Abstract
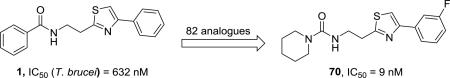
2-(2-Benzamido)ethyl-4-phenylthiazole (1) was one of 1035 molecules (grouped into 115 distinct scaffolds) found to be inhibitory to Trypanosoma brucei, the pathogen causing human African trypanosomiasis, at concentrations below 3.6 μM and non-toxic to mammalian (Huh7) cells in a phenotypic high-throughput screen of a 700,000 compound library performed by the Genomics Institute of the Novartis Research Foundation (GNF). Compound 1 and 72 analogues were synthesized in this lab by one of two general pathways. These plus 10 commercially available analogues were tested against T. brucei rhodesiense STIB900 and L6 rat myoblast cells (for cytotoxicity) in vitro. Forty-four derivatives were more potent than 1, including eight with IC50 values below 100 nM. The most potent and most selective for the parasite was the urea analogue 2-(2-piperidin-1-ylamido)ethyl-4-(3-fluorophenyl)thiazole (70, IC50 = 9 nM, SI > 18,000). None of 33 compounds tested were able to cure mice infected with the parasite; however, six compounds caused temporary reductions of parasitemia (≥97%) but with subsequent relapses. The lack of in vivo efficacy was at least partially due to their poor metabolic stability, as demonstrated by the short half-lives of 15 analogues against mouse and human liver microsomes.
Keywords: Thiazole, Amide, Urea, Antitrypanosomal, Metabolic stability
1. Introduction
Human African trypanosomiasis (HAT) occurs in 36 nations of sub-Saharan Africa. In 2015 the World Health Organization (WHO) estimated 20,000 actual cases with 65 million people at risk. Transmitted by tsetse flies, the disease is due to a chronic infection of Trypanosoma brucei gambiense (in western and Central Africa, over 98% of reported cases) or an acute infection of Trypanosoma brucei rhodesiense (in southern and eastern Africa). The T. b. gambiense infection is characterized by a slow progression from early (hemolymphatic) stage—where many patients are asymptomatic—to late stage disease, after the parasites have entered the central nervous system (CNS). The T. b. rhodesiense infection is characterized by earlier onset of symptoms and a more rapid progression from early to late stage. In either case, late stage HAT is always fatal if untreated.1,2
The need for new anti-HAT drugs continues to persist, as current drugs are few, antiquated, toxic, prone to resistance, and require parenteral administration. Treatments for T. b. rhodesiense infections are limited to suramin (a polysulfonated naphthylurea) for early stage and melarsoprol (an organoarsenical) for late stage disease. First line treatments for T. b. gambiense infections include pentamidine (an aromatic diamidine) for early stage and nifurtimox-eflornithine combination therapy (NECT) for late stage disease.1-4
This laboratory has prepared a large number of aromatic diamidines that were assayed against the trypanosome. Among the most promising were the 3,5-bis(amidinoaryl)isoxazoles5,6 and the 3,3-bis(amidinoaryl)benzenes,7,8 which are all analogues of 2,5-bis(4-amidinophenyl)furan (furamidine)9,10 having different central rings. After the failure of pafuramidine11,12 (an orally active prodrug of furamidine) in clinical trials against early stage HAT,13 our attention has shifted to non-amidine treatments of the disease. Two promising non-amidine anti-HAT drug candidates, the nitrated imidazole derivative fexninidzole14 and the benzoxaborole SCYX,15 have entered clinical trials in recent years. A phenotypic high-throughput screen of a 700,000 compound library performed by the Genomics Institute of the Novartis Research Foundation (GNF) led to the identification of 1035 compounds that inhibited growth of T. brucei in vitro at concentrations below 3.6 μM and were non-toxic to mammalian cells (Huh7). The 1035 hits could be grouped into 115 distinct scaffolds. These scaffolds was further refined based in part on their relative ease of synthesis, a lack of chiral carbon atoms, and druglike features including low molecular weight and adherence to Lipinski's rules. Work toward optimization of one of these scaffolds in the lab of a collaborator has been reported.16
2. Chemistry
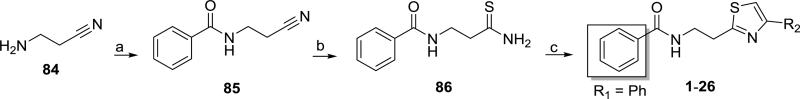
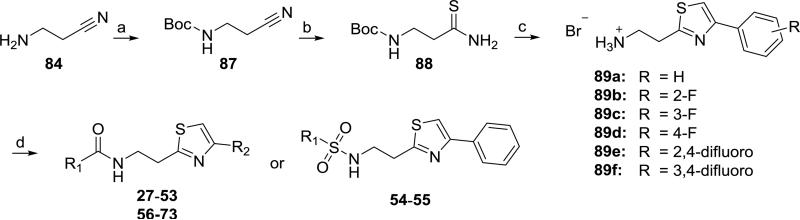
The lead compound, 2-(2-benzamido)ethyl-4-phenylthiazole (1, Table 1) was one of several other scaffolds from the GNF library selected for optimization in this laboratory or those of our collaborators. The syntheses of more than 70 analogues are described herein. Analogues 2–73 have modifications of either the 4-thiazolyl aromatic ring (R2, 2-26, Table 1), the carbonyl substituent (R1) or the carbonyl group itself (27–55, Table 2), or at both R1 and R2 (56–73, Table 3). The commercially available 4-alkyl-2-arylthiazoles 74–83 (Table 4) are regioisomers of 10 synthesized compounds with reversed substitution patterns on the thiazole rings.
Table 1.
Structures, antitrypanosomal activities, and selectivities of thiazole derivatives 1-26
| ||||
|---|---|---|---|---|
| Compd | R2 | T. b. rhodesiense a | Cytotox.b | |
| IC50c (μM) | SId | IC50c (μM) | ||
| 1 | phenyl | 0.632 | 162 | 103 |
| 2 | 3-cyanophenyl | 1.90 | 69 | 131 |
| 3 | 4-cyanophenyl | 42.7 | 1 | 44.1 |
| 4 | 4-(trifluoromethyl)phenyl | 145 | <1 | 24.6 |
| 5 | 3-nitrophenyl | 2.72 | 47 | 127 |
| 6 | 4-nitrophenyl | 29.3 | >9 | >255 |
| 7 | 3-methoxyphenyl | 6.11 | 11 | 69.2 |
| 8 | 4-methoxyphenyl | 18.3 | 5 | 99.7 |
| 9 | 2-fluorophenyl | 0.156 | 624 | 97.2 |
| 10 | 3-fluorophenyl | 0.233 | 239 | 55.8 |
| 11 | 4-fluorophenyl | 0.218 | 1130 | 247 |
| 12 | 3-chlorophenyl | 0.452 | 125 | 56.4 |
| 13 | 4-chlorophenyl | 13.3 | 7 | 95.9 |
| 14 | 3-bromophenyl | 0.553 | 94 | 51.9 |
| 15 | 4-bromophenyl | 34.7 | 1 | 46.3 |
| 16 | 2,4-difluorophenyl | 0.162 | >1590 | >257 |
| 17 | 2,5-difluorophenyl | 1.63 | 40 | 65.3 |
| 18 | 2,6-difluorophenyl | 1.22 | 133 | 162 |
| 19 | 3,4-difluorophenyl | 0.145 | 211 | 30.6 |
| 20 | 3,5-difluorophenyl | 1.62 | 36 | 58.1 |
| 21 | 2,4,5-trifluorophenyl | 2.13 | 116 | 248 |
| 22 | 2-furanyl | 2.53 | 53 | 134 |
| 23 | 2-thiophenyl | 1.62 | 63 | 102 |
| 24 | 2-pyridyl | 3.63 | 57 | 209 |
| 25 | 3-pyridyl | 4.64 | 45 | 207 |
| 26 | 4-pyridyl | 10.3 | 21 | 217 |
| pentamidine | 2.8 | 11400 | 31.8 | |
| melarsoprol | 4.0 | 1280 | 5.12 | |
| podophyllotoxin | 0.017 | |||
Trypanosoma brucei rhodesiense (STIB900)23
Cytotoxicity to L6 rat myoblast cells23
The IC50 values are the mean of two independent assays. Coefficients of variation were less than 50%.
Selectivity index for T. b. rhodesiense expressed as the ratio IC50 (L6 cells) / IC50 (T. b. rhodesiense). Values are rounded to the nearest integer or to the third significant figure.
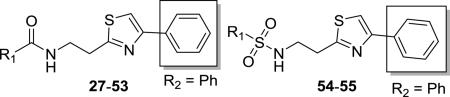
Table 2.
Structures, antitrypanosomal activities, and selectivities of thiazole derivatives 27-55a
| ||||
|---|---|---|---|---|
| Compd | R1 | T. b. rhodesiense | Cytotoxb | |
| IC50 (μM) | SI | IC50 (μM) | ||
| 1 | phenyl | 0.632 | 162 | 69.0 |
| 27 | 3-cyanophenyl | 0.171 | 404 | 58.0 |
| 28 | 4-cyanophenyl | 40.6 | 1 | 42.4 |
| 29 | 3-nitrophenyl | 0.150 | 283 | 79.0 |
| 30 | 2-fluorophenyl | 0.460 | 172 | 77.8 |
| 31 | 3-fluorophenyl | 0.383 | 203 | 35.4 |
| 32 | 4-fluorophenyl | 1.42 | 25 | 93.0 |
| 33 | 2-chlorophenyl | 0.483 | 193 | 47.8 |
| 34 | 3-chlorophenyl | 1.50 | 32 | >237 |
| 35 | 4-chlorophenyl | 34.5 | >7 | 42.5 |
| 36 | 2-pyrrolyl | 2.01 | 21 | 87.7 |
| 37 | 2-furanyl | 0.482 | 182 | 71.2 |
| 38 | 2-thiophenyl | 0.255 | 280 | 49.1 |
| 39 | 3-thiophenyl | 0.190 | 259 | 120 |
| 40 | 3-pyridyl | 1.59 | 75 | 60.1 |
| 41 | 4-pyridyl | 16.5 | 4 | 174 |
| 42 | cyclopentyl | 0.268 | 648 | 205 |
| 43 | cyclohexyl | 0.164 | 1250 | 244 |
| 44 | N-pyrrolidinyl | 0.125 | 1960 | 62.8 |
| 45 | N-pyrrolyl | 1.74 | 36 | 243 |
| 46 | N-piperidinyl | 0.0204 | 11900 | >248 |
| 47 | N-morpholinyl | 0.510 | >486 | 229 |
| 48 | N-azepanyl | 0.0516 | 4450 | 98.9 |
| 49 | tert-butylamino | 1.83 | 54 | >267 |
| 50 | benzylamino | 90.1 | >3 | 91.1 |
| 51 | cyclohexylamino | 10.1 | 9 | >278 |
| 52 | phenylamino | >309 | nde | 183 |
| 53 | phenoxy | 61.8 | 3 | 89.4 |
| 54 | phenyl | 75.8 | 1 | 69.3 |
| 55 | 4-methylphenyl | 52.9 | 1 | 69.0 |
Experimental parameters and controls are shown at the bottom of Table 1.
Not determinable.
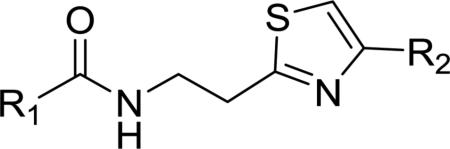
Table 3.
Structures, antitrypanosomal activities, and selectivities of thiazole derivatives 54-73a
| |||||
|---|---|---|---|---|---|
| Compd | R1 | R2 | T. b. rhodesiense a | Cytotox.b | |
| IC50c (nM) | SId | IC50c (μM) | |||
| 56 | 3-cyanophenyl | 2-fluorophenyl | 0.393 | 473 | 186 |
| 57 | 3-cyanophenyl | 3-fluorophenyl | 0.104 | 302 | 31.3 |
| 58 | 3-cyanophenyl | 4-fluorophenyl | 0.130 | 303 | 39.2 |
| 59 | 3-nitrophenyl | 2-fluorophenyl | 0.133 | 252 | 33.5 |
| 60 | 3-nitrophenyl | 3-fluorophenyl | 0.129 | 176 | 22.7 |
| 61 | 3-nitrophenyl | 4-fluorophenyl | 0.147 | 120 | 17.7 |
| 62 | 3-fluorophenyl | 3-fluorophenyl | 0.325 | 142 | 46.2 |
| 63 | 3-fluorophenyl | 4-fluorophenyl | 0.340 | 124 | 42.1 |
| 64 | 2-thiophenyl | 2-fluorophenyl | 0.177 | 230 | 40.8 |
| 65 | 2-thiophenyl | 3-fluorophenyl | 0.149 | 326 | 48.6 |
| 66 | 2-thiophenyl | 4-fluorophenyl | 0.177 | 189 | 33.6 |
| 67 | N-pyrrolidinyl | 2-fluorophenyl | 0.0495 | 4790 | 237 |
| 68 | N-pyrrolidinyl | 3-fluorophenyl | 0.0517 | 3370 | 174 |
| 69 | N-pyrrolidinyl | 4-fluorophenyl | 0.316 | 474 | 150 |
| 70 | N-piperidinyl | 3-fluorophenyl | 0.0090 | 18800 | 169 |
| 71 | N-piperidinyl | 4-fluorophenyl | 0.0120 | 10200 | 123 |
| 72 | N-piperidinyl | 2,4-difluorophenyl | 0.0099 | 9960 | 98.2 |
| 73 | N-piperidinyl | 3,4- difluorophenyl | 0.0097 | 11700 | 114 |
Experimental parameters and controls are shown at the bottom of Table 1.
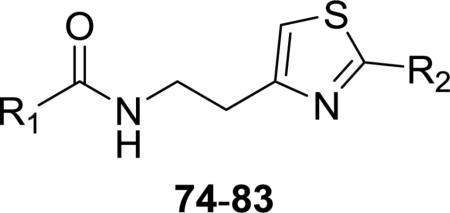
Table 4.
Comparison of thiazole geometry to antitrypanosomal activitya
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Compd | T. b. rhodesiense | Compd | R1 | R2 | T. b. rhodesiense | Cytotox. | ||
| IC50 (μM)b | SIb | IC50 (μM) | SI | IC50 (μM) | ||||
| 10 | 0.233 | 239 | 74 | phenyl | 3-fluorophenyl | 0.201 | 325 | 65.3 |
| 11 | 0.218 | 1133 | 75 | phenyl | 4-fluorophenyl | 0.165 | 261 | 43.2 |
| 13 | 13.3 | 7 | 76 | phenyl | 4-chlorophenyl | 11.4 | >23 | >263 |
| 30 | 0.460 | 172 | 77 | 2-fluorophenyl | phenyl | 0.536 | 130 | 69.6 |
| 31 | 0.383 | 203 | 78 | 3-fluorophenyl | phenyl | 0.843 | 40 | 33.5 |
| 33 | 0.483 | 193 | 79 | 2-chlorophenyl | phenyl | 1.52 | 61 | 92.8 |
| 34 | 1.50 | 32 | 80 | 3-chlorophenyl | phenyl | 0.642 | 99 | 63.3 |
| 35 | 34.5 | >7 | 81 | 4-chlorophenyl | phenyl | 30.6 | 1 | 30.1 |
| 38 | 0.255 | 280 | 82 | 2-thiophenyl | phenyl | 0.223 | 427 | 95.1 |
| 43 | 0.164 | 1254 | 83 | cyclohexyl | phenyl | 0.192 | 1050 | 202 |
The synthesis of 1, based upon that of its [14C] isotopomer,17 is depicted in Scheme 1. The benzoylation of 3-aminopropionitrile (84) gave the cyano-amide 85,18 which was converted to thioamide 8618 using sodium hydrosulfide and magnesium chloride in DMF.19 A Hantzsch thiazole synthesis involving 86 and 2-bromoacetophenone17 gave the lead compound 1. Similar reactions of 86 with other 1-aryl-2-bromoethanones gave substituted phenyl (2–21) and heteroaromatic (22–26) analogues. Initially these reactions were performed in refluxing ethanol.17 However, it was later found that these reactions proceed equally well at room temperature.
Scheme 1.
Synthesis of compounds 1-26. Reagents and conditions: a) benzoyl chloride, Et3N, THF, −5 °C to rt, overnight; b) NaHS·xH2O, MgCl2, 15-crown-5, DMF, rt; c) appropriate 1-aryl-2-bromoethanone, EtOH, reflux or rt. Structures 1-26 are defined in Table 1.
A variation of this pathway was employed for compounds 27–73 (Scheme 2). Treatment of 84 with di-tert-butyl dicarbonate in the presence of Montmorillonite K1020 gave intermediate 87,21 which was converted to thioamide 8822 under the same conditions described above for 86. The reaction of 88 with the appropriate 2-bromoacetophenone derivative in refluxing ethanol effected both thiazole ring closure and amine deprotection to give the 4-arylthiazol-2-ethylamine synthons 89a–f, which conveniently precipitated from the reaction mixture as their hydrobromide salts. The reaction of 89a with the appropriate acyl chloride and triethylamine in THF gave amides 27–43 and trisubstituted ureas 44, 46–48 (Table 2). Analogous reactions involving anhydrides, isocyanates, chloroformates, or sulfonyl chlorides gave trisubstituted urea 45, N,N′-disubstituted ureas 49–52, carbamate 53, and sulfonamides 54–55, respectively (Table 2). The reaction of synthons 89b–f with the appropriate acyl halides gave amides 57–66 and ureas 67–73 (Table 3).
Scheme 2.
Synthesis of compounds 27-73. Reagents and conditions: a) Boc2O, Montmorillonite K-10; b) NaHS·xH2O, MgCl2, 15-crown-5-, DMF, rt; c) appropriate 1-aryl-2-bromoethanone, EtOH, reflux;; d) appropriate carbonyl or sulfonyl chloride, anhydride, or isocyanate, Et3N (or DIEA), THF (or DCM). Structures 27-55 and 56–73 are defined in Tables 2 and 3, respectively.
3. Biological Results
3.1. Antiparasitic activities
3.1.1. In vitro potencies
Synthesized compounds 1–73 along with commercial compounds 74–83 were tested for activity against human pathogenic T. b. rhodesiense as well as toxicity to L6 rat myoblast cells23 (Tables 1–4). The selectivity index (SI, the ratio of cytotoxic to antitrypanosomal IC50 values) was also determined for each compound. Standard drugs included pentamidine and melarsoprol (against the parasite) and podophyllotoxin (for cytotoxicity).
The effects of modification of the 4-thiazolyl substituent (R2) are shown in Table 1. This subset of compounds includes substituted phenyl (2–21) and heteroaromatic (22–26) analogues. The cyano, trifluoromethyl, nitro, and methoxy derivatives (2–8) were all less potent than 1 (IC50 = 632 nM, SI = 162). Only members of the halogenated analogue subset (9–21) exhibited enhanced potencies, with the greatest enhancements found with the fluorinated derivatives. The 2-fluorophenyl derivative 9 (IC50 = 156 nM) was the most potent of the monosubstituted compounds, followed by the 4-fluoro (11, IC50 = 218 nM), 3-fluoro (10, IC50 = 233 nM), the 3-chloro (12, IC50 = 452 nM) and 3-bromo (14, IC50 = 553 nM) analogues. The 3-fluoro and 4-fluoro isomers 10 and 11 were similarly potent, but in all other cases the 3-substituted analogues 2, 5, 7, 12, and 14 were more potent than their 4-substituted isomers. The 2,4– and 3,4-difluoro derivatives 16 and 19 were similar in potency to 2-fluoro analogue 9. The other difluoro isomers 17, 18, and 20, as well as the trifluoro analogue 21 were all less potent than 1. Replacement of the benzene ring with furan, thiophene or pyridine (compounds 22–26) resulted in diminished potency in all instances. In summary, seven halogenated phenyl analogues were more potent than 1, including fluorinated derivatives 9, 16, and 19 with IC50 values below 200 nM. The 3,4-difluoro analogue 19 (IC50 = 145 nM, SI = 211) was the most potent of this subset, while its 2,4-difluoro isomer 16 (IC50 = 162 nM, SI > 1593) was the third most potent and the most highly selective for the parasite. Modifications at this site resulted in less than fivefold enhancements of potency.
Enhancements of potency of up to 30–fold were observed upon modification of the ring attached to the carbonyl carbon (R1, compounds 27–55, Table 2). This subset includes amides 27–43, ureas 44–52, carbamate 53, and sulfonamides 54–55. Among the substituted benzamides 27–35, the 3-nitro (29, IC50 = 150 nM) and 3-cyano (27, IC50 = 171 nM) derivatives were the most potent followed by 3-fluoro (31), 2-fluro (30) and 2-chloro (33) analogues having IC50 values between 350 and 500 nM. The 3-cyano (27), – fluoro (31), and –chloro (34) substituted analogues were more potent than their 4-substituted isomers. While the replacement of benzene with furan (37) or thiophene (38 and 39) resulted in enhanced potency relative to 1, replacement with pyrrole (36) or pyridine (40 and 41) did not. The 3-thiophenyl amide 39 (IC50 = 190 nM) was the most potent in this group, followed by 2-thiophenyl (38) and furanyl (37) analogues with IC50 values between 250 and 500 nM. Enhanced potencies were also observed in cyclohexyl (43, IC50 = 164 nM, SI = 1250) and cyclopentyl (42, IC50 = 268 nM, SI = 648), in which the benzene ring is replaced with cycloalkanes. These two compounds were also the most highly selective for the parasite among compounds 27-43, and the only ones with selectivity indices above 500.
The greatest enhancements in activities were observed in the trisubstituted ureas, in which the benzene ring is replaced with a cyclic amine. The most potent example was piperidinyl urea 46 (IC50 = 20.4 nM, SI = 11900), followed by azepanyl urea 48 (IC50 = 51.6 nM, SI = 4450), and pyrrolidinyl urea 44 (IC50 = 125 nM, SI = 1960). The aromatization of the pyrrolidine ring to pyrrole led to decreased potency (44 vs 45), as did the replacement of piperidine with morpholine (46 vs 47). By contrast, all of the N,N’-disubstituted ureas 49-52, which are derived from primary amines, were less potent than the lead compound 1, as was carbamate derivative 53.
An amide or urea group proved to be essential to activity. Replacement of the carbonyl group with a sulfonyl (54–55) resulted in 80– to 120-fold decreases in potency when compared to 1. Elimination of the benzoyl groups led to diminished activity but to a lesser extent compared to the sulfonamides. The primary amino synthetic intermediates 89a–c exhibited IC50 values of 2.2, 16.3, and 15.9 μM, respectively (data not shown), being less potent than the corresponding benzamides 1, 9 and 10. The difference in potency between 89a and 1 was less than 4-fold, but much greater disparities (over 100-fold and nearly 70-fold) existed in the other two pairs.
Incremental enhancements of potency were achieved by incorporating modifications at both ends of the molecule (56–73, Table 3). The potencies of amides 27, 29, 31, and 38 were enhanced by almost 2-fold by fluorination of the 4-thiazolyphenyl ring (56–58, 59–61, 62–63, and 64–66, respectively) in all instances but the 2-fluorophenyl analogue 56, where diminished activity was observed. However, none of the amides 56–66 exhibited IC50 values below 100 nM or selectivity indices above 500. The effects of aromatic fluorination were more pronounced in the urea analogues. The potency of pyrrolidinyl urea 44 was enhanced more than twofold, accompanied by higher selectivity, in the cases of 2–and 3-fluorophenyl analogues 67 and 68 but not the 4-fluorophenyl isomer 69. By contrast, enhanced potency relative to piperidinyl urea 46 was observed with both the 3- and 4-fluoro analogues 70 and 71. Attempts at isolating the 2-fluorophenyl analogue in this series were unsuccessful. The potency of 46 was also enhanced in the cases of the 2,4- and 3,4-difluorophenyl derivatives 72 and 73. The piperidine ureas 46 and 70–73, in addition to being the five most potent compounds, were also the most highly selective for the parasite, with selectivity indices between 9,000 and 19,000. The 3-fluoro analogue 70 (IC50 = 9 nM, SI > 18,800), was the most potent and most highly selective overall, being 70 times more potent than 1.
Commercially available compounds 74–83 are regioisomers of 10 synthesized analogues with reversed substitution patterns on the thiazole rings. The activities and selectivities of each pair of compounds are shown side by side (Table 4). Differences in potency were less than 2-fold in seven out of 10 cases. The greatest difference (3-fold) existed between 79 and 33. The correlations of potency within each pair suggest that that activity is indifferent to a reversed substitution pattern on the thiazole ring. Subsequent to the time when these experiments was performed, the corresponding regioiosmer of 1 and a number of closely related analogues have been identified as hits against T. b. brucei in other high throughput screens.24,2 A patent25 published while this manuscript was in progress gave activities of a number of 2-arylthiazoles, 26 of which are regioisomers of the test compounds in this study. A similar table showing side-by-side comparisons of activities of a total of 29 pairs of regioisomers is available in the supplemental data file. While the data derived from different strains of the trypanosome are not directly comparable, the results are qualitatively similar. The SAR trends in the 4-arylthiazoles were mirrored by the 2-arylthiazoles with respect to modifications to both R1 and R2. For example, fluorination of the R2 aromatic ring led to slightly enhanced activity, while replacement of the benzene ring with heterocycles led to diminished acitivity in both groups. Similar enhancements or decreases in activity were obtained by modification of the R1 group, most notably by the replacement of this benzene ring with a piperidine, and activity was lost by replacement of the carbonyl group with a sulfonyl function.
Overall, antiparasitic IC50 values ranged from 9 nM to >300 μM, and 44 analogues were more potent than the lead compound. Potencies were below 100 nM for eight compounds, below 200 nM for another 18 analogues, and below 500 nM for another 15 derivatives. The ureas were clearly significantly more potent than the amides, as well as being more selective for the parasite. Urea 70 (IC50 = 9 nM, SI > 18,000) was the most potent and the most selective for the parasite. The other seven urea derivatives with potencies below 100 nM had selectivity indices between 3,000 and 12,000. By contrast, compound 57 was the most potent amide analogue (IC50 = 104 nM); however, its SI was only around 300.
3.1.2. In vivo efficacies
Thirty-three compounds (1, 9–12, 16, 27, 29–31, 33, 37–39, 42–44, 46, 47, 56–69) having IC50 values below 0.2 μg/mL were administered intraperitoneally (ip) to mice infected with T. b. rhodesiense STIB900 following a modification of an established protocol8 in which groups of two mice were treated with three consecutive daily 40 mg/kg doses beginning 24 hours post infection. Despite their in vitro potencies, none of compounds cured any infected mice (data not shown). Seven compounds (amides 12, 16, 42, 56, 61, and 65 and urea 44) did cause reductions in parasitemia in both mice by at least 97% (compared to the untreated control group) as observed at either the 24 or 96 hour timepoint following the final dose, or by at least 90% in the case of urea 46. However, subsequent relapses occurred in all cases.
3.2. Metabolic stability
To determine if metabolic instability contributed to the lack of in vivo efficacy, we examined the stability of 15 select compounds (1, 9, 10, 16, 30, 43, 44, 46, 57, 60, 62, 65, 67, 68, and 70, Table 5) in mouse liver microsomes (MLM) and human liver microsomes (HLM) with and without the NADPH cofactor. Fourteen of these analogues (all but 70) had been tested against the parasite in vivo. As a whole, the compounds exhibited poor metabolic stability in MLM (containing NADPH) with half-lives ranging from 0.3 to 11 minutes. Moreover, eight of the compounds (1, 9, 10, 16, 30, 43, 62 and 65) underwent significant NADPH-independent metabolism (defined as with less than 85% substrate remaining) in MLM with 0.4% to 83% substrate remaining after a 60-minute incubation. The poor metabolic stability of these compounds in MLM likely contributed to their lack of efficacy in mice infected with T. b. rhodesiense STIB900. In contrast, these compounds exhibited improved metabolic stability in HLM (containing NADPH) with half-lives ranging from 4.9 to 50 min. Several compounds (1, 10, 16, 62, and 65) also underwent significant NADPH-independent metabolism in HLM with 24% to 80% substrate remaining after 60 minutes incubation. As a whole, the 10 amide analogues (1, 9, 10, 16, 30, 43, 57, 60, 62, and 65) were more stable to both MLM and HLM than the five urea analogues (44, 46, 67, 68, and 70). Only three molecules (amides 16, 57, and 60 having fluorophenyl substituents on the thiazole ring and/or electron withdrawing benzoyl substituents) exhibited better metabolic stability in both MLM (t1/2 > 5 min) and HLM (t1/2 > 25 min). The piperidyl urea derivative 70, which was the most potent against the parasite in vitro (IC50 = 9 nM), exhibited low metabolic stability in MLM (t1/2 = 1.6 min) and was the least stable to HLM (t1/2 = 4.9 min). This compound was synthesized after the completion of the efficacy studies of the other compounds. Based upon its poor metabolic stability and the fact that the in vivo efficacy of the pyrrolidyl urea was not enhanced an aromatic 3-fluoro substituent (44 vs 68), the in vivo evaluation of 70 was deemed to be unnecessary.
Table 5.
Stability of select compounds to mouse and human liver microsomes
|
T. b. rhodesiense
a
|
MLM |
HLM |
|||
|---|---|---|---|---|---|
| Compd |
IC50 (μM) |
Microsomal t1/2 (min)b |
Substrate remainingc |
Microsomal t1/2 (min)b |
Substrate remainingc |
| 1 | 0.632 | 1.1 | 43% | 28 | 80% |
| 9 | 0.156 | 1.9 | 29% | 34 | 96% |
| 10 | 0.233 | 3.2 | 48% | 22 | 75% |
| 16 | 0.162 | 5.5 | 22% | 50 | 67% |
| 30 | 0.460 | 1.5 | 60% | 36 | 93% |
| 43 | 0.164 | 0.6 | 0.4% | 8.0 | 104% |
| 44 | 0.125 | 0.5 | 89% | 13 | 88% |
| 46 | 0.0204 | 1.7 | 102% | 9.1 | 104% |
| 57 | 0.104 | 7.6 | 97% | 34 | 98% |
| 60 | 0.129 | 11 | 106% | 27 | 107% |
| 62 | 0.325 | 4.7 | 62% | 24 | 69% |
| 65 | 0.149 | 1.6 | 83% | 9.4 | 24% |
| 67 | 0.0495 | 0.3 | 101% | 11 | 100% |
| 68 | 0.0517 | 1.8 | 105% | 17 | 103% |
| 70 | 0.0090 | 1.6 | 90% | 4.9 | 102% |
Trypanosoma brucei rhodesiense (STIB900), data reproduced from Table 1
Microsomal t1/2 was determined in the presence of the NADPH cofactor.
Substrate concentrations were determined in incubations without NADPH after 60 min and normalized to concentrations at time zero.
4. Discussion
These results were promising regarding the preparation of a set of analogues and their in vitro activities against the parasite. A relatively facile synthetic pathway was amenable to a large number of functional groups, giving rise to a structurally diverse set of analogues, many of which were more potent than the lead compound, including eight with IC50 values below 100 nM. The high potencies (IC50 < 25 nM) of the five piperidine urea derivatives 46 and 70–73, were especially promising. However, none of the compounds tested in vivo were able to cure infected mice. At best, several analogues brought about significant reductions in parasitemia, only to be followed by relapses. The microsomal studies indicated that these compounds, as a whole, had poor metabolic stability in MLM and likely had limited exposure in mouse plasma after intraperitoneal administration. With future analogues, the extent of plasma exposure may be confirmed by pharmacokinetic studies. These results further underline the importance of considering both in vitro activity and metabolic stability in selecting candidates for in vivo efficacy studies, while at the same time they provide a rationale for continued work on this scaffold.
The analogues in this study had modifications primarily at either end of the molecule. In all of the synthesized derivatives 2–73, the thiazol-2-ethylamine core of the lead compound was left intact. Only in the commercial compounds 74–83 was this portion of the molecule altered by reversal of the substituents on the thiazole ring, and none of these analogues were tested for metabolic stability. Whether the internal core of compounds 1–73, particularly the ethylene bridge between the thiazole ring and the amide nitrogen, contributes to their poor metabolic stability remains largely unexplored. Work is currently underway, with particular emphasis on structural modifications of the internal portion of the molecule, to retain high potency while at the same time increasing metabolic stability, with the ultimate goal of obtaining in vivo efficacy against the trypanosome.
5. Experimental
5.1. Biological protocols
5.1.1. Antiparasitic activity
In vitro antitrypanosomal activities against T. b. rhodesiense (STIB900) and cytotoxicities against L6 rat myoblast cells were measured following established protocols.23 In vivo experiments were performed as previously reported with modifications to reduce the stringency of the mouse model of infection for the new chemical scaffolds and with a smaller mouse group size.8 Female NMRI mice were infected intraperitoneally (ip) with 104 STIB900 bloodstream trypanosome forms. Experimental groups of two mice were treated with the test compounds administered at 40 mg/kg ip on three consecutive days from day 1 to day 3 post infection (120 mg/kg ip total dose). A control group was infected but remained untreated. The tail blood of all mice was checked for parasitemia reduction (versus untreated control mice) at 24 and 96 hour timepoints after the final dose of the compounds. Mice were euthanized after 96 hours if the tail blood was not parasite free. The tail blood of aparasitemic mice was examined twice per week for 30 days post infection, and mice with detected parasitemia relapses were euthanized. The mice that remained aparasitemic until day 30 were considered as cured. All protocols and procedures were reviewed and approved by the local veterinary authroites of the Canton Base-Stadt, Switzerland.
5.1.2. Metabolic stability
Metabolic stability was evaluated using liver microsomes derived from mouse and human sources. Microsomal incubations were carried out according to a protocol described previously26 with modifications. Briefly, substrate stock solutions were prepared in DMSO and DMSO content was kept at 0.5% (v/v) in final incubations. Incubation mixtures (final volume 0.2 mL) consisted of substrate (3 μM), liver microsomes (0.5 mg/mL) from mouse (pool of 1000, CD-1 male mouse) or human (pool of 50, mixed gender) (XenoTech LLC, Lenexa, KS) in a phosphate buffer (100 mM, pH 7.4) containing 3.3 mM MgCl2. After a 5-minute pre-equilibration period at 37 °C, reactions (in triplicate) were initiated by adding the NADPH cofactor (1 mM). For NADPH-independent reactions, the cofactor was replaced with water. Aliquots (10 μl) of the reaction mixtures were removed at 0, 15, 30, and 60 minutes and individually mixed with 200 μL of ice-cold acetonitrile containing internal standard. The mixtures were vortex-mixed, and precipitated protein was removed by centrifugation at 2,250 × g for 15 min. The supernatant fractions were dried using a 96-well microplate evaporator (Apricot Designs Inc., Covina, CA) under N2 at 50 °C and reconstituted with 100 μL 50% methanol containing 0.1% trifluoroacetic acid before UPLC-MS/MS analysis. In vitro half-life (t1/2) was obtained by analyzing the substrate concentration vs. incubation time curve using the one-phase exponential decay model (GraphPad Prism® 5.0, San Diego, CA).
5.2. Chemistry
Uncorrected melting points were measured on a Thomas–Hoover Capillary or a Thermo Scientific 9200 melting point apparatus. 1H NMR spectra were recorded in DMSO-d6 on a Varian Inova 400 MHz spectrometer. Anhydrous solvents were purchased from Aldrich Chemical Co., Milwaukee, WI, in Sure-seal® containers and were used without further purification. .Organic starting materials were purchased from Aldrich Chemical Co. or were prepared by established procedures as noted. Compounds 74-83 were purchased from ChemDiv, San Diego, CA.
Reaction mixtures were monitored by TLC on silica gel or by reverse phase HPLC. Combined organic layers of extraction mixtures were neutralized as necessary with acidic or basic washes, washed with saturated NaCl solution and dried over MgSO4 before being evaporated under reduced pressure. Normal phase flash column chromatography was performed using Davisil grade 633, type 60A silica gel (200–425 mesh). Analytical HPLC chromatograms were recorded on an Agilent 1200 series chromatograph using a Zorbax Rx C8 column (4.6 × 75 mm, 3.5 μm) maintained at 40 °C and UV photodiode array detection at 230, 254, 265, 290, and 320 nm. Area % values are reported at the wavelengths where the strongest signals of the products were observed. Mobile phases consisted of mixtures of methanol (0–95%) in water containing formic acid (80 mM), ammonium formate (20 mM) and triethylamine (15 mM). Samples were eluted at appropriate gradients at a flow rate of 1.5 mL/min. Low resolution ESI mass spectra were recorded on an Agilent Technologies 1100 Series LC/MSD Trap mass spectrometer. In cases of hydrochloride salts, the m/z values reported are those of the free bases. Elemental analyses were measured by Atlantic Microlab, Norcross, GA, and unless stated otherwise, were within ± 0.4% of calculated values. The compounds frequently analyzed correctly for fractional moles of water and/or other solvents; in each case the 1H NMR spectra was consistent with the analysis.
5.2.1. General procedure for preparation of benzamides 1-26
A mixture of 3-(benzamido)thiopropionamide 86 (0.9-1.5 mmol) and the appropriate 1-aryl-2-bromoethanone (1-1.6 equiv) in EtOH (10 mL) was stirred at reflux for 2-3 hours (for 1, 4-8, and 10-15) or overnight at room temperature (for all others). Reaction mixtures were extracted into EtOAc (3× from basified aqueous phases as needed) or were diluted with water to give precipitated product. Compound 1 was purified by column chromatography; all others were purified by direct recrystallization or precipitation.
5.2.1.1. 2-(2-Benzamido)ethyl-4-phenylthiazole (1)
was prepared from 86 and 2-bromoacetophenone. After column chromatography (silica, hexanes/EtOAc 1:1), the product was recrystallized from EtOAc and hexane to give white crystals (272 mg, 59%): mp 112-113 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.6 Hz, 1H), 7.98 (d, J = 0.7 Hz, 1H), 7.97 – 7.92 (m, 2H), 7.87 – 7.81 (m, 2H), 7.58 – 7.51 (m, 1H), 7.50 – 7.41 (m, 4H), 7.39 – 7.29 (m, 1H), 3.69 (q, J = 6.6 Hz, 2H), 3.40 – 3.25 (m, 2H); EIMS m/z 309.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H16N2OS: C, 70.10; H, 5.23; N, 9.08; S, 10.40. Found: C, 69.81; H, 5.13; N, 9.00; S, 10.25.
5.1.1.2. 2-(2-Benzamido)ethyl-4-(3-cyanophenyl)thiazole (2)
was prepared from 86 and 2-bromo-3′-cyanoacetophenone.27 The reaction mixture was diluted with water to give a white solid (369 mg, 91%): mp 148-149 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.69 (t, J = 5.6 Hz, 1H), 8.38 (t, J = 1.8 Hz, 1H), 8.28 (ddd, J = 8.0, 1.8, 1.2 Hz, 1H), 8.21 (s, 1H), 7.85 – 7.81 (m, 2H), 7.80 (dt, J = 7.7, 1.4 Hz, 1H), 7.65 (t, J = 7.8 Hz, 1H), 7.57 – 7.48 (m, 1H), 7.51 – 7.41 (m, 2H), 3.69 (td, J = 6.8, 5.5 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H); EIMS m/z 334. 0 (M + 1)+; HPLC 100 area% (265 nm). Anal. Calcd for C19H15N3OS: C, 68.45; H, 4.53; N, 12.60. Found: C, 68.15; H, 4.35; N, 12.37.
5.2.1.3. 2-(2-Benzamido)ethyl-4-(4-cyanophenyl)thiazole (3)
was prepared from 86 and 2-bromo-4′-cyanoacetophenone. After extraction, the crude product was recrystallized from EtOH and water to give a white powder (347 mg, 86%): mp 128-129 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.27 (s, 1H), 8.14 (d, J = 8.5 Hz, 2H), 7.91 (d, J = 9.0 Hz, 2H), 7.87 – 7.79 (m, 2H), 7.57 – 7.48 (m, 1H), 7.51 – 7.41 (m, 2H), 3.69 (q, J = 6.8, 5.5 Hz, 2H), 3.33 (t, J = 6.9 Hz, 2H); EIMS m/z 334. 0 (M + 1)+; HPLC 100 area% (290 nm). Anal. Calcd for C19H15N3OS: C, 68.45; H, 4.53; N, 12.60. Found: C, 68.16; H, 4.46; N, 12.30.
5.2.1.4. 2-(2-Benzamido)ethyl-4-(4-trifluoromethylphenyl)thiazole (4)
was prepared from 86 and 2-bromo-4′-trifluoromethylacetophenone. After extraction, the product was recrystallized from EtOH to give a white solid (78 mg, 17%): 1H NMR (400 MHz, DMSO-d6) δ 8.69 (t, J = 5.7 Hz, 1H), 8.22 (s, 1H), 8.20 – 8.13 (m, 2H), 7.88 – 7.81 (m, 2H), 7.81 – 7.76 (m, 2H), 7.58 – 7.50 (m, 1H), 7.47 (ddt, J = 8.4, 6.5, 1.5 Hz, 2H), 3.69 (q, J = 6.9 Hz, 2H), 3.34 (t, J = 6.9 Hz, 2H); EIMS m/z 377.6 (M + 1)+; HPLC 100 area% (265 nm). Anal. Calcd for C19H15F3N2OS: C, 60.63; H, 4.02; N, 7.44. Found: C, 60.51; H, 3.93; N, 7.42.
5.2.1.5. 2-(2-Benzamido)ethyl-4-(3-nitrophenyl)thiazole (5)
was prepared from 86 and 2-bromo-3′-nitroacetophenone). The reaction mixture was diluted with water (10 mL) to give a white precipitate (347 mg, 80%): mp 161-162 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.76 (t, J = 2.0 Hz, 1H), 8.70 (t, J = 5.6 Hz, 1H), 8.39 (ddd, J = 7.8, 1.8, 1.0 Hz, 1H), 8.31 (s, 1H), 8.19 (ddd, J = 8.2, 2.4, 1.0 Hz, 1H), 7.87 – 7.80 (m, 2H), 7.74 (t, J = 8.0 Hz, 1H), 7.57 – 7.49 (m, 1H), 7.49 – 7.41 (m, 2H), 3.70 (q, J = 6.8 Hz, 2H), 3.35 (t, J = 6.8 Hz, 2H); EIMS m/z 354.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15N3O3S·0.2H2O: C, 60.56; H, 4.35; N, 11.77. Found: C, 60.51; H, 4.18; N, 11.80.
5.2.1.6. 2-(2-Benzamido)ethyl-4-(4-nitrophenyl)thiazole (6)28,29
was prepared from 86 and 2-bromo-4′-nitroacetophenone . After. After extraction, the product was recrystallized from EtOH to give off-white crystals (318 mg, 75%): mp 156-157 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.6 Hz, 1H), 8.35 (s, 1H), 8.33 – 8.26 (m, 2H), 8.25 – 8.18 (m, 2H), 7.88 – 7.80 (m, 2H), 7.56 – 7.50 (m, 1H), 7.49 – 7.42 (m, 2H), 3.70 (td, J = 6.8, 5.5 Hz, 2H), 3.34 (t, J = 6.9 Hz, 2H); EIMS m/z 354.1 (M + 1)+; HPLC 100 area% (230 nm). Anal. Calcd for C18H15N3O3S: C, 61.18; H, 4.28; N, 11.89; S, 9.07. Found: C, 61.16; H, 4.27; N, 11.70; S, 9.13.
5.2.1.7. 2-(2-Benzamido)ethyl-4-(3-methoxyphenyl)thiazole (7)
was prepared from and 2-bromo-3′-methoxyacetophenone. The extract was concentrated to a small volume and was triturated with hexanes to give a white precipitate (304 mg, 75%): mp 89-90°C; 1H NMR (400 MHz, DMSO-d6) δ 8.69 (t, J = 5.5 Hz, 1H), 8.00 (s, 1H), 7.88 – 7.78 (m, 2H), 7.58 – 7.49 (m, 3H), 7.49 – 7.41 (m, 2H), 7.34 (dd, J = 8.3, 7.6 Hz, 1H), 6.91 (ddd, J = 8.2, 2.6, 1.0 Hz, 1H), 3.80 (s, 3H), 3.68 (td, J = 6.9, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 339.6(M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H18N2OS·0.1H2O: C, 67.07; H, 5.39; N, 8.23. Found: C, 66.91; H, 5.41; N, 8.28.
5.2.1.8. 2-(2-Benzamido)ethyl-4-(4-methoxyphenyl)thiazole (8)
was prepared from 86 and 2-bromo-4′-methoxyacetophenone. The reaction mixture was diluted with water to give a white precipitate (311 mg, 75%): mp 135-136 °C); 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 7.92 – 7.86 (m, 2H), 7.86 – 7.81 (m, 2H), 7.80 (s, 1H), 7.56 – 7.50 (m, 1H), 7.50 – 7.42 (m, 2H), 7.03 – 6.94 (m, 2H), 3.79 (s, 3H), 3.67 (td, J = 6.9, 5.5 Hz, 2H), 3.29 (t, J = 7.0 Hz, 2H); EIMS m/z 339.1(M + 1)+; HPLC 99.0 area% (265 nm). Anal. Calcd for C19H18N2OS·0.3H2O: C, 66.37; H, 5.45; N, 8.15. Found: C, 66.18; H, 5.30; N, 8.07.
25.2.1.9. 2-(2-Benzamido)ethyl-4-(2-fluorophenyl)thiazole hydrochloride (9)
was prepared from 86 and 2-bromo-2′-fluoroacetophenone.30 An ethanolic solution of the evaporated extract was treated with saturated ethanolic HCl and diluted with ether to give a precipitate (324 mg, 74%); mp 120-126 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.72 (t, J = 5.6 Hz, 1H), 8.12 (td, J = 7.9, 2.0 Hz, 1H), 7.91 – 7.84 (m, 2H), 7.83 (d, J = 1.6 Hz, 1H), 7.57 – 7.50 (m, 1H), 7.49 – 7.43 (m, 2H), 7.43 – 7.36 (m, 1H), 7.35 – 7.24 (m, 2H), 3.69 (td, J = 6.9, 5.5 Hz, 2H), 3.33 (t, J = 6.9 Hz, 2H); EIMS m/z 327.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS·HCl: C, 59.58; H, 4.44; N, 7.72; Cl, 9.77. Found: C, 59.82; H, 4.35; N, 7.89; Cl, 9.57.
5.2.1.10. 2-(2-Benzamido)ethyl-4-(3-fluorophenyl)thiazole (10)
was prepared from 86 and 2-bromo-3′-fluoroacetophenone. Incremental dilution of the reaction mixture with water gave a white precipitate (223 mg, 57%): mp 88-89 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.69 (t, J = 5.6 Hz, 1H), 8.10 (s, 1H), 7.88 – 7.82 (m, 2H), 7.82 – 7.78 (m, 1H), 7.75 (ddd, J = 10.6, 2.7, 1.5 Hz, 1H), 7.58 – 7.49 (m, 1H), 7.49 – 7.41 (m, 3H), 7.21 – 7.11 (m, 1H), 3.68 (q, J = 6.8 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 327.4 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS: C, 66.24; H, 4.63; N, 8.58. Found: C, 65.95; H, 4.55; N, 8.56.
5.2.1.11. 2-(2-Benzamido)ethyl-4-(4-fluorophenyl)thiazole (11)
was prepared from and 2-bromo-4′-fluoroacetophenone. The reaction mixture was diluted with water to give a white precipitate (323 mg, 82%): mp 145-146 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.04 – 7.96 (m, 2H), 7.96 (s, 1H), 7.88 – 7.80 (m, 2H), 7.58 – 7.48 (m, 1H), 7.47 (ddt, J = 8.3, 6.6, 1.4 Hz, 2H), 7.31 – 7.20 (m, 2H), 3.68 (td, J = 6.9, 5.6 Hz, 2H), 3.30 (t, J = 7.0 Hz, 2H); EIMS m/z 327.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS·0.1H2O: C, 65.87; H, 4.67; N, 8.54. Found: C, 65.72; H, 4.62; N, 8.47.
5.2.1.12. 2-(2-Benzamido)ethyl-4-(3-chlorophenyl)thiazole (12)
was prepared from and 2-bromo-3′-chloroacetophenone.. Addition of water to the reaction mixture gave a white precipitate (320 mg, 75%); mp 109 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.13 (s, 1H), 8.00 (t, J = 1.9 Hz, 1H), 7.92 (dt, J = 7.7, 1.4 Hz, 1H), 7.87 – 7.80 (m, 2H), 7.58 – 7.49 (m, 1H), 7.46 (dtd, J = 11.8, 7.1, 5.2 Hz, 3H), 7.39 (ddd, J = 8.0, 2.2, 1.1 Hz, 1H), 3.68 (td, J = 6.8, 5.5 Hz, 2H), 3.31 (t, J = 7.0 Hz, 2H); EIMS m/z 343.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15ClN2OS: C, 63.06; H, 4.41; N, 8.17. Found: C, 63.04; H, 4.42; N, 8.23.
5.2.1.13. 2-(2-Benzamido)ethyl-4-(4-chlorophenyl)thiazole (13)
was prepared from and 2-bromo-4′-chloroacetophenone. The extract was evaporated to a white solid (380 mg), which was recrystallized from EtOH to give off-white crystals (155 mg, 37%): mp 154-155 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.04 (s, 1H), 8.01 – 7.93 (m, 2H), 7.88 – 7.80 (m, 2H), 7.58 – 7.42 (m, 5H), 3.68 (td, J = 6.9, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 343.1 (M + 1)+; HPLC 100 area% (230 nm). Anal. Calcd for C18H15ClN2OS: C, 63.06; H, 4.41; N, 8.17. Found: C, 62.67; H, 4.26; N, 8.07.
5.2.1.14. 2-(2-Benzamido)ethyl-4-(3-bromophenyl)thiazole (14)
was prepared from 86 and 2,3′-dibromoacetophenone The extract was concentrated to a small volume and triturated with hexanes to give a white solid (311 mg, 65%); mp 96-97 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.15 (t, J = 1.8 Hz, 1H), 8.13 (s, 1H), 7.96 (ddd, J = 7.8, 1.6, 1.0 Hz, 1H), 7.88 – 7.80 (m, 2H), 7.58 – 7.50 (m, 2H), 7.50 – 7.43 (m, 2H), 7.40 (t, J = 7.9 Hz, 1H), 3.68 (td, J = 6.9, 5.6 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 387.0 (M + 1)+, 389.0 (M + 3)+; HPLC 100 area% (230 nm). Anal. Calcd for C18H15BrN2OS·0.1H2O: C, 55.56; H, 3.94; N, 7.20. Found: C, 55.42; H, 3.76; N, 7.29.
5.2.1.15. 2-(2-Benzamido)ethyl-4-(4-bromophenyl)thiazole (15)
was prepared from 86 and 2,4′-dibromoacetophenone. The extract was evaporated to a white solid (440 mg), which was recrystallized from EtOH to give off-white crystals (207 mg, 44%): mp 158-159 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.05 (s, 1H), 7.95 – 7.86 (m, 2H), 7.88 – 7.80 (m, 2H), 7.69 – 7.58 (m, 2H), 7.57 – 7.48 (m, 1H), 7.51 – 7.41 (m, 2H), 3.67 (td, J = 6.9, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 387.2 (M + 1)+, 389.1 (M + 3)+; HPLC 100 area% (265 nm). Anal. Calcd for C18H15BrN2OS: C, 55.82; H, 3.90; N, 7.23; S, 8.28. Found: C, 55.86; H, 3.31; N, 7.17; s, 8.14.
5.2.1.16. 2-(2-Benzamido)ethyl-4-(2,4-difluorophenyl)thiazole (16)
was prepared from 86 (416 mg, 2.00 mmol) and 2-bromo-2′,4′-difluoroacetophenone (470 mg, 2.00 mmol). The evaporated extract was recrystallized from EtOH/H2O (2:1, 75 mL) to give white crystals (340 mg, 49%): mp 124-125 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.69 (t, J = 5.6 Hz, 1H), 8.15 (td, J = 8.9, 6.8 Hz, 1H), 7.88 – 7.80 (m, 3H), 7.58 – 7.50 (m, 1H), 7.50 – 7.43 (m, 2H), 7.38 (ddd, J = 11.8, 9.3, 2.6 Hz, 1H), 7.23 – 7.13 (m, 1H), 3.68 (td, J = 6.9, 5.5 Hz, 2H), 3.32 (t, J = 6.9 Hz, 2H); EIMS m/z 345.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS·0.3H2O: C, 61.81; H, 4.21; N, 8.01. Found: C, 61.56; H, 4.21; N, 7.94.
5.2.1.17. 2-(2-Benzamido)ethyl-4-(2,5-difluorophenyl)thiazole (17)
was prepared from 86 and 2-bromo-2′,5′-difluoroacetophenone31 The reaction mixture was concentrated by heating, and water was added to give white crystals (274 mg, 91%): mp 123-124 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 7.95 (d, J = 2.6 Hz, 1H), 7.89 – 7.80 (m, 3H), 7.57 – 7.50 (m, 1H), 7.49 – 7.43 (m, 2H), 7.39 (ddd, J = 10.8, 9.1, 4.6 Hz, 1H), 7.24 (ddt, J = 9.0, 7.3, 3.5 Hz, 1H), 3.69 (q, J = 6.5 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H); EIMS m/z 345.4 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS: C, 62.78; H, 4.10; N, 8.13. Found: C, 62.60; H, 4.28; N, 8.04.
5.2.1.18. 2-(2-Benzamido)ethyl-4-(2,6-difluorophenyl)thiazole hydrochloride (18)
was prepared from 86 and 2-bromo-2′,6′-difluoroacetophenone.32 The evaporated extract was recrystallized twice from EtOH, saturated ethanolic HCl, and ether to give white crystals (305 mg, 73%): mp 158-167 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.71 (t, J = 5.6 Hz, 1H), 7.89 – 7.80 (m, 3H), 7.58 – 7.42 (m, 4H), 7.28 – 7.16 (m, 2H), 3.66 (td, J = 7.0, 5.5 Hz, 2H), 3.32 (t, J = 7.0 Hz, 2H); EIMS m/z 345.4 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS·HCl: C, 56.77; H, 3.97; N, 7.36; Cl, 8.93. Found: C, 56.56; H, 4.04; N, 7.27; Cl, 9.17.
5.2.1.19. 2-(2-Benzamido)ethyl-4-(3,4-difluorophenyl)thiazole (19)
was prepared from 86 and 2-bromo-3′,4′-difluoroacetophenone.32 Concentration of the reaction mixture followed by incremental dilution with water gave white crystals (351 mg, 93%): mp 103-103 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.5 Hz, 1H), 8.07 (s, 1H), 7.96 (ddd, J = 12.2, 7.9, 2.2 Hz, 1H), 7.87 – 7.81 (m, 2H), 7.81 – 7.76 (m, 1H), 7.57 – 7.41 (m, 4H), 3.67 (td, J = 6.8, 5.5 Hz, 2H), 3.30 (t, J = 6.9 Hz, 2H); EIMS m/z 345.1 (M + 1)+; HPLC 98.2 area% (254 nm). Anal. Calcd for C18H14F2N2OS: C, 62.78; H, 4.10; N, 8.13. Found: C, 62.52; H, 3.99; N, 7.95.
5.2.1.20. 2-(2-Benzamido)ethyl-4-(3,5-difluorophenyl)thiazole (20)
was prepared from 86 and 2-bromo-3′,5′-difluoroacetophenone.33 The reaction mixture was concentrated by heating, and water was added to give white crystals (326 mg, 91%): mp 118-119 °C;1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.22 (s, 1H), 7.87 – 7.79 (m, 2H), 7.71 – 7.60 (m, 2H), 7.57 – 7.47 (m, 1H), 7.50 – 7.41 (m, 2H), 7.20 (tt, J = 9.3, 2.4 Hz, 1H), 3.68 (td, J = 6.8, 5.5 Hz, 2H), 3.31 (t, J = 6.8 Hz, 2H); EIMS m/z 345.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS·0.1H2O: C, 62.45; H, 4.13; N, 8.09. Found: C, 62.42; H, 4.04; N, 8.03.
5.2.1.21. 2-(2-Benzamido)ethyl-4-(2,4,5-trifluorophenyl)thiazole (21)
was prepared from 86 and 2-bromo-2′,4′,5′-trifluoroacetophenone. 34 The reaction mixture was heated and diluted with more EtOH to dissolve precipitated product and was then diluted with water to give white needles (337 mg, 87%); mp 139-140 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 8.04 (ddd, J = 11.7, 9.2, 7.0 Hz, 1H), 7.92 (d, J = 2.7 Hz, 1H), 7.87 – 7.75 (m, 2H), 7.70 (td, J = 10.9, 6.8 Hz, 1H), 7.53 (ddt, J = 8.1, 6.4, 1.4 Hz, 1H), 7.46 (ddt, J = 8.3, 6.5, 1.4 Hz, 2H), 3.68 (td, J = 6.8, 5.6 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H); EIMS m/z 363.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H13F3N2OS: C, 59.66; H, 3.62; N, 7.73. Found: C, 59.48; H, 3.48; N, 7.70.
5.2.1.22. 2-(2-Benzamido)ethyl-4-(furan-2-yl)thiazole (22)
was prepared from and 2-bromo-1-(furan-2-yl)ethanone.35 The evaporated extract was recrystallized from hexanes containing the minimum volume of EtOAc to give white crystals (231 mg, 77%): mp 88-89 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 7.88 – 7.81 (m, 2H), 7.73 (dd, J = 1.7, 0.7 Hz, 1H), 7.67 (s, 1H), 7.58 – 7.50 (m, 1H), 7.50 – 7.40 (m, 2H), 6.78 (dd, J = 3.4, 0.8 Hz, 1H), 6.59 (dd, J = 3.4, 1.9 Hz, 1H), 3.65 (td, J = 6.9, 5.5 Hz, 2H), 3.28 (t, J = 6.9 Hz, 2H); EIMS m/z 299.1 (M + 1)+; HPLC 100 area% (265 nm). Anal. Calcd for C16H14N2O2S: C, 64.41; H, 4.73; N, 9.39. Found: C, 64.39; H, 4.69; N, 9.38.
5.2.1.23. 2-(2-Benzamido)ethyl-4-(thiophen-2-yl)thiazole (23)
was prepared from 86 and 2-bromo-1-(thiophen-2-yl)ethanone.35 The evaporated extract was recrystallized from hexanes containing the minimum volume of EtOAc to give a white solid (251 mg, 78%): mp 116-117 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (t, J = 5.6 Hz, 1H), 7.86 – 7.82 (m, 2H), 7.81 (s, 1H), 7.58 – 7.50 (m, 3H), 7.50 – 7.42 (m, 2H), 7.11 (dd, J = 5.1, 3.6 Hz, 1H), 3.65 (td, J = 6.9, 5.6 Hz, 2H), 3.28 (t, J = 6.9 Hz, 2H); EIMS m/z 315.1 (M + 1)+; HPLC 98.1 area% (290 nm). Anal. Calcd for C16H14N2OS2: C, 61.12; H, 4.49; N, 8.91. Found: C, 60.91; H, 4.47; N, 8.98.
5.2.1.24. 2-(2-Benzamido)ethyl-4-(pyridin-2-yl)thiazole (24)
was prepared from 86 and 2-bromo-1-(pyridin-2-yl)ethanone hydromide.36 A decolorized (Norit) ethanolic solution of the evaporated filtrated was treated with saturated ethanolic HCl (1 mL) and diluted with ether. This solution was evaporated to dryness. The residue was dissolved in a mixture of aqueous HCl and EtOH and then basified with 1 N NaOH to give a precipitate (226 mg, 61%): mp 129-132 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.6 Hz, 1H), 8.64 – 8.57 (m, 1H), 8.16 (s, 1H), 8.06 (dd, J = 7.9, 1.1 Hz, 1H), 7.89 (tdd, J = 7.7, 4.4, 1.8 Hz, 1H), 7.86 – 7.80 (m, 2H), 7.57 – 7.49 (m, 1H), 7.49 – 7.39 (m, 2H), 7.35 (ddd, J = 7.5, 4.8, 1.3 Hz, 1H), 3.69 (q, J = 6.7 Hz, 2H), 3.33 (t, J = 6.9 Hz, 2H); EIMS m/z 309.7 (M + 1)+; HPLC 99.3 area% (254 nm). Anal. Calcd for C17H15N3OS·H2O: C, 62.36; H, 5.23; N, 12.83. Found: C, 62.15; H, 5.14; N, 12.68.
5.2.1.25. 2-(2-Benzamido)ethyl-4-(pyridin-3-yl)thiazole hydrochloride (25)
was prepared from 86 and 2-bromo-1-(pyridin-3-yl)ethanone hydromide (prepared analogously to its 2-pyridyl analogue).36 The evaporated extract was dissolved in EtOH, filtered, treated with saturated ethanolic HCl, and diluted with ether to give a solid (201 mg, 48%): mp 174-176 °C dec.; 1H NMR (400 MHz, DMSO-d6) δ 9.36 (d, J = 2.0 Hz, 1H), 8.91 (dt, J = 8.2, 1.7 Hz, 1H), 8.81 (dd, J = 5.5, 1.4 Hz, 1H), 8.76 (t, J = 5.6 Hz, 1H), 8.47 (s, 1H), 8.00 (dd, J = 8.1, 5.6 Hz, 1H), 7.88 – 7.80 (m, 2H), 7.57 – 7.49 (m, 1H), 7.49 – 7.41 (m, 2H), 3.71 (q, J = 6.6 Hz, 2H), 3.36 (t, J = 6.9 Hz, 2H); EIMS m/z 309.7 (M + 1)+ of free base; HPLC 99.1 area% (254 nm). Anal. Calcd for C17H15N3OS·1.1HCl·0.25H2O: C, 57.68; H, 4.73; N, 11.87; Cl, 11.02. Found: C, 57.49; H, 4.72; N, 11.69; Cl, 11.25.
5.2.1.26 2-(2-Benzamido)ethyl-4-(pyridin-4-yl)thiazole hydrochloride (26)
was prepared from 86 and 2-bromo-1-(pyridin-4-yl)ethanone hydromide (prepared analogously to its 2-pyridyl analogue).36 The evaporated extract was dissolved in EtOH, treated with Norit, and filtered. The filtrate was treated with saturated ethanolic HCl, and diluted with ether to give a solid (346 mg, 81%): mp 215-218 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.90 (d, J = 5.8 Hz, 2H), 8.84 (s, 1H), 8.75 (t, J = 5.6 Hz, 1H), 8.45 (d, J = 6.7 Hz, 2H), 7.87 – 7.80 (m, 2H), 7.57 – 7.49 (m, 1H), 7.49 – 7.41 (m, 2H), 3.76 – 3.66 (m, 2H), 3.38 (t, J = 6.8 Hz, 2H); EIMS m/z 310.0 (M + 1)+; HPLC 100 area% (230 nm). Anal. Calcd for C17H15N3OS·HCl·0.6H2O: C, 57.25; H, 4.86; N, 11.78; Cl, 9.94. Found: C, 57.06; H, 4.77; N, 11.74; Cl, 10.22.
5.2.2. General procedure for preparation of amides 27-43
A suspension of 4-phenylthiazol-2-ethylamine hydrobromide (89a, 1-1.1 mmol, 1 equiv) and the appropriate acyl halide (minimum of 1.2 equiv) in THF (10 mL) was chilled to 0 °C. Triethylamine (minimum of 3 equiv) was added and the mixture was allowed to warm to room temperature and stirred overnight. The reaction mixture was diluted with water and extracted into EtOAc (3×), and the crude product obtained was recrystallized from an appropriate solvent, unless stated otherwise.
5.2.2.1. 2-[2-(3-Cyanobenzamido)]ethyl-4-phenylthiazole (27)
was prepared from 89a and 3-cyanobenzoyl chloride. The product was recrystallized from EtOAc and hexanes to give a solid (300 mg, 85%): mp 125 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.91 (t, J = 5.6 Hz, 1H), 8.28 – 8.19 (m, 1H), 8.15 (dt, J = 8.0, 1.6 Hz, 1H), 8.01 (dt, J = 7.8, 1.4 Hz, 1H), 7.98 (s, 1H), 7.96 – 7.91 (m, 2H), 7.70 (t, J = 7.8 Hz, 1H), 7.47 – 7.39 (m, 2H), 7.37 – 7.28 (m, 1H), 3.70 (td, J = 6.8, 5.5 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H); EIMS m/z 334.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H15N3OS: C, 68.45; H, 4.53; N, 12.60. Found: C, 68.18; H, 4.53; N, 12.48.
5.2.2.2. 2-[2-(4-Cyanobenzamido)]ethyl-4-phenylthiazole (28)25
was prepared from 89a and 4-cyanobenzoyl chloride. The product was recrystallized from EtOH and water to give a solid (256 mg, 70%): mp 119 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.95 (t, J = 5.6 Hz, 1H), 8.05 – 7.88 (m, 7H), 7.48 – 7.39 (m, 2H), 7.38 – 7.29 (m, 1H), 3.70 (td, J = 6.9, 5.5 Hz, 2H), 3.32 (t, J = 63.9 Hz, 2H); EIMS m/z 334.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H15N3OS·01H2O: C, 68.08; H, 4.57; N, 12.54. Found: C, 68.00; H, 4.40; N, 12.41.
5.2.2.3. 2-[2-(3-Nitrobenzamido)]ethyl-4-phenylthiazole (29)
was prepared from 89a and 3-nitrobenzoyl chloride. The product was recrystallized from EtOAc and hexanes to give a solid (271 mg, 73%): mp 111-112 °C;1H NMR (400 MHz, DMSO-d6) δ 9.08 (t, J = 5.6 Hz, 1H), 8.68 (t, J = 2.0 Hz, 1H), 8.39 (ddd, J = 8.2, 2.3, 1.1 Hz, 1H), 8.28 (ddd, J = 7.8, 1.8, 1.0 Hz, 1H), 7.98 (d, J = 0.8 Hz, 1H), 7.96 – 7.90 (m, 2H), 7.79 (t, J = 8.0 Hz, 1H), 7.42 (td, J = 7.1, 1.1 Hz, 2H), 7.37 – 7.28 (m, 1H), 3.73 (q, J = 6.9 Hz, 2H), 3.34 (t, J = 6.8 Hz, 2H); EIMS m/z 354.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15N3O3S: C, 61.18; H, 4.28; N, 11.89. Found: C, 61.31; H, 4.24; N, 11.99.
5.2.2.4. 2-[2-(2-Fluorobenzamido)]ethyl-4-phenylthiazole (30)
was prepared from 89a and 2-fluorobenzoyl chloride. The product was recrystallized from EtOAc and hexanes to give a solid (246 mg, 71%): mp 70 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.55 – 8.49 (m, 1H), 7.99 (s, 1H), 7.98 – 7.93 (m, 2H), 7.63 (td, J = 7.5, 1.8 Hz, 1H), 7.53 (dddd, J = 8.4, 7.2, 5.3, 1.9 Hz, 1H), 7.47 – 7.39 (m, 2H), 7.38 – 7.31 (m, 1H), 7.31 – 7.22 (m, 2H), 3.69 (td, J = 6.9, 5.5 Hz, 2H), 3.29 (t, J = 7.0 Hz, 2H); EIMS m/z 327.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS: C, 66.24; H, 4.63; N, 8.58. Found: C, 66.11; H, 4.61; N, 8.46.
5.2.2.5. 2-[2-(3-Fluorobenzamido)]ethyl-4-phenylthiazole (31)
was prepared from 89a and 3-fluorobenzoyl chloride. The product was recrystallized from EtOAc and hexanes to give a solid (239 mg, 69%): mp 108-109 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.79 (t, J = 5.6 Hz, 1H), 7.97 (s, 1H), 7.97 – 7.90 (m, 2H), 7.70 (dt, J = 7.7, 1.3 Hz, 1H), 7.63 (ddd, J = 10.1, 2.7, 1.5 Hz, 1H), 7.53 (td, J = 8.0, 5.8 Hz, 1H), 7.46 – 7.36 (m, 3H), 7.36 – 7.29 (m, 1H), 3.68 (td, J = 6.9, 5.6 Hz, 2H), 3.31 (t, J = 7.0 Hz, 2H);EIMS m/z 327.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS: C, 66.24; H, 4.63; N, 8.58. Found: C, 65.95; H, 4.63; N, 8.55.
5.2.2.6. 2-[2-(4-Fluorobenzamido)]ethyl-4-phenylthiazole (32)
was prepared from 89a and 4-fluorobenzoyl chloride. The product was recrystallized from EtOH and water to give a solid (292 mg, 85%): mp 114 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.72 (t, J = 5.6 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.86 (m, 4H), 7.48 – 7.38 (m, 2H), 7.38 – 7.24 (m, 3H), 3.67 (td, J = 6.9, 5.5 Hz, 2H), 3.30 (t, J = 7.0 Hz, 2H); EIMS m/z 327.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15FN2OS: C, 66.24; H, 4.63; N, 8.58. Found: C, 66.14; H, 4.54; N, 8.44.
5.2.2.7. 2-[2-(2-Chlorobenzamido)]ethyl-4-phenylthiazole (33)
was prepared from 89a and 2-chlorobenzoyl chloride. The mixture was evaporated, and the residue was dissolved, with heating, in a mixture of aqueous HCl and EtOH to give, after cooling, white needles (270 mg, 73%) as the free base: mp 119 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (t, J = 5.6 Hz, 1H), 7.99 (s, 1H), 7.99 – 7.92 (m, 2H), 7.53 – 7.39 (m, 5H), 7.39 – 7.29 (m, 2H), 3.66 (q, J = 6.4 Hz, 2H), 3.34 – 3.25 (m, 2H); EIMS m/z 343.5 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15ClN2OS: C, 63.06; H, 4.41; N, 8.17; Cl, 10.34. Found: C, 63.04; H, 4.38; N, 8.44 ; Cl, 10.08.
5.2.2.8. 2-[2-(3-Chlorobenzamido)]ethyl-4-phenylthiazole (34)
was prepared from 89a and 3-chlorobenzoyl chloride. The product was recrystallized from EtOAc and hexanes (205 mg, 57%): mp 84-85 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.82 (t, J = 5.6 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.90 (m, 2H), 7.87 (t, J = 1.8 Hz, 1H), 7.80 (ddd, J = 7.7, 1.7, 1.1 Hz, 1H), 7.61 (ddd, J = 8.0, 2.2, 1.1 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.47 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 3.68 (td, J = 6.9, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 343.3 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15ClN2OS: C, 63.06; H, 4.41; N, 8.17. Found: C, 62.96; H, 4.31; N, 8.14.
5.2.2.9. 2-[2-(4-Chlorobenzamido)]ethyl-4-phenylthiazole hydrochloride (35)
was prepared from 89a and 4-chlorobenzoyl chloride. The product was crystallized as the HCl salt from EtOH and saturated ethanolic HCl (1 mL) to give white needles (94.3 mg, 23%): mp 162-165 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 8.81 (t, J = 5.6 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.91 (m, 2H), 7.89 – 7.83 (m, 2H), 7.58 – 7.51 (m, 2H), 7.47 – 7.38 (m, 2H), 7.38 – 7.29 (m, 1H), 3.68 (td, J = 6.9, 5.5 Hz, 2H), 3.30 (d, J = 7.0 Hz, 2H); EIMS m/z 343.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H15ClN2OS·HCl: C, 57.00; H, 4.25; N, 7.369; Cl, 18.69. Found: C, 57.23; H, 4.33; N, 7.46; Cl, 18.44.
5.2.2.10. 2-(2-Pyrrol-2-ylamido)ethyl-4-phenylthiazole (36)
A suspension of pyrrole-2-carboxylic acid (335 mg, 3.02 mmol) in dichloromethane (10 mL) was treated with EDCI·HCl (302 mg, 1.58 mmol). After 1 hour the mixture was poured into water and extracted into ether to give the anhydride as a white solid (210 mg, 68%). This was dissolved in THF (10 mL) and transferred to a stirred mixture of 89a (301 mg, 1.06 mmol) and triethylamine (175 μL, 1.26 mmol) in THF (10 mL). After 2.5 hours, more triethylamine (225 μL) was added, and the mixture was stirred overnight. Additional acid (112 mg, 1.01 mmol) and EDCI·HCl (191 mg, 1.00 mmol) were added and the mixture was stirred overnight. The reaction was extracted into ether (3×). The product was recrystallized from EtOAc/hexanes and then from EtOH/water to give precipitated product (180 mg, 57%): mp 113 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.43 (s, 1H), 8.19 (t, J = 5.7 Hz, 1H), 7.96 (s, 1H), 7.96 – 7.90 (m, 2H), 7.48 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 6.84 (td, J = 2.7, 1.4 Hz, 1H), 6.74 (ddd, J = 3.9, 2.5, 1.5 Hz, 1H), 6.07 (dt, J = 3.6, 2.4 Hz, 1H), 3.63 (q, J = 6.7 Hz, 2H), 3.27 (t, J = 7.0 Hz, 2H); EIMS m/z 298.0 (M + 1)+; HPLC 99.0 area% (265 nm). Anal. Calcd for C16H15lN2OS: C, 64.62; H, 5.08; N, 14.13. Found: C, 64.87; H, 5.05; N, 14.34.
5.2.2.11. 2-(2-Furan-2-ylamido)ethyl-4-phenylthiazole (37)
was prepared from 89a and 2-furanoyl chloride. The mixture reaction was extracted into ether. The product was recrystallized from ethanolic HCl and ether to give a white solid (262 mg, 72%): mp 130-140 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.59 (t, J = 5.8 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.90 (m, 2H), 7.83 (dd, J = 1.8, 0.8 Hz, 1H), 7.48 – 7.38 (m, 2H), 7.38 – 7.29 (m, 1H), 7.10 (dd, J = 3.5, 0.8 Hz, 1H), 6.62 (dd, J = 3.5, 1.8 Hz, 1H), 3.64 (td, J = 7.0, 5.7 Hz, 2H), 3.28 (t, J = 7.0 Hz, 2H); EIMS m/z 299.4 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H14lNO2S·0.6HCl: C, 60.01; H, 4.60; N, 8.75; Cl, 6.64. Found: C, 60.12; H, 4.53; N, 8.68; Cl, 6.56.5.2.2.12. 2-(2-Thiophen-2-ylamido)ethyl-4-phenylthiazole (38) was prepared from 89a and thiophene-2-carbonyl chloride. The product was recrystallized from ethanolic HCl and ether as a white solid (246 mg, 66%): mp 110-114 °C dec; 1H NMR (400 MHz, DMSO-d6) δ 8.72 (t, J = 5.6 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.90 (m, 2H), 7.78 – 7.71 (m, 2H), 7.47 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 7.14 (dd, J = 5.0, 3.8 Hz, 1H), 3.65 (td, J = 7.0, 5.6 Hz, 2H), 3.29 (t, J = 7.0 Hz, 2H); EIMS m/z 315.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H14NO2S·0.1HCl: C, 60.42; H, 4.44; N, 8.81; Cl, 1.11. Found: C, 60.48; H, 4.53; N, 8.68; Cl, 1.17.
5.2.2.13. 2-(2-Thiophen-3-ylamido)ethyl-4-phenylthiazole (39)
A mixture of thiophene-3-carboxylic acid (201 mg, 1.95 mmol) and thionyl chloride (1.0 mL, 13.7 mmol) in toluene (10 mL) was refluxed for 2 hours. The mixture was evaporated, and the acyl halide was dissolved in dichlormethane (10 ml), followed by the addition of 89a (301 mg, 1.06 mmol) and triethylamine (0.5 mL, 3.6 mmol). The mixture was stirred overnight and worked up by dilution with water and extraction into dichloromethane. EtOAc/hexanes to give white crystals (208 mg, 63%): mp 98-118 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.52 (t, J = 5.7 Hz, 1H), 8.11 (dd, J = 3.0, 1.3 Hz, 1H), 7.97 (s, 1H), 7.96 – 7.90 (m, 2H), 7.58 (dd, J = 5.0, 3.0 Hz, 1H), 7.49 (dd, J = 5.1, 1.3 Hz, 1H), 7.43 (dd, J = 8.4, 6.9 Hz, 2H), 7.38 – 7.28 (m, 1H), 3.64 (td, J = 7.0, 5.6 Hz, 2H), 3.29 (t, J = 7.0 Hz, 2H); EIMS m/z 315.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H14NO2S: C, 61.12 H, 4.49; N, 8.91. Found: C, 60.88; H, 4.73; N, 8.88.
5.2.2.14. 2-(2-Pyridin-3-ylamido)ethyl-4-phenylthiazole dihydrochloride (40)
was prepared from 89a and nicotinoyl chloride hydrochloride. The product was recrystallized from ethanolic HCl a white solid (309 g, 81%):mp 175-178 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.29 (t, J = 5.5 Hz, 1H), 9.20 (dd, J = 2.1, 0.8 Hz, 1H), 8.92 (dd, J = 5.4, 1.5 Hz, 1H), 8.65 (dt, J = 8.1, 1.8 Hz, 1H), 7.99 (s, 1H), 7.97 – 7.93 (m, 2H), 7.91 (dd, J = 8.0, 5.2 Hz, 1H), 7.48 – 7.38 (m, 2H), 7.38 – 7.29 (m, 1H), 3.74 (td, J = 6.9, 5.5 Hz, 2H), 3.35 (t, J = 6.9 Hz, 2H); EIMS m/z 310.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H15N 3 OS·2HCl·0.25H2O: C, 52.79; H, 4.56; N, 10.86; Cl, 18.33. Found: C, 52.72; H, 4.73; N, 10.74; Cl, 18.06.
5.2.2.15. 2-(2-Pyridin-4-ylamido)ethyl-4-phenylthiazole (41)
was prepared from 89a and isonicotinoyl chloride hydrochloride. The reaction mixture was diluted with water to give a white solid (220 mg, 67%): mp 116 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.98 (t, J = 5.6 Hz, 1H), 8.76 – 8.69 (m, 2H), 7.97 (s, 1H), 7.96 – 7.90 (m, 2H), 7.77 – 7.70 (m, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.38 – 7.28 (m, 1H), 3.70 (q, J = 6.8 Hz, 2H), 3.32 (t, J = 6.9 Hz, 2H); EIMS m/z 310.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H15N3OS·.1H2O: C, 65.61; H, 4.92; N, 13.50. Found: C, 65.62; H, 5.00; N, 13.28.
5.2.2.16. 2-(2-Cyclopentylamido)ethyl-4-phenylthiazole (42)
was prepared from 89a and cyclopentanecarbonyl chloride. The product was recrystallized from EtOAc/hexanes as ivory crystals (161 mg, 52%); mp 106-107 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H), 7.96 – 7.89 (m, 3H), 7.47 – 7.41 (m, 2H), 7.38 – 7.29 (m, 1H), 3.45 (td, J = 6.8, 5.6 Hz, 2H), 3.16 (t, J = 6.9 Hz, 2H), 2.59 – 2.46 (m, 1H), 1.77 – 1.66 (m, 2H), 1.66 – 1.54 (m, 4H), 1.53 – 1.43 (m, 2H); EIMS m/z 301.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H20N2OS: C, 67.97; H, 6.71; N, 9.32. Found: C, 67.81; H, 6.70; N, 9.29.
5.2.2.17. 2-(2-Cyclohexylamido)ethyl-4-phenylthiazole (43)
was prepared from 89a and cyclohexanecarbonyl chloride. The product was recrystallized from EtOAc/hexanes as white crystals (249 mg, 78%); mp 112-114 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (s, 1H), 7.96 – 7.91 (m, 2H), 7.88 (t, J = 5.6 Hz, 1H), 7.48 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 3.43 (q, J = 6.7 Hz, 2H), 3.15 (t, J = 6.8 Hz, 2H), 2.07 (tt, J = 11.5, 3.2 Hz, 1H), 1.68 (dd, J = 10.0, 6.8 Hz, 4H), 1.60 (d, J = 9.3 Hz, 1H), 1.38 – 1.06 (m, 5H); EIMS m/z 315.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H22N2OS: C, 68.75; H, 7.05; N, 8.91. Found: C, 68.45; H, 7.08; N, 8.80.
5.2.3. General procedure for preparation of trisubstituted ureas 44-48
A suspension of 4-phenylthiazol-2-ethylamine hydrobromide (89a, 1.05-1.1 mmol) and the appropriate carbamoyl chloride or anhydride (minimum of 1.2 equivalents) in THF (10 mL) was chilled to 0 °C. Triethylamine (minimum of 3 equivalents) was added, and the mixture was allowed to warm to room temperature and stirred overnight. The reaction mixture was diluted with water and extracted into EtOAc (3×), and the crude product obtained was recrystallized from an appropriate solvent, unless stated otherwise.
5.2.3.1. 2-(2-Pyrrolidin-1-ylamido)ethyl-4-phenylthiazole hydrochloride sesquihydrochloride (44)
was prepared from 89a and pyrrolidine-1-carbonyl chloride. The crude product was recrystallized from ethanolic HCl/ ether to give a solid (244 mg, 68%): mp 129-154 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H), 7.97 – 7.90 (m, 2H), 7.48 – 7.39 (m, 2H), 7.38 – 7.29 (m, 1H), 3.42 (t, J = 7.2 Hz, 2H), 3.25 – 3.13 (m, 6H), 1.84 – 1.71 (m, 4H); EIMS m/z 302.0 (M + 1)+; HPLC 98.1 area% (254 nm). Anal. Calcd for C16H19N3OS·1.5HCl·0.7H2O: C, 52.12; H, 5.99; N, 11.40; Cl, 14.42. Found: C, 51.79; H, 6.01; N, 11.29; Cl, 14.70.
5.2.3.2. 2-(2-Pyrrol-1-ylamido)ethyl-4-phenylthiazole (45)
was prepared from 89a and 1H-pyrrole-1-carboxylic anhydride37, and the reaction mixture was worked up by extraction into ether. The product was recrystallized from EtOAc/hexanes (262 mg, 84%): mp 100-101 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.40 (t, J = 5.6 Hz, 1H), 7.98 (s, 1H), 7.96 – 7.89 (m, 2H), 7.46 – 7.39 (m, 2H), 7.39 – 7.35 (m, 2H), 7.35 – 7.28 (m, 1H), 6.21 (t, J = 2.2 Hz, 2H), 3.65 (td, J = 6.9, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 298.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H15N3OS: C, 64.62; H, 5.08; N, 14.13. Found: C, 64.40; H, 5.04; N, 14.19.
5.2.3.3. 2-(2-Piperidin-1-ylamido)ethyl-4-phenylthiazole (46)25
was prepared from 89a and piperidine-1-carbonyl chloride. The crude product was recrystallized from EtOAc/hexanes to give a white solid (162 mg, 49%); mp 105-106 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (s, 1H), 7.96 – 7.90 (m, 2H), 7.48 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 6.64 (t, J = 5.4 Hz, 1H), 3.41 (td, J = 7.0, 5.4 Hz, 2H), 3.28 – 3.23 (m, 4H), 3.16 (t, J = 7.0 Hz, 2H), 1.51 (dt, J = 11.2, 5.6 Hz, 2H), 1.46 – 1.35 (m, 4H); EIMS m/z 316.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H21N3OS·0.2H2O: C, 64.00; H, 6.76; N, 13.17. Found: C, 64.02; H, 6.69; N, 13.12.
5.2.3.4. 2-(2-Morpholin-4-ylamido)ethyl-4-phenylthiazole hydrochloride (47)
was prepared from 89a and morpholine-4-carbonyl chloride, and the reaction mixture diluted with water and extracted into ether (3×). The product was recrystallized from ethanolic HCl/ether as a white solid (271 mg, 70%): mp 103-135 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H), 7.95 (dd, J = 8.3, 1.3 Hz, 2H), 7.48 – 7.39 (m, 2H), 7.38 – 7.29 (m, 1H), 6.81 (s, 1H), 3.57 – 3.50 (m, 4H), 3.43 (t, J = 7.0 Hz, 2H), 3.26 (dd, J = 5.6, 4.1 Hz, 4H), 3.22 – 3.14 (m, 2H); EIMS m/z 318.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H19N3O2S·0.95HCl·0.6H2O: C, 52.96; H, 5.88; N, 11.58; Cl, 9.28. Found: C, 52.89; H, 5.64; N, 11.54; Cl, 9.01.
5.2.3.5. 2-(2-Azepan-1-ylamido)ethyl-4-phenylthiazole (48)
was prepared from 89a and azepane-1-carbonyl chloride (prepared from azepane by modification of the method of McGhee.38 The product was recrystallized from EtOAc/hexanes as white needles (173 mg, 50%): mp 81-82 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 0.6 Hz, 1H), 7.96 – 7.90 (m, 2H), 7.48 – 7.38 (m, 2H), 7.37 – 7.28 (m, 1H), 6.39 (t, J = 5.5 Hz, 1H), 3.42 (td, J = 6.8, 5.4 Hz, 2H), 3.34 – 3.24 (m, 4H), 3.17 (t, J = 6.9 Hz, 2H), 1.57 (pt, J = 4.7, 2.8, 2.3 Hz, 4H), 1.44 (dt, J = 7.3, 2.7 Hz, 4H); EIMS m/z 330.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H23N3OS: C, 65.62; H, 7.04; N, 12.75. Found: C, 65.83; H, 7.16; N, 12.54.
5.2.4. General procedure for preparation of disubstituted ureas 49-52 and carbamate 53
The appropriate isocyanate or chloroformate (minimum of 1 equivalent) was added to a mixture of 4-phenylthiazol-2-ethylamine hydrobromide (89a, 1-1.05 mmol) and N,N-diisopropylethylamine (minimum of 2 equivalents) in dichloromethane (10 mL). After stirring overnight at room temperature, the reaction mixture was diluted with water and extracted into dichloromethane (3×). The product was recrystallized from an appropriate solvent.
5.2.4.1. 1-(tert-Butyl)-3-[2-(4-phenylthiazol-2-yl)ethyl]urea (49)
was prepared from 89a and tert-butylisocyanate using triethylamine in place of N,N-diisopropylethylamine. Recrystallization of the product from EtOAc/hexanes gave white needles (230 mg, 75): mp 162-163 C°; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (d, J = 0.9 Hz, 1H), 7.97 – 7.91 (m, 2H), 7.48 – 7.38 (m, 2H), 7.33 (dddd, J = 9.3, 6.5, 2.0, 1.2 Hz, 1H), 5.80 (t, J = 5.9 Hz, 1H), 5.74 (s, 1H), 3.39 (q, J = 6.5 Hz, 2H), 3.11 (t, J = 6.8 Hz, 2H), 1.21 (d, J = 0.6 Hz, 9H); EIMS m/z 304.1 (M + 1)+; HPLC 98.6 area% (265 nm). Anal. Calcd for C16H21N3OS: C, 63.33 H, 6.98; N, 13.85. Found: C, 63.14; H, 7.09; N, 13.73.
5.2.4.2. 1-Benzyl-3-[2-(4-phenylthiazol-2-yl)ethyl]urea (50)
was prepared from 89a and benzyl isocyanate. The product was recrystallized from EtOH/water as white granules (294 mg, 87%): mp 131-134 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.98 (d, J = 0.5 Hz, 1H), 7.97 – 7.90 (m, 2H), 7.48 – 7.40 (m, 2H), 7.36 – 7.26 (m, 3H), 7.25 – 7.16 (m, 3H), 6.47 (t, J = 6.0 Hz, 1H), 6.12 (t, J = 5.9 Hz, 1H), 4.20 (d, J = 6.0 Hz, 2H), 3.47 (q, J = 6.5 Hz, 2H), 3.16 (t, J = 6.8 Hz, 2H); EIMS m/z 338.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H19N3OS: C, 67.63 H, 5.68; N, 12.45. Found: C, 67.85; H, 5.79; N, 12.54.
5.2.4.3. 1-Cyclohexyl-3-[2-(4-phenylthiazol-2-yl)ethyl]urea (51)
was prepared from 89a and cyclohexyl isocyanate. The product was recrystallized from EtOAc and then from EtOH/water to give a white powder (199 mg, 60%): mp 139-140 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (d, J = 0.6 Hz, 1H), 7.97 – 7.92 (m, 2H), 7.48 – 7.39 (m, 2H), 7.38 – 7.29 (m, 1H), 5.91 – 5.82 (m, 2H), 3.43 (q, J = 6.6 Hz, 2H), 3.38 – 3.29 (m, 1H), 3.12 (t, J = 6.8 Hz, 2H), 1.73 (dd, J = 12.6, 3.8 Hz, 2H), 1.62 (dt, J = 12.8, 4.0 Hz, 2H), 1.55 – 1.46 (m, 1H), 1.31 – 1.18 (m, 2H), 1.17 – 0.98 (m, 3H); EIMS m/z 330.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H23N3OS: C, 65.62 H, 7.04; N, 12.75. Found: C, 65.47; H, 7.16; N, 12.70.
5.2.4.4. 1-Phenyl-3-[2-(4-phenylthiazol-2-yl)ethyl]urea (52)
was prepared from 89a and phenyl isocyanate. The product was recrystallized from EtOH/water as white needles (283 mg, 83%): mp 193-195 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.56 (s, 1H), 7.99 (d, J = 0.8 Hz, 1H), 7.98 – 7.92 (m, 2H), 7.48 – 7.40 (m, 2H), 7.40 – 7.29 (m, 3H), 7.21 (ddd, J = 8.5, 7.4, 0.7 Hz, 2H), 6.88 (tq, J = 7.5, 1.1 Hz, 1H), 6.29 (t, J = 5.8 Hz, 1H), 3.55 (q, J = 6.5 Hz, 2H), 3.21 (t, J = 6.7 Hz, 2H; EIMS m/z 324.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H17N3OS: C, 66.85 H, 5.30; N, 12.99. Found: C, 66.97; H, 5.35; N, 13.10.
5.2.4.5. Phenyl [2-(4-phenylthiazol-2-yl)ethyl]carbamate (53)
was prepared from 89a and phenyl chloroformate using triethylamine in place of N,N-diisopropylethylamine. The product was recrystallized from EtOH/water to give white needles (261 mg, 80%); mp 101-104 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.00 (s, 1H), 7.99 – 7.92 (m, 3H), 7.53 – 7.40 (m, 2H), 7.40 – 7.31 (m, 3H), 7.26 – 7.14 (m, 1H), 7.12 – 7.03 (m, 2H), 3.51 (q, J = 6.8 Hz, 2H), 3.25 (t, J = 6.9 Hz, 2H); EIMS m/z 325.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H16N3O2S: C, 66.64 H, 4.97; N, 8.64. Found: C, 66.40 H, 5.17; N, 8.48.
5.2.5. General procedure for compounds 54-73
A suspension of one of the 4-arylthiazol-2-ethylamine hydrobromides 89a-f (1 mmol) in THF (10 mL) was treated with triethylamine (minimum of 2.5 equivalents). The appropriate sulfonyl, acyl, or carbamoyl chloride was added (minimum of 1.1 equivalents), and the mixture was stirred overnight at room temperature before being diluted with water and extracted into EtOAc (3×. The products were recrystallized from EtOAc/hexane unless stated otherwise.
5.2.5.1. 2-(2-Benzenesulfonamido)ethyl-4-phenylthiazole hydrochloride (54)
was prepared 89a and benzenesulfonyl chloride. The product was converted to the HCl salt using ethanolic HCl followed by recrystallization from EtOH to give a solid (342 mg, 82%): mp 166 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H), 7.94 – 7.88 (m, 3H), 7.84 – 7.79 (m, 2H), 7.67 – 7.62 (m, 1H), 7.62 – 7.56 (m, 2H), 7.48 – 7.38 (m, 2H), 7.38 – 7.28 (m, 1H), 3.25 – 3.10 (m, 4H); EIMS m/z 345.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H16N2O2S·HCl: C, 53.60 H, 4.50; N, 7.35; Cl, 9.31. Found: C, 53.59 H, 4.45; N, 7.23; Cl, 9.19.
5.2.5.2. 2-(2-p-Toluenesulfonamido)ethyl-4-phenylthiazole (55)
was prepared from 89a and tosyl chloride. The product was converted to the HCl salt using ethanolic HCl and ether to give a white solid (363 mg, 86%): mp 165-173 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (s, 1H), 7.94 – 7.87 (m, 2H), 7.84 – 7.76 (m, 1H), 7.71 – 7.64 (m, 2H), 7.47 – 7.40 (m, 2H), 7.37 (d, J = 8.0 Hz, 2H), 7.35 – 7.28 (m, 1H), 3.15 (q, J = 3.9, 3.0 Hz, 4H), 2.36 (s, 3H); EIMS m/z 359.0 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H18N2O2S·HCl·0.25H2O: C, 54.12 H, 4.92; N, 7.01; Cl, 8.88. Found: C, 54.28; H, 4.80; N, 7.06; Cl, 8.59.
5.2.5.3. 2-[2-(3-Cyanobenzamido)]ethyl-4-(2-fluorophenyl)thiazole (56)
was prepared from 89b and 3-cyanobenzoyl chloride as a white solid (283 mg, 78%): mp 115-116 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.91 (t, J = 5.5 Hz, 1H), 8.24 (t, J = 1.7 Hz, 1H), 8.14 (dt, J = 7.9, 1.4 Hz, 1H), 8.10 (td, J = 7.9, 1.9 Hz, 1H), 8.01 (dt, J = 7.5, 1.2 Hz, 1H), 7.87 (dd, J = 2.6, 0.6 Hz, 1H), 7.70 (t, J = 7.8 Hz, 1H), 7.45 – 7.36 (m, 1H), 7.36 – 7.24 (m, 2H), 3.71 (q, J = 6.7 Hz, 2H), 3.33 (t, J = 6.8 Hz, 2H); EIMS m/z 352.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H14FN3OS: C, 64.94 H, 4.02; N, 11.96. Found: C, 64.64; H, 4.11; N, 11.81.
5.2.5.4. 2-[2-(3-Cyanobenzamido)]ethyl-4-(3-fluoropheny)lthiazole (57)
was prepared from 89c and 3-cyanobenzoyl chloride as white crystals (303 mg, 86%): mp 114-115 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.91 (t, J = 5.6 Hz, 1H), 8.24 (td, J = 1.7, 0.6 Hz, 1H), 8.14 (ddd, J = 7.9, 1.8, 1.2 Hz, 1H), 8.11 (s, 1H), 8.01 (ddd, J = 7.7, 1.7, 1.2 Hz, 1H), 7.79 (ddd, J = 7.8, 1.6, 0.9 Hz, 1H), 7.75 – 7.66 (m, 2H), 7.47 (td, J = 8.0, 6.1 Hz, 1H), 7.16 (dddd, J = 9.0, 8.2, 2.7, 0.9 Hz, 1H), 3.70 (td, J = 6.8, 5.5 Hz, 2H), 3.32 (t, J = 6.39 Hz, 2H); EIMS m/z 352.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H14FN3OS: C, 64.94 H, 4.02; N, 11.96. Found: C, 64.75; H, 4.19; N, 11.83.
5.2.5.5. 2-[2-(3-Cyanobenzamido)]ethyl-4-(4-fluorophenyl)thiazole (58)
was prepared from 89d and 3-cyanobenzoyl chloride as white crystals (68 mg, 19%): mp 126-128 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.90 (t, J = 5.6 Hz, 1H), 8.24 (t, J = 1.8 Hz, 1H), 8.14 (ddd, J = 7.9, 1.9, 1.2 Hz, 1H), 8.05 – 7.93 (m, 4H), 7.70 (t, J = 7.9 Hz, 1H), 7.33 – 7.18 (m, 2H), 3.70 (q, J = 6.8 Hz, 2H), 3.31 (t, J = 6.8 Hz, 2H); EIMS m/z 352.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C19H14FN3OS·0.2H2O: C, 64.28 H, 4.09; N, 11.84. Found: C, 64.21; H, 4.11; N, 11.66.
5.2.5.6. 2-[2-(3-Nitrobenzamido)]ethyl-4-(2-fluorophenyl)thiazole (59)
was prepared from 89b and 3-nitrobenzoyl chloride as white crystals (276 mg, 74%): mp 114-115 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.09 (t, J = 5.6 Hz, 1H), 8.70 – 8.64 (m, 1H), 8.39 (ddd, J = 8.3, 2.4, 1.0 Hz, 1H), 8.28 (dt, J = 7.8, 1.4 Hz, 1H), 8.10 (td, J = 7.9, 1.9 Hz, 1H), 7.87 (d, J = 2.5 Hz, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.39 (dddd, J = 8.8, 7.2, 5.2, 1.8 Hz, 1H), 7.35 – 7.22 (m, 2H), 3.73 (td, J = 6.8, 5.5 Hz, 2H), 3.35 (t, J = 6.8 Hz, 2H); EIMS m/z 372.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14FN3O3S: C, 58.21 H, 3.80; N, 11.31. Found: C, 58.18; H, 3.85; N, 11.27.
5.2.5.7. 2-[2-(3-Nitrobenzamido)]ethyl-4-(3-fluorophenyl)thiazole (60)
was prepared from 89c and 3-nitrobenzoyl chloride as white crystals (301 mg, 81%): mp 106 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.08 (t, J = 5.6 Hz, 1H), 8.67 (t, J = 2.0 Hz, 1H), 8.39 (ddd, J = 8.2, 2.3, 1.1 Hz, 1H), 8.28 (ddd, J = 7.8, 1.7, 1.0 Hz, 1H), 8.11 (s, 1H), 7.83 – 7.73 (m, 2H), 7.72 (ddd, J = 10.7, 2.6, 1.5 Hz, 1H), 7.46 (td, J = 8.0, 6.1 Hz, 1H), 7.16 (dddd, J = 9.1, 8.3, 2.7, 1.0 Hz, 1H), 3.73 (q, J = 6.9, 2H), 3.34 (t, J = 6.9 Hz, 2H); EIMS m/z 372.1 (M + 1)+; HPLC 98.6 area% (254 nm). Anal. Calcd for C18H14FN3O3S·0.25H2O: C, 57.51 H, 3.89; N, 11.18. Found: C, 57.32; H, 3.71; N, 11.05.
5.2.5.8. 2-[2-(3-Nitrobenzamido)]ethyl-4-(4-fluorophenyl)thiazole (61)
was prepared from 89d and 3-nitrobenzoyl chloride as white crystals (261 mg, 70%): mp 143-144 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.08 (t, J = 5.6 Hz, 1H), 8.67 (ddd, J = 2.3, 1.7, 0.5 Hz, 1H), 8.39 (ddd, J = 8.2, 2.3, 1.0 Hz, 1H), 8.28 (ddd, J = 7.8, 1.7, 1.1 Hz, 1H), 8.05 – 7.93 (m, 3H), 7.79 (dd, J = 8.3, 7.7 Hz, 1H), 7.32 – 7.17 (m, 2H), 3.72 (td, J = 6.9, 5.5 Hz, 2H), 3.33 (d, J = 6.8 Hz, 2H); EIMS m/z 372.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14FN3O3S: C, 58.21 H, 3.80; N, 11.31. Found: C, 58.35; H, 3.93; N, 11.17.
5.2.5.9. 2-[2-(3-Fluorobenzamido)]ethyl-4-(3-fluorophenyl)thiazole (62)
was prepared from 89c and 3-fluorobenzoyl chloride as white crystals (266 mg, 77%): mp 106-107 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.78 (t, J = 5.6 Hz, 1H), 8.10 (s, 1H), 7.80 (ddd, J = 7.8, 1.6, 0.9 Hz, 1H), 7.74 (ddd, J = 10.7, 2.7, 1.5 Hz, 1H), 7.69 (dt, J = 7.7, 1.2 Hz, 1H), 7.62 (ddd, J = 10.1, 2.7, 1.5 Hz, 1H), 7.50 (dtd, J = 22.2, 8.0, 6.0 Hz, 2H), 7.39 (tdd, J = 8.3, 2.6, 1.0 Hz, 1H), 7.16 (dddd, J = 9.0, 8.2, 2.7, 0.9 Hz, 1H), 3.68 (td, J = 6.8, 5.5 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 345.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS: C, 62.78 H, 4.10; N, 8.13. Found: C, 62.59; H, 4.16; N, 8.03.
5.2.5.10. 2-[2-(3-Fluorobenzamido)]ethyl-4-(4-fluorophenyl)thiazole (63)
was prepared from 89d and 3-fluorobenzoyl chloride as white crystals (266 mg, 77%): mp 122 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.78 (t, J = 5.6 Hz, 1H), 7.99 (dd, J = 8.9, 5.6 Hz, 2H), 7.96 (s, 1H), 7.69 (dt, J = 7.8, 1.2 Hz, 1H), 7.62 (ddd, J = 10.1, 2.7, 1.5 Hz, 1H), 7.53 (td, J = 8.0, 5.8 Hz, 1H), 7.39 (tdd, J = 8.4, 2.7, 1.0 Hz, 1H), 7.30 – 7.21 (m, 2H), 3.68 (td, J = 6.9, 5.6 Hz, 2H), 3.30 (t, J = 7.0 Hz, 2H); EIMS m/z 345.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C18H14F2N2OS: C, 62.78 H, 4.10; N, 8.13. Found: C, 62.72; H, 4.17; N, 8.06.
5.2.5.11. 2-(2-Thiophen-2-ylamido)ethyl-4-(2-fluorophenyl)thiazole (64)
was prepared from 89b and thiophene-2-carbonyl chloride as white crystals (267 mg, 80%): mp 139-141 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.7 Hz, 1H), 8.12 (td, J = 7.9, 1.9 Hz, 1H), 7.86 (d, J = 2.6 Hz, 1H), 7.75 (dd, J = 5.0, 1.1 Hz, 1H), 7.73 (dd, J = 3.7, 1.2 Hz, 1H), 7.45 – 7.37 (m, 1H), 7.36 – 7.24 (m, 2H), 7.15 (dd, J = 5.0, 3.7 Hz, 1H), 3.66 (td, J = 6.9, 5.6 Hz, 2H), 3.31 (t, J = 6.9 Hz, 2H); EIMS m/z 333.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H13FN2OS2: C, 57.81 H, 3.94; N, 8.43. Found: C, 57.72; H, 4.06; N, 8.37.
5.2.5.12. 2-(2-Thiophen-2-ylamido)ethyl-4-(3-fluorophenyl)thiazole (65)
was prepared from 89c and thiophene-2-carbonyl chloride as ivory needles (266 mg, 79%): mp 137-138 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.7 Hz, 1H), 8.10 (d, J = 0.6 Hz, 1H), 7.84 – 7.77 (m, 1H), 7.77 – 7.69 (m, 3H), 7.47 (td, J = 8.0, 6.1 Hz, 1H), 7.17 (ddd, J = 8.1, 2.7, 0.8 Hz, 1H), 7.16 – 7.11 (m, 1H), 3.65 (q, J = 6.6 Hz, 2H), 3.30 (t, J = 6.9 Hz, 2H); EIMS m/z 333.2 (M + 1)+; HPLC 98.9 area% (265 nm). Anal. Calcd for C16H13FN2OS2: C, 57.81 H, 3.94; N, 8.43. Found: C, 57.68; H, 3.97; N, 8.31.
5.2.5.13. 2-(2-Thiophen-2-ylamido)ethyl-4-(4-fluorophenyl)thiazole (66)
was prepared from 89d and thiophene-2-carbonyl chloride as a white solid (268 mg, 80%): mp 124-125 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (t, J = 5.7 Hz, 1H), 8.03 – 7.96 (m, 2H), 7.96 (s, 1H), 7.75 (dd, J = 5.0, 1.1 Hz, 1H), 7.72 (dd, J = 3.8, 1.2 Hz, 1H), 7.31 – 7.18 (m, 2H), 7.14 (dd, J = 5.0, 3.7 Hz, 1H), 3.65 (td, J = 6.9, 5.6 Hz, 2H), 3.29 (t, J = 7.0 Hz, 2H); EIMS m/z 333.2 (M + 1)+; HPLC 100 area% (265 nm). Anal. Calcd for C16H13FN2OS2: C, 57.81 H, 3.94; N, 8.43. Found: C, 57.61; H, 4.03; N, 8.39.
5.2.5.14. 2-(2-Pyrrolidin-1-ylamido)ethyl-4-(2-fluorophenyl)thiazole (67)
was prepared from 89b and pyrrolidine-1-carbonyl chloride as a white solid (111 mg, 34%): mp 102-103 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.12 (td, J = 7.8, 1.7 Hz, 1H), 7.85 (d, J = 2.6 Hz, 1H), 7.45 – 7.36 (m, 1H), 7.35 – 7.26 (m, 2H), 6.32 (t, J = 5.6 Hz, 1H), 3.42 (td, J = 7.0, 5.5 Hz, 2H), 3.25 – 3.14 (m, 6H), 1.84 – 1.73 (m, 4H); EIMS m/z 320.1 (M + 1)+; HPLC 99.1 area% (254 nm). Anal. Calcd for C16H18FN3OS·0.2H2O: C, 59.50; H, 5.74; N, 13.01. Found: C, 59.39; H, 5.71; N, 13.00.
5.2.5.15. 2-(2-Pyrrolidin-1-ylamido)ethyl-4-(3-fluorophenyl)thiazole (68)
was prepared from 89c and pyrrolidine-1-carbonyl chloride as white crystals (256 mg, 80%): mp 118-120 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.10 (s, 1H), 7.80 (ddd, J = 7.8, 1.6, 0.9 Hz, 1H), 7.75 (ddd, J = 10.7, 2.7, 1.5 Hz, 1H), 7.48 (td, J = 8.0, 6.2 Hz, 1H), 7.17 (dddd, J = 9.0, 8.3, 2.7, 0.9 Hz, 1H), 6.31 (t, J = 5.6 Hz, 1H), 3.41 (td, J = 7.0, 5.6 Hz, 2H), 3.25 – 3.13 (m, 6H), 1.85 – 1.71 (m, 4H); EIMS m/z 320.3 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C16H18FN3OS: C, 60.17; H, 5.68; N, 13.16. Found: C, 60.02; H, 5.66; N, 13.11.
5.2.5.16. 2-(2-Pyrrolidin-1-ylamido)ethyl-4-(4-fluorophenyl)thiazole (69)
was prepared from 89d and pyrrolidine-1-carbonyl chloride as ivory crystals (246 mg, 77%): mp 128-129 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.04 – 7.96 (m, 2H), 7.95 (s, 1H), 7.32 – 7.21 (m, 2H), 6.31 (t, J = 5.6 Hz, 1H), 3.41 (td, J = 7.1, 5.5 Hz, 2H), 3.24 – 3.18 (m, 4H), 3.16 (t, J = 7.0 Hz, 2H), 1.85 – 1.73 (m, 4H); EIMS m/z 320.2 (M + 1)+; HPLC 98.3 area% (254 nm). Anal. Calcd for C16H18FN3OS: C, 60.17; H, 5.68; N, 13.16. Found: C, 60.12; H, 5.73; N, 13.15.
5.2.5.17. 2-(2-Piperidin-1-ylamido)ethyl-4-(3-fluorophenylt)hiazole (70)
was prepared from 89c and piperidine-1-carbonyl chloride as a white solid (278 mg, 83%): mp 102-103 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (s, 1H), 7.80 (dt, J = 7.8, 1.2 Hz, 1H), 7.74 (ddd, J = 10.7, 2.7, 1.5 Hz, 1H), 7.48 (td, J = 8.0, 6.2 Hz, 1H), 7.21 – 7.11 (m, 1H), 6.63 (t, J = 5.4 Hz, 1H), 3.45 – 3.36 (m, 2H), 3.28 – 3.22 (m, 4H), 3.16 (t, J = 6.9 Hz, 2H), 1.57 – 1.46 (m, 2H), 1.45 – 1.34 (m, 4H); EIMS m/z 334.2 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H20FN3OS: C, 61.24; H, 6.05; N, 12.60. Found: C, 61.22; H, 6.11; N, 12.60.
5.2.5.18. 2-(2-Piperidin-1-ylamido)ethyl-4-(4-fluorophenyl)thiazole (71)
was prepared from 89d and piperidine-1-carbonyl chloride as a white powder (203 mg, 60%): mp 107-108 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.04 – 7.96 (m, 2H), 7.95 (d, J = 0.7 Hz, 1H), 7.31 – 7.22 (m, 2H), 6.63 (t, J = 5.5 Hz, 1H), 3.41 (td, J = 6.9, 5.3 Hz, 2H), 3.25 (dd, J = 6.5, 4.4 Hz, 4H), 3.16 (t, J = 7.0 Hz, 2H), 1.56 – 1.46 (m, 2H), 1.45 – 1.35 (m, 4H); EIMS m/z 334.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H20FN3OS: C, 61.24; H, 6.05; N, 12.60. Found: C, 61.15; H, 5.98; N, 12.39.
5.2.5.19. 2-(2-Piperidin-1-ylamido)ethyl-4-(2,4-difluorophenyl)thiazole (72)
was prepared from 89e and piperidine-1-carbonyl chloride as an off-white powder (232 mg, 65%): mp 104 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.15 (td, J = 8.9, 6.8 Hz, 1H), 7.83 (d, J = 2.6 Hz, 1H), 7.38 (ddd, J = 11.8, 9.2, 2.6 Hz, 1H), 7.20 (td, J = 8.4, 2.5 Hz, 1H), 6.64 (t, J = 5.4 Hz, 1H), 3.41 (td, J = 6.9, 5.3 Hz, 2H), 3.25 (dd, J = 6.5, 4.4 Hz, 4H), 3.17 (t, J = 6.9 Hz, 2H), 1.57 – 1.46 (m, 2H), 1.45 – 1.34 (m, 4H); EIMS m/z 352.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H19F2N3OS·0.2H2O: C, 57.51; H, 5.51; N, 11.84. Found: C, 57.47; H, 5.31; N, 11.70.
5.2.5.20. 2-(2-Piperidin-1-ylamido)ethyl-4-(3,4-difluorophenyl)thiazole (73)
was prepared from 89f and piperidine-1-carbonyl chloride as ivory crystals (287 mg, 81%): mp 100-103 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.06 (s, 1H), 7.97 (ddd, J = 12.2, 7.8, 2.1 Hz, 1H), 7.86 – 7.76 (m, 1H), 7.50 (dt, J = 10.7, 8.6 Hz, 1H), 6.63 (t, J = 5.4 Hz, 1H), 3.40 (td, J = 6.8, 5.3 Hz, 2H), 3.29 – 3.21 (m, 4H), 3.16 (t, J = 6.9 Hz, 2H), 1.51 (q, J = 6.2 Hz, 2H), 1.45 – 1.34 (m, 4H); EIMS m/z 352.1 (M + 1)+; HPLC 100 area% (254 nm). Anal. Calcd for C17H19F2N3OS·0.5H2O: C, 56.65; H, 5.59; N, 11.66. Found: C, 56.54; H, 5.59; N, 11.57.
5.2.6. N-(2-Cyanoethyl)benzamide (85)18
A solution of benzoyl chloride (11.98 g, 85.22 mmol) in THF (50 mL) was added dropwise to a solution of 3-aminopropionitrile (84, 5.98 g, 85.3 mmol) and triethylamine (15 mL, 107.6 mmol) in THF (50 mL) at −5 °C (ice-salt bath). The reaction mixture was stirred overnight at room temperature before being poured into water and extracted into EtOAC (3×). The dried, evaporated extract was recrystallized from hexanes and the minimum volume of EtOAC to give white crystals (12.36 g, 83%): mp 94 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.83 (t, J = 5.7 Hz, 1H), 7.90 – 7.82 (m, 2H), 7.60 – 7.50 (m, 1H), 7.53 – 7.44 (m, 2H), 3.50 (td, J = 6.5, 5.7 Hz, 2H), 2.78 (t, J = 6.5 Hz, 2H); HPLC 100 area% (230 nm). Anal. Calcd for C10H10N2O: C, 68.95; H, 5.79; N, 16.08. Found: C, 68.86; H, 5.80; N, 16.22.
5.2.7. 3-(Benzamido)thiopropionamide (86)18
A mixture of sodium hydrosulfide hydrate (1.68 g, 22.54 mmol) and magnesium chloride (1.97 g, 20.69 mmol) in DMF (20 mL) was stirred for 20 minutes at room temperature before the addition of 15-crown-5 (0.2 mL, 1.01 mmol) and N-(2-cyanoethyl)benzamide (85, 1.75 mg, 10.05 mmol). The mixture was stirred overnight before being poured over ice to give a white precipitate. This material was recrystallized from toluene/EtOH to give white crystals (1.31 g, 63%): mp 171-172 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 9.25 (s, 1H), 8.53 (t, J = 5.7 Hz, 1H), 7.87 – 7.79 (m, 2H), 7.56 – 7.47 (m, 1H), 7.50 – 7.41 (m, 2H), 3.59 (dt, J = 7.7, 5.8 Hz, 2H), 2.75 (dd, J = 8.0, 6.6 Hz, 2H); HPLC 99.3 area % (254 nm). Anal. Calcd for C10H12N2OS: C, 57.67; H, 5.81; N, 13.45. Found: C, 57.91; H, 5.74; N, 13.31.
5.2.8. 3-(Boc-amino)propionitrile (87)21
3-Aminopropionitrile (1.30 g, 18.53 mmol) was added dropwise to a stirred mixture of Montmorillonite K10 (199 mg) in molten di-tert-butyl dicarbonate (4.07 g, 18.7 mmol). After 1 hour, the mixture was diluted with EtOAC and filtered, The filtrate was evaporated to an oil, which was triturated with hexanes to give white crystals (2.63 g, 83%): mp 45 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.16 (t, J = 6.0 Hz, 1H), 3.15 (q, J = 6.3 Hz, 2H), 2.59 (t, J = 6.5 Hz, 2H), 1.39 (s, 9H). Anal. Calcd for C8H14N2O2: C, 56.45; H, 8.29; N, 16.46. Found: C, 56.51; H, 8.23; N, 16.25.
5.2.9. 3-(Boc-amino)thiopropionamide (88)22
The title compound was prepared analogously to compound 86 above from 3-(Boc-amino)propionitrile (87, 4.35 g. 25.56 mmol). The reaction mixture was poured over ice-water and extracted into dichloromethane. The extract was evaporated under reduced pressure followed by vacuum distillation (to remove residual DMF). The residue was recrystallized from EtOH/water to give white crystals (4.52 g, 87%): mp 111-112 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.39 (s, 1H), 9.17 (s, 1H), 6.79 (t, J = 5.4 Hz, 1H), 3.24 (ddd, J = 9.0, 7.5, 5.8 Hz, 2H), 2.63 – 2.52 (m, 2H), 1.37 (s, 9H). Anal. Calcd for C8H16N2OS: C, 47.03; H, 7.89; N, 13.71. Found: C, 47.13; H, 7.90; N, 13.44.
5.2.10. General procedure for amine hydrobromides 89a-f
A solution of 3-(boc-amino)thiopropionamide (88) and the appropriate α-bromoacetophenone (1 equivalent) in EtOH (10- 20 mL) was refluxed until the reaction was complete by HPLC (3-5 hours). Upon cooling, product precipitated from solution. The mixture was diluted was ether and the product was filtered off.
5.2.10.1. 4-Phenylthiazol-2-ethylamine hydrobromide (89a)
was prepared from 88 (2.05 g, 10.03 mmol) and 2-bromoacetophenone (2.00 g, 10.04 mmol) as a white solid (2.62 g, 92%): mp 187-188 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.06 (s, 1H), 8.02 – 7.94 (m, 2H), 7.88 (s, 3H), 7.50 – 7.40 (m, 2H), 7.40 – 7.31 (m, 1H), 3.40 – 3.26 (m, 4H); HPLC 100 area% (254 nm). Anal. Calcd for C11H12N2S·HBr: C, 46.32; H, 4.59; N, 9.82; Br, 28.02. Found: C, 46.38; H, 4.63; N, 9.77; Br, 27.85.
5.2.10.2. 4-(2-Fluorophenyl)thiazol-2-ethylamine hydrobromide (89b)
was prepared from 88 (1.33 g, 6.51 mmol) and 2-bromo-2′-fluoroacetophenone30 (1.88 g, 8.66 mmol) as a white solid (1.88 g, 95%): mp 164-166 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.16 (td, J = 7.8, 1.7 Hz, 1H), 7.94 (d, J = 2.6 Hz, 1H), 7.91 (s, 3H), 7.42 (tdd, J = 7.1, 5.3, 2.6 Hz, 1H), 7.37 – 7.27 (m, 2H), 3.42 – 3.34 (m, 2H), 3.34 – 3.26 (m, 2H); HPLC 100 area% (254 nm). Anal. Calcd for C11H11FN2S·HBr: C, 43.58; H, 3.99; N, 9.24; Br, 26.35. Found: C, 43.30; H, 4.02; N, 9.25; Br, 26.10.
5.2.10.3. 4-(3-Fluorophenyl)thiazol-2-ethylamine hydrobromide (89c)
was prepared from 88 (1.06 g, 5.19 mmol) and 2-bromo-3′-fluoroacetophenone (1.14 g, 5.25 mmol) as a white solid (1.49 g, 100%): mp 178-179 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.19 (s, 1H), 7.91 (s, 3H), 7.85 – 7.79 (m, 2H), 7.50 (td, J = 8.0, 6.1 Hz, 1H), 7.19 (dddd, J = 9.1, 8.3, 2.6, 1.0 Hz, 1H), 3.41 – 3.28 (m, 4H); HPLC 100 area% (254 nm). Anal. Calcd for C11H11FN2S·HBr: C, 43.58; H, 3.99; N, 9.24; Br, 26.35. Found: C, 43.32; H, 4.03; N, 908; Br, 26.26.
5.2.10.4. 4-(4-Fluorophenyl)thiazol-2-ethylamine hydrobromide (89d)
was prepared from 88 (3.07 g, 15.03 mmol) and 2-bromo-4′-fluoroacetophenone (3.26 g, 15.02 mmol) as a white solid (4.16 g, 86%): mp 219 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.04 (s, 1H), 8.07 – 7.97 (m, 2H), 7.91 (s, 3H), 7.36 – 7.21 (m, 2H), 3.40 – 3.24 (m, 4H); HPLC 100 area% (254 nm). Anal. Calcd for C11H11FN2S·HBr: C, 43.58; H, 3.99; N, 9.24; Br, 26.35. Found: C, 43.52; H, 4.00; N, 9.05; Br, 26.16.
5.2.10.5. 4-(2,4-Difluorophenyl)thiazol-2-ethylamine hydrobromide (89e)
was prepared from 88 (331 mg, 1.62 mmol) and 2-bromo-2′,4′-fluoroacetophenone (380 mg, 1.62 mmol) as a white solid (468 mg, 90%): mp 197 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.19 (td, J = 8.9, 6.8 Hz, 1H), 7.91 (d, J = 2.7 Hz, 1H), 7.90 (s, 3H); 7.40 (ddd, J = 11.8, 9.3, 2.6 Hz, 1H), 7.27 – 7.17 (m, 1H), 3.41 – 3.28 (m, 4H); HPLC 100 area% (254 nm). Anal. Calcd for C11H10F2N2S·HBr: C, 41.13; H, 3.45; N, 8.72. Found: C, 40.88; H, 3.56; N, 8.59.
5.2.10.6. 4-(3,4-Difluorophenyl)thiazol-2-ethylamine hydrobromide (89f)
was prepared from 88 (1.75 g, 8.57 mmol) and 2-bromo-3′,4′-fluoroacetophenone323232 (1.98 g, 8.44 mmol) as a white solid (2.49 mg, 92%): mp 191-193 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.11 (d, J = 0.8 Hz, 1H), 8.01 (dddd, J = 12.2, 7.9, 2.2, 0.8 Hz, 1H), 7.86 (s, 3H), 7.80 (dddd, J = 8.7, 4.3, 2.2, 1.3 Hz, 1H), 7.48 (ddd, J = 10.7, 9.0, 8.2 Hz, 1H), 3.36 – 3.21 (m, 4H); HPLC 100 area% (254 nm). Anal. Calcd for C11H10F2N2S·HBr: C, 41.13; H, 3.45; N, 8.72. Found: C, 41.16 H, 3.46; N, 8.74.
Supplementary Material
Acknowledgments
This work was supported by the Consortium for Parasitic Drug Development (CPDD), and National Institutes of Health (NIH) gran number 5R01AI106850-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [5 January 2016]; http://www.who.int/mediacentre/factsheets/fs259/en/.
- 2.Sykes ML, Baell JB, Kaiser M, Chatelain E, Moawad SR, Ganame D, Ioset J-R, Avery VM. PLoS Negl Trop Dis. 2012;6:e1896. doi: 10.1371/journal.pntd.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astelbauer F, Walochnik J. Int. J. Antimicrob. Agents. 2011;38:118. doi: 10.1016/j.ijantimicag.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Burri C. Parasitology. 2010;137:1987. doi: 10.1017/S0031182010001137. [DOI] [PubMed] [Google Scholar]
- 5.Patrick DA, Bakunov SA, Bakunova SM, Kumar EVKS, Lombardy RJ, Jones SK, Bridges AS, Zhirnov O, Hall JE, Wenzler T, Brun R, Tidwell RR. J. Med. Chem. 2007;50:2468. doi: 10.1021/jm0612867. [DOI] [PubMed] [Google Scholar]
- 6.Patrick DA, Bakunov SA, Bakunova SM, Wenzler T, Brun R, Tidwell RR. Bioorg. Med. Chem. 2014;22:559. doi: 10.1016/j.bmc.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Patrick DA, Ismail MA, Arafa RK, Wenzler T, Zhu X, Pandharkar T, Jones SK, Werbovetz KA, Brun R, Boykin DW, Tidwell RR. J. Med. Chem. 2013;56:5473. doi: 10.1021/jm400508e. [DOI] [PubMed] [Google Scholar]
- 8.Wenzler T, Yang S, Patrick DA, Braissant O, Ismail MA, Tidwell RR, Boykin DW, Wang MZ, Brun R. Antimicrob. Agents Chemother. 2014;58:4452. doi: 10.1128/AAC.02309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dann O, Fick H, Pietzner B, Walkenhorst E, Fernbach R, Zeh D. Justus Liebigs Ann. Chem. 1975:160. [Google Scholar]
- 10.Das BP, Boykin DW. J. Med. Chem. 1977;20:531. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 11.Anbazhagan M, Boykin DW. Heterocycl. Commun. 2003;9:117. [Google Scholar]
- 12.Ansede JH, Anbazhagan M, Brun R, Easterbrook JD, Hall JE, Boykin DW. J. Med. Chem. 2004;47:4335. doi: 10.1021/jm030604o. [DOI] [PubMed] [Google Scholar]
- 13.Harrill AH, DeSmet KD, Wolf KK, Bridges AS, Eaddy JS, Kurtz CL, Hall JE, Paine MF, Tidwell RR, Watkins PB. Toxicol. Sci. 2012;130:416. doi: 10.1093/toxsci/kfs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torreele E, Trunz BB, Tweats D, Kaiser M, Brun R, Mazue G, Bray MA, Pecoul B. PLoS Neglected Trop. Dis. 2010;4:e923. doi: 10.1371/journal.pntd.0000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs RT, Nare B, Wring SA, Orr MD, Chen D, Sligar JM, Jenks MX, Noe RA, Bowling TS, Mercer LT, Rewerts C, Gaukel E, Owens J, Parham R, Randolph R, Beaudet B, Bacchi CJ, Yarlett N, Plattner JJ, Freund Y, Ding C, Akama T, Zhang YK, Brun R, Kaiser M, Scandale I, Don R. PLoS Neglected Trop. Dis. 2011;5:e1151. doi: 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatipaka HB, Gillespie JR, Chatterjee AK, Norcross NR, Hulverson MA, Ranade RM, Nagendar P, Creason SA, McQueen J, Duster NA, Nagle A, Supek F, Molteni V, Wenzler T, Brun R, Glynne R, Buckner FS, Gelb MH. J. Med. Chem. 2014;57:828. doi: 10.1021/jm401178t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young RC, Ganellin CR, Griffiths R, Mitchell RC, Parsons ME, Saunders D, Sore NE. Eur. J. Med. Chem. 1993;28:201. doi: 10.1021/jm00398a028. [DOI] [PubMed] [Google Scholar]
- 18.Zee-Cheng K-Y, Cheng C-C. J. Heterocycl. Chem. 1970;7:1439. [Google Scholar]
- 19.Manaka A, Sato M. Synth. Commun. 2005;35:761. [Google Scholar]
- 20.Chankeshwara SV, Chakraborti AK. J. Mol. Catal. A: Chem. 2006;253:198. [Google Scholar]
- 21.Houssin R, Bernier JL, Henichart JP. Synthesis. 1988:259. [Google Scholar]
- 22.Barker PL, Gendler PL, Rapoport H. J. Org. Chem. 1981;46:2455. [Google Scholar]
- 23.Bakunova SM, Bakunov SA, Patrick DA, Kumar EVKS, Ohemeng KA, Bridges AS, Wenzler T, Barszcz T, Kilgore Jones S, Werbovetz KA, Brun R, Tidwell RR. J. Med. Chem. 2009;52:2016. doi: 10.1021/jm801547t. [DOI] [PubMed] [Google Scholar]
- 24.Peña I, Pilar Manzano M, Cantizani J, Kessler A, Alonso-Padilla J, Bardera AI, Alvarez E, Colmenarejo G, Cotillo I, Roquero I, de Dios-Anton F, Barroso V, Rodriguez A, Gray DW, Navarro M, Kumar V, Sherstnev A, Drewry DH, Brown JR, Fiandor JM, Martin J. J. Sci. Rep. 2015;5:8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baell J, Piggott M, Russell S, Toynton A, Rahmani R, Ferrins L, Nguyen N. 2015 WO2015172196A1.
- 26.Wang MZ, Zhu X, Srivastava A, Liu Q, Sweat JM, Pandharkar T, Stephens CE, Riccio E, Parman T, Munde M, Mandal S, Madhubala R, Tidwell RR, Wilson WD, Boykin DW, Hall JE, Kyle DE, Werbovetz KA. Antimicrob. Agents Chemother. 2010;54:2507. doi: 10.1128/AAC.00250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogarth PM, Pietersz GA, Moloney GP. WO2004058747A1. 2004 [Google Scholar]
- 28.Silberg A, Benko A, Panczel IA. Stud. Cercet. Chim. 1965;13:655. [Google Scholar]
- 29.Silberg A, Benko A, Panczel IA. Rev. Roum. Chim. 1965;10:617. [Google Scholar]
- 30.Nishida H, Arikawa Y, Hirase K. 2009 WO2009041705A2.
- 31.Eberle M, Bachmann F, Strebel A, Roy S, Saha G, Sadhukhan SK, Saxena R, Srivastava S. 2005 WO2005061476A2.
- 32.Ridge DN, Hanifin JW, Harten LA, Johnson BD, Menschik J, Nicolau G, Sloboda AE, Watts DE. J. Med. Chem. 1979;22:1385. doi: 10.1021/jm00197a020. [DOI] [PubMed] [Google Scholar]
- 33.Biagetti M, Contini SA, Genski T, Guery S, Leslie CP, Mazzali A, Pizzi DA, Sabbatini FM, Seri C. 2009 WO2009095377A1.
- 34.Borhani DW, Calderwood DJ, Frank KE, Davis HM, Josephsohn NS, Skinner BS. 2008 WO2008063287A2.
- 35.Kajigaeshi S, Kakinami T, Okamoto T, Fujisaki S. Bull. Chem. Soc. Jpn. 1987;60:1159. [Google Scholar]
- 36.Hoover DJ, Witter KG. 2008 WO2008004117A1.
- 37.Boger DL, Patel M. J. Org. Chem. 1987;52:2319. [Google Scholar]
- 38.McGhee WD, Pan Y, Talley JJ. Tetrahedron Lett. 1994;35:839. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.