|---|---|---|
| Rabbit anti-Tuj1 | 1:10,000 | PRB-435P | Covance*; RRID:AB_10063850 |
| Goat anti-RET | 1:100 | GT15002 | Neuromics; RRID:AB_2179886 |
| Mouse anti-BRDU (Conjugated to Alexa-594) | 1:100 | A21304 | Invitrogen; RRID:AB_221472 |
| Rabbit anti-cleaved caspase-3 | 1:100 | 9661 | Cell Signaling Technology; RRID:AB_2314091 |
| Mouse anti-PPARγ | 1:100 | sc-7273 | Santa Cruz; RRID:AB_628115 |
| Phalloidin (Conjugated to Alexa-488) | 1:50 | A12379 | Invitrogen; RRID:AB_2315147 |
| Rabbit anti-GST | 1:100 | sc-35614 | Santa Cruz; RRID:AB_647587 |
| Mouse anti-vinculin | 1:100 | V9131 | Sigma; RRID:AB_477629 |
| Mouse anti-HuC/HuD (Biotin Conjugate (16A11)) | 1:800 | A21272 | Invitrogen; RRID:AB_10375876 |
| Mouse anti-chicken N- cadherin antibody (clone 6B3) | 1:5 | 6B3 | Developmental Studies Hybridoma Bank, Iowa; RRID:AB_528118 |
| Rabbit anti-nerve growth factor (NGF-receptor), P75 | 1:1000 | AB1554 | EMD Millipore RRID:AB_90760 |
| Anti-rabbit IgG MicroBeads | 1:50 | 130-048-602 | Miltenyi Biotec RRID:AB_244362 |
| Alexa Fluor® 594 Donkey anti-mouse | 1:400 | A21203 | Molecular Probes (Invitrogen); RRID: AB_141633 |
| Alexa Fluor® 594 Donkey anti-rabbit | 1:400 | A21207 | Life Technologies: RRID:AB_10049744 |
| Alexa Fluor® 488 Donkey anti-rabbit | 1:400 | A21206 | Life Technologies; RRID:AB_10049650 |
| Alexa Fluor® 594 Donkey anti-goat | 1:400 | A11058 | Life Technologies; RRID:AB_10563390 |
| Alexa Fluor® 647 Donkey anti-rabbit | 1:400 | A31573 | Life Technologies; RRID:AB_10561706 |
Covance was acquired by BioLegend in 2014 (new catalog # 802001; RRID not yet assigned)
RRID = Research Resource Identifiers (https://www.force11.org/node/4856)
Avian bowel was fixed (4% paraformaldehyde in PBS, 1 hour), rinsed (PBS), incubated in 15% sucrose/PBS (overnight, 4°C), then in 7.5% gelatin,15% sucrose/PBS (37°C, 1 hour), before freezing (−50°C, 2-Methylbutane, Sigma). 10 μm frozen sections on Probe On Plus slides (Fisher Scientific) were stained with ENCDC specific anti-chicken N-cadherin antibody (Nagy et al., 2012) Nuclei were DAPI stained. EdU was detected using Clik-iT EdU Imaging Kit (Invitrogen).
Active RAC1/CDC42 and RHOA Measurements
NIH3T3 (ATCC) for scratch test were grown on glass 8-well chamber slides to 100% confluence in DMEM (high glucose), 10% fetal calf serum (FCS), 100 IU/mL penicillin, and 100 μg/mL streptomycin. 250 μM ibuprofen was added two hours before scratching to remove some cells using a 10 μL pipette tip. Media was changed after the scratch. For RAC1/CDC42 and RHOA studies, NIH3T3 were starved (18 hours in 1 % FCS, then 24 hours in 0 % FCS), then stimulated with 100 ng/mL RAC1/CDC42 Activator II (3 min, Cytoskeleton, Denver) or 100 μg/mL Rho Activator I (30 min, Cytoskeleton, Denver). During stimulation, RAC1/CDC42 control cells were kept in 0% FCS media while RHOA control cells were in 1 % FCS media.
After culture ENCDC and NIH3T3 were fixed (4% paraformaldehyde, 20 min, 25°C), permeabilized in TBST (55°C, one hour) and incubated with 20 μg GST-tagged PAK-PBD protein (PBD-GST) (Cytoskeleton, Denver) or 100 μg GST-tagged Rhotekin-RBD protein (RBD-GST) (Cytoskeleton, Denver) (1 hour, 4°C) in 100 μl TBST. Cells were PBS washed, post-fixed (4% paraformaldehyde, 15 min, 25°C), and washed again (PBS) before anti-GST immunohistochemistry.
Microscopy and Analysis
Micrographs were obtained with 1) Olympus BX60 or IX71, Axiocam CCD, Axiovision software, 2) Olympus FV1000 confocal with Fluoview software, 3) Zeiss Axio Imager.A2, AxioCam MRm Rev.3, ZEN software, 4) Zeiss LSM 710 confocal, Zen software, 5) Nikon Eclipse 80i, Spot camera, software version 3.3.1 (Diagnostic Instruments). FIJI (NIH ImageJ) and Photoshop CS6 (Adobe) were used to process images (Schindelin et al., 2012) including only cropping, stitching (Preibisch et al., 2009), rotating, centering, and uniform adjustments of brightness, contrast and saturation. Confocal images show flattened Z–stacks. Time-lapse imaging used an AxioObserver.Z1 microscope (Zeiss) with motorized stage and incubator with temperature and CO2 controls. Live-imaging was at 37°C and 5% CO2.
ENCDC colonization of mouse bowel was measured from cecal tip to most distal TuJ1+ cell or neurite. For midgut slices, migration was measured in octants from slice edges to the most distal ENCDC or mesenchymal cell (Supplemental Figure 1). Octants adjacent to other slices were not evaluated. ENCDC were RET+ by immunohistochemistry. Mesenchymal cells were identified by absent RET immunostaining and an actin-rich cytoskeleton after Alexa488-phalloidin staining. Each slice was considered a single replicate.
Lamellipodia were defined as regions of obvious F-actin ruffling wider than the cell body and were analyzed in phalloidin-stained ENCDC furthest from gut slices (Supplemental Figure 1). The longest neurite in RET+ cells were measured using FIJI (NIH ImageJ). Cell speed was measured in time-lapse images using MTrackJ manually (Meijering et al., 2012). Immunofluorescent pixel intensity was measured using FIJI on images taken at fixed exposure times. Only RET+ cells that migrated furthest from slices were analyzed for fluorescence intensity. All samples for which fluorescence intensity was compared were stained on the same chamber slide.
Statistical Analysis
SigmaPlot 11 (Systat Software), student’s t-test or one-way ANOVA was used for comparisons. All studies include at least three biological replicates. Non-parametric data were analyzed by ranks. Post-hoc Holm-Sidak or Dunn’s tests were used for multiple comparisons. Data are plotted as mean +/− SEM unless noted. P<0.05 was considered significant.
Approvals
All studies were approved by Animal Studies Committee at Washington University School of Medicine, Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia, and by Massachusetts General Hospital’s Institutional Subcommittee on Research Animal Care.
Results
Ibuprofen inhibited enteric nervous system development in zebrafish
Zebrafish embryos were exposed to selected medicines that are used by > 0.5 % of women during early pregnancy (Thorpe et al., 2013) (Table 1). Fish were treated during the period that ENCDC migrate through bowel (34 to 96 hours post fertilization (hpf)) and then stained with Elavl3/4 (HuC/D) antibody to show enteric neurons (Kuhlman and Eisen, 2007; Lake et al., 2013). Ibuprofen and acetylsalicylic acid specifically reduced bowel colonization by ENCDC at concentrations found in human blood with therapeutic dosing (Table 1). We focused on ibuprofen because it inhibited bowel colonization by ENCDC in a dose dependent manner (Figure 1A–E) and because acetylsalicylic acid caused death at 660 μM (i.e., twice the dose that caused ENS defects) suggesting more generalized toxicity. In contrast, at an ibuprofen dose that reduced bowel colonization by ENCDC (25 μM), fish looked grossly normal (Figure 1A – D). Body length was slightly reduced (Figure 1A, C, F), but dorsal root ganglia (DRG), another neural crest-derivative, were in a normal position after ibuprofen treatment (Figure 1A, C, G).. Furthermore fish treated with 25 μM ibuprofen had a normal number of DRG neurons per ganglion (DMSO 2.2 +/− 0.09, ibuprofen 2.1 +/− 0.1, p = 0.3, 2 tailed t-test). These data suggest that ibuprofen inhibits zebrafish bowel colonization by ENCDC at concentrations that do not globally disrupt development or block migration of other neural crest derivatives.
Figure 1. Ibuprofen reduced ENCDC colonization of zebrafish bowel.
(A–D) Zebrafish were treated with vehicle (1%DMSO) or ibuprofen (Ibu) from 34–96 hpf and then stained with HuC/D antibody. (A) White arrows indicate dorsal root ganglia. (C) White arrows highlight enteric neurons. Scale bar=500μm. (B, D) Higher magnification of fish midsection. Scale bar=100μm. (E) Length of bowel from most distal HuC/D+ cell (arrows in B, D) to bowel terminus (arrowheads in B, D). *P<0.05 (ANOVA on Ranks) (F) Length of whole zebrafish (1% DMSO n=4, 25 μM Ibuprofen n=6). *P=0.025 (t-test). (G) Distance between DRG and dorsal zebrafish edge (1% DMSO n=3, 25 μM ibuprofen n=4). *P>0.05 (t-test).
Ibuprofen inhibits ENCDC colonization of chick bowel in organ culture
To determine if ibuprofen affects ENS development in other species we cultured E6 chick hindgut for 48 hours with or without ibuprofen (250μM) and then stained with N-cadherin antibody (Figure 2A–B). This ibuprofen concentration was selected based on work in mouse culture (Figure 3) where higher ibuprofen doses are needed to affect ENCDC migration. This ibuprofen level is comparable to human serum levels after consuming three over-the-counter ibuprofen tablets (i.e., 600 mg, a commonly used dose) (Ritschel and Kerns, 2009). Remarkably, ibuprofen-treated hindgut was almost devoid of ENCDC, whereas control bowel was almost fully colonized by ENCDC (n=6 per treatment group). Proliferation of ENCDC was also reduced by ibuprofen based on EdU incorporation into N-Cadherin expressing cells at the leading edge of the migration wavefront (N-Cadherin+ EdU+/N-Cadherin x 100: Control 58 +/− 0.06%, ibuprofen 38 +/− 0.05%, P = 0.03, n = 6/group, t-test) as was proliferation in gut mesenchymal cells (DAPI+ N-Cadherin- EdU+/DAPI+ N-Cadherin- x 100: Controls 20 +/− 1.5%, ibuprofen 13 +/− 0.9%, P = 0.03, n = 6/group, t-test). Thus in chick and in fish, ibuprofen dramatically reduced colon colonization by ENCDC. In chick, this was accompanied by reduced proliferation of ENCDC and surrounding mesenchymal cells.
Figure 2. Ibuprofen inhibited hindgut colonization by chick ENCDC ex vivo and mouse ENCDC in vivo.
(A, B) Ibuprofen (250 μM) almost completely blocked E6 chick colon colonization by N-cadherin+ (green) ENCDC during 48 hour culture. (Scale bar=200 μm, n=6 per group). Insets show the migration wavefront with EdU (red) and N-cadherin (green) immunohistochemistry. (Scale bar=35 μm). mg=midgut, hg=hindgut. (C–E) Ibuprofen feeding from E8.5 to E12.5 reduced Ret +/− mouse colon colonization by ENCDC visualized with TuJ1 antibody (red). Scale bars=400 μm. (E, F) Quantitative data show the proportion of the colon colonized by ENCDC (i.e., length of colon containing ENCDC divided by total colon length) (Control n=4, ibuprofen n=9). *P =0.042 (t-test). (F) Sox10 +/− colon was normally colonized by ENCDC when dams were fed ibuprofen from E8.5 to E12.5 (Control n = 19, ibuprofen n=13). P=0.79 (t-test).
Figure 3. Ibuprofen reduced murine ENCDC migration, but did not affect proliferation, caspase-3 activation, or neuronal differentiation.
E12.5 CF-1 midgut slices cultured with or without ibuprofen for 16 hours after GDNF addition were stained for (A, B) RET (red), phalloidin (green), DAPI (blue) (scale bar=200μm), (C, D) RET (green), BrdU (red), DAPI (blue) (Scale bar=100μm), (E, F) RET (red), cleaved-caspase 3 (green), DAPI (blue) (Scale bar=100μm), or (G, H) RET (green), TuJ1 (red), DAPI (blue) (scale bar=100μm). (I) Ibuprofen reduced the distance ENCDC migrated from gut explants at 250 μM and 500 μM. Control n=275 slices, 29 biological replicates; 50 μM ibuprofen n = 48 slices, 3 biological replicates; 100 μM ibuprofen n = 55 slices, 3 biological replicates; 250 μM ibuprofen n=111 slices, 5 biological replicates; 500 μM ibuprofen n = 102 slices, 8 biological replicates) but not at lower ibuprofen concentrations. **P<0.001 (ANOVA). (J) Mean percentage BrdU+ ENCDC (RET+ cells) (Control n=21 slices, 7 biological replicates; ibuprofen n=9 slices, 3 biological replicates). P=0.24 (t-test). (K) Mean percentage cleaved-caspase 3+ ENCDC (RET+ cells) (Control n=15 slices, ibuprofen n=15 slices; both 3 biological replicates). P=0.36 (t-test). (L) Mean percentage TuJ1+ ENCDC (RET+ cells) (Control n=19 slices, ibuprofen n=23 slices; both 3 biological replicates). P=0.35 (t-test).
Ibuprofen slows ENCDC colonization of mouse bowel in vivo
To determine if ibuprofen affects mammalian ENCDC development, we fed ibuprofen to two mouse lines with genetic defects that predispose to HSCR-like disease. Pregnant mice with Ret or Sox10 mutations received ibuprofen containing or control chow from E8.5 to E12.5. We selected these dates because E8.5 is one day before ENCDC enter fetal bowel and by E12.5 ENCDC have normally migrated to mid-colon. We then stained the bowel with an antibody to neuron specific β3 tubulin (TuJ1) (Figure 2C–F). Ibuprofen-exposed Ret+/− mice had a small, but significant reduction in bowel colonization by ENCDC (% colon colonization: untreated Ret +/− 73.6 ± 3.9%, n=4; ibuprofen treated Ret+/− 65.2 ± 5.2% n=9, P<0.042, t-test). In contrast, colon colonization by ENCDC was normal in ibuprofen treated E12.5 Sox10+/− mice and in E12.5 WT littermates of Sox10+/− and Ret+/− animals (% colon colonization: untreated Sox10+/− 59.4 ± 2.4% n=19; ibuprofen Sox10+/− 60.3 ± 2.3% n=13, p=0.79, t-test and data not shown) (Figure 2F). These data show that in Ret+/− mice, ibuprofen can delay ENCDC colonization of fetal bowel in vivo, although the effect is more subtle than in chick and fish.
Ibuprofen specifically inhibited murine ENCDC migration in vitro
To gain insight into why ibuprofen slowed bowel colonization by ENCDC, we cultured E12.5 CF-1 mouse gut slices on fibronectin coated dishes in the presence or absence of ibuprofen (Fu et al., 2013; Fu et al., 2010; Lake et al., 2013; Wang et al., 2010). Addition of GDNF to the culture media enhanced ENCDC migration onto the dishes permitting drug effects on ENCDC migration, proliferation, cell death and differentiation to be evaluated. After 16 hours in culture, ENCDC were identified by RET immunoreactivity. RET is expressed by all ENS precursors and differentiated enteric neurons in vitro (Heuckeroth et al., 1998; Pachnis et al., 1993). ENCDC migration was assessed by measuring the distance between gut slice edges and the most distant ENCDC (Supplemental Figure 1). 250 μM ibuprofen reduced the distance ENCDC migrated from gut slices, but migration distance was normal at lower ibuprofen concentrations (50 μM, 100 μM) (Figure 3A–B, I).
Since ENCDC migration might be affected by the number of migrating ENCDC and by differentiation (Lake et al., 2013), we determined if ibuprofen altered ENCDC proliferation, apoptosis, or differentiation. Double label immunohistochemistry for RET (expressed in ENCDC and enteric neurons) plus BrdU, RET plus activated caspase-3, and RET plus TuJ1 (neuron specific β3 tubulin antibody that identifies early neurons) demonstrated ibuprofen had no effect on murine ENCDC proliferation, apoptosis, or differentiation (Figure 3C–H and 3J–L). These data suggest ibuprofen specifically affects murine ENCDC migration without altering survival, proliferation or differentiation.
NIH 3T3 and gut mesenchymal cell migration was not affected by ibuprofen
To determine if ibuprofen affects migration of other cells, we performed “scratch test migration assays” on confluent NIH3T3 fibroblasts. After two hours of ibuprofen treatment, we created a wound to induce migration (Figure 4A–D) and imaged for 24 hours. Ibuprofen did not slow NIH3T3 migration (P = 0.75) (Figure 4A–E). To determine if ibuprofen effects require specialized media or factors produced by bowel, we examined CF-1 mesenchymal cells that migrated from the gut along with ENCDC (Supplemental Figure 1). Like NIH3T3, gut mesenchymal cell migration was not affected by ibuprofen (Figure 4G). These data suggest that ENCDC migration is especially sensitive to ibuprofen, compared to other cell types.
Figure 4. Ibuprofen did not affect migration or lamellipodia of NIH 3T3 or mouse gut mesenchymal cells.
Confluent NIH 3T3 were pretreated with 250 μM ibuprofen (2 hours) before making a scratch to remove some cells. (A, B) NIH3T3 immediately after the scratch was made. (C, D) Two hours after the scratch. Cells were imaged every 5 minutes for 24 hours 250 μM ibuprofen did not affect (E) average speed of gap closure (Control n=5 assays, ibuprofen; n=7 assays), P=0.76 (t-test) or (F) percentage of NIH 3T3 with lamellipodia (Control n=8 assays, ibuprofen; n=9 assays). P=0.75 (t-test). (G, H) 250 μM ibuprofen did not affect distance CF-1 mesenchymal cells migrated from gut explants (Control n=19 slices, ibuprofen n=24 slices; 3 biological replicates). P=0.57 (t-test) or the percentage of mesenchymal cells with lamellipodia after 16 hours (Control=9 slices, ibuprofen=9 slices; 3 biological replicates). P=0.52 (t-test).
Ibuprofen-exposed ENCDC have reduced lamellipodia and filamentous actin
To assess if ibuprofen affected ENCDC morphology, we cultured CF-1 mouse fetal bowel slices with or without ibuprofen for 16 hours and stained with phalloidin. Fewer ibuprofen treated migrating ENCDC had lamellipodia than control ENCDC at all doses tested (Figure 5A–F, I). In contrast, ibuprofen did not reduce well-formed lamellipodia in NIH 3T3 (Figure 4F) nor in gut mesenchymal cells (Figure 4H). This finding may underlie ibuprofen effects on ENCDC migration because lamellipodia are thin membranous actin projections that facilitate cell migration (Hall, 2005).
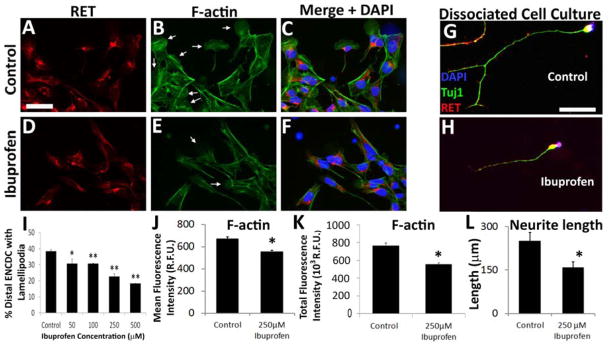
Figure 5. Ibuprofen reduced lamellipodia in migrating murine ENCDC and reduced neurite length in differentiating murine enteric neurons.
(A–F) E12.5 CF-1 midgut slices were cultured with 250 μM ibuprofen or control media for 16 hours after GDNF addition and then stained for (A, D) RET, (B, E) Alexa488-phalloidin, and (C, F) DAPI (merged image). Arrows highlight well-formed lamellipodia. (G, H) Dissociated immunoselected E12.5 CF-1 ENCDC cultured 48 hours were stained for RET (red), TuJ1 (green) and DAPI (blue). Scale bars=50 μm. (I, J). (I) The percentage of migrating ENCDC with lamellipodia was reduced by 50 μM or higher ibuprofen concentrations. RET+ ENCDC most distant from the gut slices were analyzed. (Control n=61 slices, 13 biological replicates; 50 μM Ibuprofen n = 9 slices, 3 biological replicates; 100 μM ibuprofen n=9 slices, 3 biological replicates, 250 μM ibuprofen n=27 slices, 9 biological replicates; 500 μM ibuprofen n = 12 slices, 4 biological replicates). *P <0.05, **P <0.001, (ANOVA, Holm-Sidak method). (J) Ibuprofen reduced mean Alexa488-phalloidin fluorescence intensity for RET+ ENCDC (Control n=232 cells, ibuprofen n=236 cells; both 4 biological replicates). *P<0.001 (Mann-Whitney Rank Sum). (K) Ibuprofen reduced longest neurite in cultured differentiating ENCDC (Control n=50, ibuprofen n=76, both 3 biological replicates). *P=0.009 (Mann-Whitney Rank Sum).
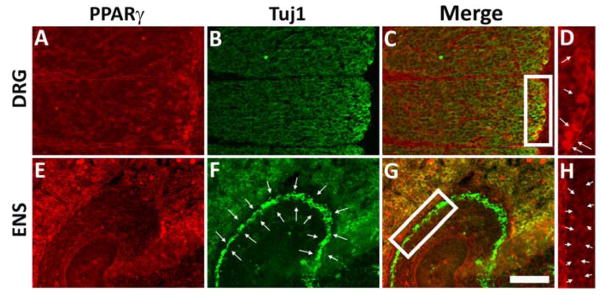
Lamellipodia formation is driven by actin polymerization and cross-linking. To assess filamentous actin (F-actin), we quantified fluorescence intensity of Alexa488-phalloidin stained ENCDC. Ibuprofen treated ENCDC had reduced Alexa488 staining suggesting that ibuprofen affects ENCDC actin dynamics (Figure 5A–F, J, K). As actin polymerization is also important for neurite growth and we assessed neurite length and found that ibuprofen reduced neurite length by 36 % when dissociated ENCDC differentiate in vitro (Figure 5G–H, L). This contrasts with studies showing ibuprofen enhanced neurite growth in corticospinal neurons, dorsal root ganglion cells (Fu et al., 2007) and raphespinal neurons (Wang et al., 2009) via PPARγ and reduced RHOA/ROCK signaling (Dill et al., 2010). Developing enteric neurons, however, have much less PPARγ than dorsal root ganglion neurons (Figure 6) providing a possible explanation for different ibuprofen effects. Collectively these data suggest ibuprofen slows ENCDC migration and reduces neurite length in ENCDC-derived neurons by altering actin cytoskeletal dynamics.
Figure 6. E12.5 CF-1 mouse ENCDC had less immunoreactive PPARγ than E12.5 dorsal root ganglion (DRG) neurons.
Sagittal sections were stained with antibodies to PPARγ (red) and neuron specific beta 3 tubulin (green, TuJ1). (A–D) PPARγ is readily detectable in DRG neurons. The boxed region in C contains DRG neurons that are immunoreactive for both PPARγ and TuJ1 antibodies (yellow cells) and is enlarged in D to show DRG neurons immunoreactive for PPARγ staining (red cells, white arrows). (E–H) Developing enteric neurons (TuJ1+ cells highlighted with arrows in F) have much lower levels of immunoreactive PPARγ (i.e., no yellow cells in the region of the ENS in G). The boxed region in G is enlarged in H to show the lack of PPARγ staining (red) in the ENCDC. Arrows highlight the region of the developing ENS. Images are from the same fetal mouse stained on the same slide. Scale bar = 100μm.
Ibuprofen slows ENCDC migration but effects are delayed
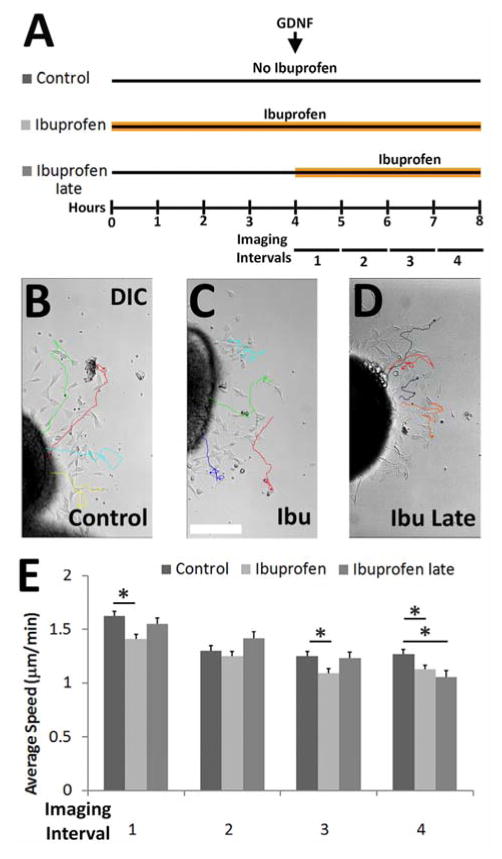
To gain insight into the mechanism by which ibuprofen slows ENCDC migration, we performed time-lapse microscopy on gut slice cultures with ibuprofen added, at the initiation of imaging (Figure 7A–D, Supplemental Movie 1–2). Analysis of sequential images showed that ENCDC persistence (net distance migrated/total distance migrated) was unaffected by ibuprofen (data not shown), but ENCDC migration speed was reduced. Interestingly, the effect on migration speed was only apparent after 3 hours of ibuprofen (i.e., during “imaging interval 4”, Figure 7A, E, Supplemental Movies 1–2). This is much longer than would be expected if ibuprofen effects were mediated by COX inhibition, the primary therapeutic effect of ibuprofen (Peppelenbosch et al., 1993; Vane, 1971). One possible explanation for delayed ibuprofen responsiveness of ENCDC is that prolonged contact with tissue culture surfaces changes ENCDC biology so that these cells become ibuprofen sensitive. To test this hypothesis, we added ibuprofen just as gut slices were placed in culture (i.e. four hours before initiation of imaging (Supplemental Movie 3)) and observed that ibuprofen-mediated effects on ENCDC migration were already apparent when imaging began. This suggests that the delayed response of ENCDC to ibuprofen was not dependent on a change in ENCDC phenotype after migration onto the culture dish, but instead is consistent with the hypothesis that ibuprofen effects on ENCDC migration were COX-independent.
Figure 7. Ibuprofen had delayed effects on murine ENCDC migration.
(A) Experimental paradigms. E12.5 CF-1 midgut slices were cultured on fibronectin. Ibuprofen was added four hours after plating (ibuprofen late) or at plating (ibuprofen). Time lapse images were then obtained every two minutes for four hours. (B–D) DIC images show final time points from time-lapse movies. Tracks show trajectories for single cells that migrated from explants. Immunohistochemistry after imaging confirmed tracked cells expressed RET. Scale bar = 100μm. (E) Mean ENCDC migration speed during each one hour interval. Ibuprofen slowed ENCDC speed, but not until 3–4 hours after ibuprofen exposure (Control n=130 cells, Ibuprofen n=180 cells, ibuprofen late n=92 cells; all 3 biological replicates). *P<0.05 (ANOVA on ranks).
Ptgs1 −/− Ptgs2 −/− mice have normal appearing ENCDC migration in vitro and in vivo
To directly test if reduced COX activity affected ENCDC, we cultured mid-small bowel slices from E12.5 Ptgs1−/− Ptgs2−/− (i.e., Cox1−/− Cox2−/−) mice on fibronectin and found no difference in ENCDC migration or lamellipodia compared to WT (Figure 8A–B, E–F). We also stained WT and Ptgs1 −/− Ptgs2 −/− E12.5 bowel with TuJ1antibody and found equivalent colon colonization by ENCDC (Figure 8C–D, G). Finally, we tried to rescue ibuprofen effects on CF-1 ENCDC using stable analogs of two prostaglandins (PGE2 and PGF2) reported to facilitate cell migration (16,16-dimethyl PGE2 or 16, 16-dimethyl PGF2 respectively), but did not observe any effect on migration or lamellipodia (Figure 8H–K). These data also suggest that ibuprofen effects on ENCDC migration may be COX-independent.
Figure 8. Ibuprofen effects on murine ENCDC migration and lamellipodia do not appear to be cyclooxygenase dependent. Ptgs1−/− Ptgs2−/− mice have normal ENCDC migration in vitro and normal bowel colonization in vivo.
(A, B) E12.5 WT C57BL/6 and Ptgs1−/− Ptgs2−/− midgut slices were cultured 16 hours after GDNF addition and then stained with RET antibody (red), phalloidin (green), and DAPI (blue). Scale bar=50 μm. (C, D) E12.5 bowel from WT and Ptgs1−/− Ptgs2−/− mice was stained with Tuj1 antibody. Scale bar=500 μm. Ptgs1−/− Ptgs2−/− mutations did not affect (E) the mean distance that ENCDC migrated from gut slices (WT n=57 slices, 5 embryos and Ptgs1−/− Ptgs2−/− n=48, slices, 7 embryos), P=0.55 (t-test), or (F) the mean percentage of ENCDC with a well-formed lamellipodia (WT n=11 slices, 4 embryos and Ptgs1−/− Ptgs2−/− n=10 slices, 5 embryos), P=0.38 (t-test), or (G) the extent of bowel colonization by ENCDC in vivo (WT n=6 and Ptgs1−/− Ptgs2−/− n=5), P=0.23 (t-test). Only ENCDC most distant from gut slices were evaluated for lamellipodia. (H–K) Stable Prostaglandin E2 (PGE2) and Prostaglandin F2 (PGF2) analogs did not rescue ibuprofen-induced ENCDC migration defects. E12.5 CF-1 midgut slices were cultured in media with GDNF with or without ibuprofen for 16 hours and then stained for RET, F-actin (phalloidin) and with DAPI. (H, I) Distance from the gut slice edge to the most distant RET+ ENCDC was measured. Neither 16, 16-dimethyl PGE2 (H) nor 16, 16-dimethyl PGF2 (I) restored ENCDC migration to control levels in the presence of ibuprofen, but 16,16-dimethyl PGE2 (H) did reduce ENCDC migration in the absence of ibuprofen. (For PGE2 studies: Control n = 165 slices, ibuprofen n = 112 slices, 16, 16-dimethyl PGE2 n = 137 slices, ibuprofen plus 16, 16-dimethyl PGE2 n = 53 slices; *P<0.05 compared to Control. #P<0.05 compared to Prostaglandin E2 (ANOVA). For PGF2 studies: Control n =103 slices, ibuprofen n = 96 slices, 16, 16-dimethyl PGF2 n = 127 slices, ibuprofen plus PGF2 n = 107 slices; *P<0.05 compared to Control. #P<0.05 compared to Prostaglandin F2 (ANOVA on Ranks)) (J, K) The percentage of ENCDC with well-formed lamellipodia was determined by examining phalloidin stained ENCDC that had migrated furthest from the gut slice edge. Neither 16, 16-dimethyl PGE2 nor 16, 16-dimethyl PGF2 rescued lamellipodia in the presence of ibuprofen (For PGE2 studies: Control n = 27 slices, ibuprofen n = 18 slices, PGE2 n = 27 slices, ibuprofen plus PGE2 n =18 slices) *P<0.05 compared to Control. #P<0.05 compared to Prostaglandin E2 (ANOVA on Ranks). For PGF2 studies: Control n = 105 slices, ibuprofen n = 83 slices, 16, 16-dimethyl PGF2 n = 105 slices, ibuprofen plus 16, 16-dimethyl PGF2 n =93 slices *P<0.05 compared to Control. #P<0.05 compared to Prostaglandin F2 (ANOVA)).
One caveat is that Ptgs mutant animals were on a C57BL/6 genetic background. We therefore cultured E12.5 small bowel slices from C57BL/6 mice on fibronectin in media with GDNF +/− ibuprofen. Ibuprofen did not reduce ENCDC migration from WT C57BL/6 gut (Distance of most distant ENCDC from gut slice: WT 335 +/− 7 μm; 250 μM ibuprofen 343 +/− 7 μm, 4 biological replicates, 128 control slices, 161 ibuprofen slices, P = 0.39, t-test), however, when this experiment was performed using C57BL/6 Ret+/− gut explants, ENCDC migration was reduced by ibuprofen (untreated: 349 +/− 7 μm, 250 μM ibuprofen 324 +/− 7 μm, 4 biological replicates 125 control slices, 147 ibuprofen slices, P = 0.015, t-test), consistent with our in vivo results. These data suggest that the C57BL/6 genetic background makes ENCDC more resistant to ibuprofen effects on migration compared to CF-1.
Ibuprofen reduces RAC1 activity in migrating murine ENCDC
Reduced F-actin, lamellipodia, and neurite growth in ibuprofen treated ENCDC suggested reduced RAC1 or increased RHOA activity (Bryan et al., 2005; Hall, 2005; Koh, 2006; Sato and Heuckeroth, 2008; Wang et al., 2003). We attempted to measure active RAC1 and RHOA in migrating ENCDC using sensitive G-LISA kits (Ferri et al., 2014; Nini and Dagnino, 2010), but could not obtain reproducible results. Furthermore, we wanted to measure RAC1 and RHOA activity only in cells that migrated furthest from cultured gut slices (i.e., distal ENCDC, Supplemental Figure 1) because these cells were examined for all other studies. Therefore we used a validated “in situ” method to analyze active RAC1/CDC42 or active RHOA based on binding to p21 PAK binding domain (PBD) or rhotekin binding domain (RBD) respectively, fused to glutathione-S-transferase (GST) (Supplemental Figure 2) (Li et al., 2002; Lindsley et al., 2011). Gut slices were cultured with or without ibuprofen, fixed and incubated with PBD-GST or RBD-GST followed by GST immunohistochemistry. Analysis of mean fluorescence intensity in ENCDC that migrated furthest from the gut edge demonstrated less PDB-GST immunoreactive protein in ibuprofen-treated ENCDC, suggesting less active RAC1/CDC42 than in control ENCDC (Figure 9A–H, Supplemental Figure 2A, C). In contrast, active RHOA levels were similar in ibuprofen-treated and control ENCDC based on RBD-GST binding (Figure 9I–P, Supplemental Figure 2 B, D). Collectively these data suggest ibuprofen hinders ENCDC migration by reducing active RAC1 without affecting RHOA activity.
Figure 9. Ibuprofen reduced active RAC1/CDC42, but did not affect active RHOA in migrating murine ENCDC.
E12.5 CF-1 midgut slices cultured 16 hours with GDNF were fixed, permeabilized and incubated with (A–H) PBD-GST that binds active GTP-RAC1 and GTP-CDC42, or (I–P) with RBD-GST that binds active GTP-RHOA. Cultures were then stained for (A, D, I, L) RET (green), (B, E, J, M) GST (red), and (C, F, K, N) DAPI (blue; merged image). Scale bar=50μm. (G, O) Mean fluorescence intensity for PBD-GST (G) and RBD-GST (O) for ENCDC that migrated furthest from gut slices. (G) 250 μM Ibuprofen treated ENCDC had less bound PBD-GST indicating reduced active RAC1/CDC42 (Control n=243 cells, ibuprofen n=205 cells, both 4 biological replicates); *P<0.001 (Mann-Whitney Rank Sum), but did not reduce (O) RBD-GST bound to active RHOA in actively migrating ENCDC (Control n=144 cells, ibuprofen n=136 cells, both 4 biological replicates). P=0.408 (Mann-Whitney Rank Sum). (H, P) Because ENCDC shape is altered by ibuprofen and this may change mean fluorescence intensity per pixel, total fluorescence intensity (mean fluorescence intensity/pixel x pixels/cell) was determine for (H) active RAC1/CDC42 (via PGD-GST binding) (Control n=243 cells, ibuprofen n=205 cells, both 4 biological replicates, and (P) active RHOA (via RBD-GST binding) (Control n =144 cells, ibuprofen n=136 cells, both 4 biological replicates) per cell. *P<0.001, Mann-Whitney Rank Sum. These data indicate that ibuprofen reduced total cellular levels of F-actin and active RAC1/CDC42 without affecting active RHOA levels. Only ENCDC furthest from the gut slice edge were analyzed.
ROCK inhibition rescues ibuprofen effects on ENCDC migration in vitro
RAC1 and RHOA are regulated in complex ways as they control actin cytoskeleton to alter cell morphology (Guilluy et al., 2011; Hall, 2005; Parri and Chiarugi, 2010). We hypothesized that if ibuprofen slowed ENCDC migration and reduced lamellipodia via reduced RAC1 activation, it might be possible to rescue ibuprofen’s effects by inhibiting ROCK, a RHOA effector kinase that inhibits RAC1. To test this hypothesis, CF-1 midgut slices were cultured with the ROCK inhibitor Y-27632 (Figure 10). This drug increased migration of ibuprofen-treated ENCDC to the level seen in control cells, but did not affect ENCDC migration in the absence of ibuprofen (Figure 10M). These data suggest that excess RHOA/ROCK activity relative to RAC1/CDC42 may underlie ibuprofen’s effect on ENCDC migration (Figure 11).
Figure 10. ROCK inhibition rescued ibuprofen effects on murine ENCDC migration.
(A–L) E12.5 CF-1 midgut slices were cultured with or without 250μM ibuprofen four hours before GDNF and 5 μM Y-27632 were added. Sixteen hours later cultures were fixed and stained for (A, D, G, J) RET (red), (B, E, H, K) phalloidin (green), and (C, F, I, L) DAPI (blue; merged image). Scale bar = 50 μm. (M) Distance from the gut slice edge to the most distant RET+ ENCDC was measured. Y-27632 increased migration of ibuprofen treated ENCDC, but not control ENCDC (Control n =43 slices, ibuprofen n=48 slices, Y-27632 n=54 slices, ibuprofen + Y-27632 n=59 slices, all from 7 biological replicates). *P<0.05 compared to control, #P<0.05 compared to ibuprofen + Y-27632 (ANOVA on ranks).
Figure 11. Model.

RAC1 organizes the actin cytoskeleton to form lamellipodia and support migration. Ibuprofen reduced ENCDC RAC1 activation. ROCK is a RHOA effector that inhibits RAC1. Y-27632 inhibits ROCK to permit RAC1 activation, enhancing ENCDC migration.
Discussion
Despite surgical treatment available for Hirschsprung disease since 1948 (Swenson and Bill, 1948), approximately 5% of children with HSCR die at an early age (Rescorla et al., 1992; Suita et al., 2005) and > 40% have problems after surgery (El-Sawaf et al., 2013; Menezes et al., 2008). It would therefore be ideal if HSCR could be prevented from occurring in the first place. We now believe this may be possible in some cases by optimizing maternal nutrition, health, and medication use before conception and during early pregnancy. If non-genetic risk factors were identified, targeted advice could be provided to families at “high genetic risk” for HSCR. From this standpoint it is particularly valuable to identify common and avoidable exposures.
Using zebrafish to test medicines commonly used by pregnant women we discovered that ibuprofen causes HSCR-like absence of neurons in developing fish bowel in vivo. Studies in mice also show ibuprofen can slow colonization of fetal bowel in vivo. Most dramatically, ibuprofen treatment of fetal chick bowel led to almost complete absence of colon colonization by ENCDC. Detailed analysis of actively migrating mouse ENCDC confirmed ibuprofen slowed migration, reduced lamellipodia, reduced filamentous actin, and reduced RAC1 activation. These in vitro and in vivo data suggest ibuprofen use during the period that ENCDC colonize fetal bowel (e.g. week 3–8 of human gestation) might increase HSCR risk.
Human studies are now needed to extend this work. Although ibuprofen reduced ENCDC colonization of fetal bowel in three species, the dose needed and severity of inhibitory effects differed between species. The reason for interspecies and interstrain differences is not known, but may reflect differences in drug metabolism, the presence of alternative migration modes, target sensitivity, or intracellular signaling. One valuable approach will be human HSCR epidemiologic studies examining maternal medication use in early pregnancy.
To put the mouse in vivo studies in context, Ret+/− mice fed 375 ppm ibuprofen in chow had a 12% reduction in ENCDC colonization of colon at E12.5. This dose was selected because it suppresses COX-dependent inflammation (Lim et al., 2000; Ritschel and Kerns, 2009) and gives serum levels (60–70 μM) (Morihara et al., 2005) within the human therapeutic dosing range (Ritschel and Kerns, 2009). Because ibuprofen use in early pregnancy is common (almost 1 in 4) (Thorpe et al., 2013) and HSCR relatively rare (about 1:5000) (Amiel et al., 2008), ibuprofen alone is unlikely to cause HSCR in humans even at higher doses. Ibuprofen might, however, increase HSCR occurrence in the context of underlying genetic risk. This is important because known genetic causes for HSCR are partially penetrant suggesting gene-gene or gene-environment interactions cause HSCR. This was demonstrated dramatically in mice by the observation that Ret+/− mice never have distal bowel aganglionosis (McCallion et al., 2003), but develop HSCR-like disease more readily than WT when other mutations or non-genetic risk factors are present (Arnold et al., 2009; Carrasquillo et al., 2002; Fu et al., 2010; Gunadi et al., 2014; Lake et al., 2013; McCallion et al., 2003; Phusantisampan et al., 2012; Wallace and Anderson, 2011). In contrast to mice, humans with a single inactive RET allele (i.e., ~30% of HSCR cases) have about a 50% chance of having HSCR (Amiel et al., 2008). The vast majority of these children have only a small region of distal aganglionic bowel, suggesting that even small changes in bowel colonization efficiency by ENCDC could have an important effect on HSCR occurrence. For example, in mice, male sex only slightly reduces bowel colonization by ENCDC similar to the effect of ibuprofen (Vohra et al., 2007b), but in humans the male sex increases HSCR occurrence four-fold.
From the standpoint of human teratogenicity, it may be important that ibuprofen effects on ENCDC appear likely to be COX-independent. COX (PTGS) enzymes that make prostaglandins (Vane, 1971) are the primary therapeutic target of ibuprofen and other NSAIDs, but Ptgs1−/− Ptgs2−/− mice had normal bowel colonization by ENCDC and normal ENCDC migration in vitro. Furthermore, prolonged ibuprofen exposure and high doses were needed to slow migration. NSAIDs should inhibit COX enzymes much faster (with effects apparent within 10 minutes) and at low micromolar doses (IC50 = 2.1 μM for PTGS1 and 1.6 μM for PTGS2) (Peppelenbosch et al., 1993; Tegeder et al., 2001). One caveat is that our Ptgs mutant mice were C57BL/6 genetic background, which appears more resistant to ibuprofen effects than CF-1.
The precise mechanisms through which ibuprofen inhibits ENCDC migration remain uncertain, but our observations suggest ibuprofen treated ENCDC have abnormal regulation of actin dynamics. Reduced neurite growth in ibuprofen treated ENCDC was not anticipated since ibuprofen increased neurite growth in several other neuron types (Fu et al., 2007; Wang et al., 2009). We hypothesize this cell-type specific difference occurs because unlike other neurons tested, ENCDC have low levels of PPARγ that is required for ibuprofen to reduce RHOA activity and enhance neurite growth (Dill et al., 2010). The lack of ibuprofen effect on NIH 3T3 or CF-1 gut mesenchymal cell migration and lamellipodia further highlights how cell-type specific gene expression impacts biology and emphasizes that extrapolation from one cell type to another may be misleading.
Our studies suggest ibuprofen reduces active RAC1/CDC42 in migrating ENCDC. This fits with emerging literature on the role of small RhoGTPases in neural crest-derived cell migration. Elegant in vivo FRET studies showed RAC1 promotes ENCDC chain migration through fetal bowel (Goto et al., 2013). Reduced RAC1 activity in ibuprofen treated ENCDC provides a reasonable explanation for reduced migration and fewer lamellipodia. The ability of ROCK inhibition to prevent ibuprofen induced changes in migration and lamellipodia, without any apparent alteration of RHOA activity, may occur because RAC1 and RHOA usually inhibit each other and ROCK is a RHOA effector kinase (Figure 9) (Nakayama et al., 2008; Sordella and Van Aelst, 2008).
Conclusions
These data demonstrate that ibuprofen, a medication commonly used in the first trimester of pregnancy, inhibits ENCDC migration and might increase HSCR risk in genetically susceptible individuals. This study extends our previous observations demonstrating environmental risk factors like vitamin A deficiency (Fu et al., 2010) and mycophenolate (Lake et al., 2013) can cause HSCR-like disease in mice, especially when combined with predisposing genetic changes. Human epidemiological studies are necessary to determine the extent to which ibuprofen contributes to HSCR risk.
Supplementary Material
Supplemental Movies 1–3. Time lapse images of migrating ENCDC. E12.5 CF-1 midgut slice explants were cultured for 4 hours before GDNF addition. Time lapse imaging began when GDNF was added and continued for 4 hours. DIC images include a time stamp in “hours:minutes” in the upper left hand corner.
Movie 1: No added ibuprofen.
Movie 2: Ibuprofen added just before imaging began.
Movie 3: Ibuprofen added 4 hours before imaging began.
E12.5 CF-1 mouse midgut slices were cultured for 16 hours on fibronectin coated dishes in media with GDNF. ENCDC migrate from gut slices onto the culture dish under these conditions. For analyses where we report the “Distance migrated” by ENCDC from the slice, the slice area was divided into octants (dotted lines) and the distance from the gut slice edge to the most distant ENCDC (RET immunoreactive cell, red) was measured for each octant. Cell morphology was also assessed in “Distal ENCDC” (i.e., the RET+ cells that had migrated furthest from the slice edge). Mesenchymal cells were identified by their lack of RET immunoreactivity and their actin-rich cytoskeleton (stained green with Alexa488-phalloidin). Phalloidin is a fungal bicyclic heptapeptide that binds filamentous, but not G-actin (Govindan et al., 1972). The distance migrated by mesenchymal cells was determined by measuring the distance from the gut slice edge to the most distant RET negative cell with distinctive intense phalloidin staining.
NIH 3T3 were serum starved and then either (A, B) left unstimulated, (C) treated with RAC1/CDC42 Activator II to activate RAC1 and CDC42, or (D) treated with RHO Activator I to activate RHOA. Cells were fixed and treated with either PBD-GST (A, C) or RBD-GST (B, D) as in Figure 9. All cells were then stained with antibodies to GST to visualize active RAC1/CDC42 or RHOA. Stimulated cells had greater GST fluorescence compared to unstimulated cells for PBD-GST and RBD-GST fusion proteins as expected after stimulation. Scale bar = 20 μm.
Highlights.
Ibuprofen inhibits ENS precursor migration in zebrafish, chick and mouse.
Ibuprofen reduces lamellipodia and F-actin in cultured murine ENS precursors.
Ibuprofen reduces RAC1 activation in migrating ENS precursors.
Ibuprofen effects on ENS precursor migration appear to be COX independent.
Ibuprofen is commonly used in early pregnancy and might increase Hirschsprung risk.
Acknowledgments
We thank Dr. Tatyana Svitkina for insightful guidance about the actin cytoskeleton, Dr. Allen Mitchell for sharing unpublished data about medicine use in early pregnancy, Ryo Hotta, Ming Fu, Elizabeth Wright-Jin, Rajarshi Sengupta, and Alisha Jamil, and the Mouse Genetics Core at Washington University School of Medicine for assistance and advice. This work was supported by Irma and Norman Braman Endowment (ROH), Suzi and Scott Lustgarten Center Endowment (ROH), The Children’s Hospital of Philadelphia Research Institute (ROH), The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (grant nos. CH-II-1008-123, CH-II-2010-390, MD-II-2013-269) (ROH), NIH grants RO1 DK087715 (ROH), R01 GM059688 (SLJ), R37 DK33165 (WFS), RO1 DK080914 (AMG), Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (grant no. 1008525) (ROH), NIH F30 DK100101 (EMS) and by the NIH Medical Scientist Training Program Training Grant T32 GM07200.
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
Author Contributions
EMS, JIL, NN, WFS, SLJ, AMG, and ROH designed experiments. EMS, OAT, NN, SKB, MA, and LF collected data. EMS, OAT, AMG, NN, SKB, and ROH analyzed data. EMS and ROH wrote the manuscript. All authors reviewed and edited.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Aluri JB, Stavchansky S. Determination of guaifenesin in human plasma by liquid chromatography in the presence of pseudoephedrine. J Pharm Biomed Anal. 1993;11:803–808. doi: 10.1016/0731-7085(93)80072-9. [DOI] [PubMed] [Google Scholar]
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- Arnold S, Pelet A, Amiel J, Borrego S, Hofstra R, Tam P, Ceccherini I, Lyonnet S, Sherman S, Chakravarti A. Interaction between a chromosome 10 RET enhancer and chromosome 21 in the Down syndrome-Hirschsprung disease association. Hum Mutat. 2009;30:771–775. doi: 10.1002/humu.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. Biomedical Publications; Seal Beach, CA, U.S.A: 1982. [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan B, Cai Y, Wrighton K, Wu G, Feng XH, Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005;579:1015–1019. doi: 10.1016/j.febslet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, McCallion AS, Puffenberger EG, Kashuk CS, Nouri N, Chakravarti A. Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet. 2002;32:237–244. doi: 10.1038/ng998. [DOI] [PubMed] [Google Scholar]
- Dasgupta R, Langer JC. Hirschsprung disease. Curr Probl Surg. 2004;41:942–988. doi: 10.1067/j.cpsurg.2004.09.004. [DOI] [PubMed] [Google Scholar]
- de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30:47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci. 2010;30:963–972. doi: 10.1523/JNEUROSCI.5045-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sawaf M, Siddiqui S, Mahmoud M, Drongowski R, Teitelbaum DH. Probiotic prophylaxis after pullthrough for Hirschsprung disease to reduce incidence of enterocolitis: A prospective, randomized, double-blind, placebo-controlled, multicenter trial. J Pediatr Surg. 2013;48:111–117. doi: 10.1016/j.jpedsurg.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Ferri N, Panariti F, Ricci C, Maiocchi G, Corsini A. Aliskiren inhibits prorenininduced human aortic smooth muscle cell migration. Journal of the renin-angiotensinaldosterone system: JRAAS. 2014 doi: 10.1177/1470320314528364. [DOI] [PubMed] [Google Scholar]
- Fu M, Landraville S, Agapova OA, Wiley LA, Shoykhet M, Harbour JW, Heuckeroth RO. Retinoblastoma protein loss in the enteric nervous system causes selective defects and early death. The Journal of Clinical Investigation. 2013 doi: 10.1172/JCI67653. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Sato Y, Lyons-Warren A, Zhang B, Kane MA, Napoli JL, Heuckeroth RO. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development. 2010;137:631–640. doi: 10.1242/dev.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Tam PK, Sham MH, Lui VC. Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol (Berl) 2004;208:33–41. doi: 10.1007/s00429-003-0371-0. [DOI] [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Ghobadi C, Mirhosseini N, Shiran MR, Moghadamnia A, Lennard MS, Ledger WL, Rostami-Hodjegan A. Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J Clin Pharmacol. 2009;49:147–154. doi: 10.1177/0091270008328096. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Hofstra RM, Burns AJ. Building a brain in the gut: development of the enteric nervous system. Clin Genet. 2013;83:307–316. doi: 10.1111/cge.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A, Sumiyama K, Kamioka Y, Nakasyo E, Ito K, Iwasaki M, Enomoto H, Matsuda M. GDNF and endothelin 3 regulate migration of enteric neural crest-derived cells via protein kinase A and Rac1. J Neurosci. 2013;33:4901–4912. doi: 10.1523/JNEUROSCI.4828-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan VM, Faulstich H, Wieland T, Agostini B, Hasselbach W. In-vitro effect of phalloidin on plasma membrane preparation from rat liver. Die Naturwissenschaften. 1972;59:521–522. doi: 10.1007/BF00609837. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunadi Kapoor A, Ling AY, Rochadi Makhmudi A, Herini ES, Sosa MX, Chatterjee S, Chakravarti A. Effects of RET and NRG1 polymorphisms in Indonesian patients with Hirschsprung disease. J Pediatr Surg. 2014;49:1614–1618. doi: 10.1016/j.jpedsurg.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO. Hirschsprung disease. In: Faure C, DiLorenzo C, Thapar N, editors. Pediatric neurogastroenterology: gastrointestinal motility and functional disorders in children. Springer; New York: 2013. pp. 271–283. [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EMJ, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Developmental Biology. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Hilbert J, Moritzen V, Parks A, Radwanski E, Perentesis G, Symchowicz S, Zampaglione N. The pharmacokinetics of loratadine in normal geriatric volunteers. The Journal of international medical research. 1988;16:50–60. doi: 10.1177/030006058801600106. [DOI] [PubMed] [Google Scholar]
- Hirschsprung H. Stuhlträgheit Neugeborener in Folge von Dilatation und Hypertrophie des Colons. Jahrbuch für Kinderheilkunde und physische Erziehung (Berlin) 1888;27:1–7. [Google Scholar]
- Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev Biol. 2000;227:146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- Kapur RP, Yost C, Palmiter RD. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116:167–175. doi: 10.1242/dev.116.Supplement.167. [DOI] [PubMed] [Google Scholar]
- Koh CG. Rho GTPases and their regulators in neuronal functions and development. Neurosignals. 2006;15:228–237. doi: 10.1159/000101527. [DOI] [PubMed] [Google Scholar]
- Kuhlman J, Eisen JS. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev Dyn. 2007;236:118–127. doi: 10.1002/dvdy.21033. [DOI] [PubMed] [Google Scholar]
- Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1–24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Tusheva OA, Graham BL, Heuckeroth RO. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J Clin Invest. 2013;123:4875–4887. doi: 10.1172/JCI69781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley TA, Shah SN, Ruggiero EA. Ethanol alters BDNF-induced Rho GTPase activation in axonal growth cones. Alcohol Clin Exp Res. 2011;35:1321–1330. doi: 10.1111/j.1530-0277.2011.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion AS, Stames E, Conlon RA, Chakravarti A. Phenotype variation in twolocus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci U S A. 2003;100:1826–1831. doi: 10.1073/pnas.0337540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley interdisciplinary reviews. Developmental biology. 2013;2:113–129. doi: 10.1002/wdev.57. [DOI] [PubMed] [Google Scholar]
- Meijering E, Dzyubachyk O, Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- Menezes M, Pini Prato A, Jasonni V, Puri P. Long-term clinical outcome in patients with total colonic aganglionosis: a 31-year review. J Pediatr Surg. 2008;43:1696–1699. doi: 10.1016/j.jpedsurg.2008.01.072. [DOI] [PubMed] [Google Scholar]
- Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, Frautschy SA, Cole GM. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer’s models. Neuropsychopharmacology. 2005;30:1111–1120. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- Murphey RD, Zon LI. Small molecule screening in the zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Nagy N, Burns AJ, Goldstein AM. Immunophenotypic characterization of enteric neural crest cells in the developing avian colorectum. Dev Dyn. 2012;241:842–851. doi: 10.1002/dvdy.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nini L, Dagnino L. Accurate and reproducible measurements of RhoA activation in small samples of primary cells. Anal Biochem. 2010;398:135–137. doi: 10.1016/j.ab.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch MP, Tertoolen LG, Hage WJ, de Laat SW. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- Phusantisampan T, Sangkhathat S, Phongdara A, Chiengkriwate P, Patrapinyokul S, Mahasirimongkol S. Association of genetic polymorphisms in the RETprotooncogene and NRG1 with Hirschsprung disease in Thai patients. J Hum Genet. 2012;57:286–293. doi: 10.1038/jhg.2012.18. [DOI] [PubMed] [Google Scholar]
- Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–1465. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla FJ, Morrison AM, Engles D, West KW, Grosfeld JL. Hirschsprung’s disease. Evaluation of mortality and long-term function in 260 cases. Arch Surg. 1992;127:934–941. doi: 10.1001/archsurg.1992.01420080068011. discussion 941–932. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Ritschel WA, Kerns GL. Handbook of basic pharmacokinetics... including clinical applications. 7. American Pharmacists Association; Washington, D.C: 2009. [Google Scholar]
- Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Sato Y, Heuckeroth RO. Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro. Dev Biol. 2008;320:185–198. doi: 10.1016/j.ydbio.2008.05.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Skinner M. Hirschsprung’s Disease. Curr Probl Surg. 1996;33:391–461. doi: 10.1016/s0011-3840(96)80009-8. [DOI] [PubMed] [Google Scholar]
- Sordella R, Van Aelst L. Dialogue between RhoA/ROCK and members of the Par complex in cell polarity. Dev Cell. 2008;14:150–152. doi: 10.1016/j.devcel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Young HM, Popoff M, Anderson RB. Effects of pharmacological inhibition of small GTPases on axon extension and migration of enteric neural crestderived cells. Dev Biol. 2007;307:92–104. doi: 10.1016/j.ydbio.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Suita S, Taguchi T, Ieiri S, Nakatsuji T. Hirschsprung’s disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. J Pediatr Surg. 2005;40:197–201. doi: 10.1016/j.jpedsurg.2004.09.052. discussion 201–192. [DOI] [PubMed] [Google Scholar]
- Swenson O, Bill AH., Jr Resection of rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolon; an experimental study. Surgery. 1948;24:212–220. [PubMed] [Google Scholar]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA National Birth Defects Prevention S. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22:1013–1018. doi: 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirinlike drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Fu M, Heuckeroth RO. Protein kinase Czeta and glycogen synthase kinase-3beta control neuronal polarity in developing rodent enteric neurons, whereas SMAD specific E3 ubiquitin protein ligase 1 promotes neurite growth but does not influence polarity. J Neurosci. 2007a;27:9458–9468. doi: 10.1523/JNEUROSCI.0870-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BP, Planer W, Armon J, Fu M, Jain S, Heuckeroth RO. Reduced endothelin converting enzyme-1 and endothelin-3 mRNA in the developing bowel of male mice may increase expressivity and penetrance of Hirschsprung disease-like distal intestinal aganglionosis. Dev Dyn. 2007b;236:106–117. doi: 10.1002/dvdy.21028. [DOI] [PubMed] [Google Scholar]
- Wallace AS, Anderson RB. Genetic interactions and modifier genes in Hirschsprung’s disease. World J Gastroenterol. 2011;17:4937–4944. doi: 10.3748/wjg.v17.i45.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hughes I, Planer W, Parsadanian A, Grider JR, Vohra BP, Keller-Peck C, Heuckeroth RO. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. J Neurosci. 2010;30:1523–1538. doi: 10.1523/JNEUROSCI.3861-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Wang X, Budel S, Baughman K, Gould G, Song KH, Strittmatter SM. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma. 2009;26:81–95. doi: 10.1089/neu.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chan AK, Sham MH, Burns AJ, Chan WY. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141:992–1002. e1001–1006. doi: 10.1053/j.gastro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movies 1–3. Time lapse images of migrating ENCDC. E12.5 CF-1 midgut slice explants were cultured for 4 hours before GDNF addition. Time lapse imaging began when GDNF was added and continued for 4 hours. DIC images include a time stamp in “hours:minutes” in the upper left hand corner.
Movie 1: No added ibuprofen.
Movie 2: Ibuprofen added just before imaging began.
Movie 3: Ibuprofen added 4 hours before imaging began.
E12.5 CF-1 mouse midgut slices were cultured for 16 hours on fibronectin coated dishes in media with GDNF. ENCDC migrate from gut slices onto the culture dish under these conditions. For analyses where we report the “Distance migrated” by ENCDC from the slice, the slice area was divided into octants (dotted lines) and the distance from the gut slice edge to the most distant ENCDC (RET immunoreactive cell, red) was measured for each octant. Cell morphology was also assessed in “Distal ENCDC” (i.e., the RET+ cells that had migrated furthest from the slice edge). Mesenchymal cells were identified by their lack of RET immunoreactivity and their actin-rich cytoskeleton (stained green with Alexa488-phalloidin). Phalloidin is a fungal bicyclic heptapeptide that binds filamentous, but not G-actin (Govindan et al., 1972). The distance migrated by mesenchymal cells was determined by measuring the distance from the gut slice edge to the most distant RET negative cell with distinctive intense phalloidin staining.
NIH 3T3 were serum starved and then either (A, B) left unstimulated, (C) treated with RAC1/CDC42 Activator II to activate RAC1 and CDC42, or (D) treated with RHO Activator I to activate RHOA. Cells were fixed and treated with either PBD-GST (A, C) or RBD-GST (B, D) as in Figure 9. All cells were then stained with antibodies to GST to visualize active RAC1/CDC42 or RHOA. Stimulated cells had greater GST fluorescence compared to unstimulated cells for PBD-GST and RBD-GST fusion proteins as expected after stimulation. Scale bar = 20 μm.