Abstract
In cancer cells, telomere length maintenance occurs largely via the direct synthesis of TTAGGG repeats at chromosome ends by telomerase, or less frequently by the recombination-dependent alternative lengthening of telomeres (ALT) pathway. The latter is characterized by the atypical clustering of telomeres within promyelocytic leukemia (PML) nuclear bodies, which harbor proteins that are linked with DNA repair and recombination activity. For this reason, it is speculated that these associated PML bodies represent the sites of the recombination that maintains telomere length. The protocols described here can be employed for the routine investigation of the structural integrity of telomeres and the association of proteins at telomeres in normal cells, challenged cells, and archived formalin-fixed paraffin-embedded clinical tissue specimens that may have activated the ALT pathway.
Keywords: telomeres, metaphase, DNA damage, ALT pathway
INTRODUCTION
Mammalian telomeres are defined by the TTAGGG DNA sequence that is repeated over several kilobases (kb) and a terminal lasso-type structure termed the T-loop (Griffith et al., 1999). A specialized protein complex known as shelterin maintains telomere protection and regulates telomere length maintenance pathways (Palm and de Lange, 2008). Telomere protection is mediated, for the most part, by TRF1, TRF2, and POT1, core subunits of shelterin (Karlseder et al., 1999; Denchi and de Lange, 2007; Sfeir et al., 2009). For instance, genetic depletion of TRF2 profoundly alters telomere structure and renders telomeres de-protected. Similarly, telomere shortening that occurs in somatic cells due to the “end-replication” problem diminishes the protective function of shelterin. In either case, the loss of TRF2 compromises telomere structure and leads to a DNA damage response (DDR) at chromosome ends (identified by H2AX phosphorylation and 53BP1 accumulation), and potentially DNA repair activities that direct fusion of chromosomes (Denchi and de Lange, 2007; Cesare et al., 2013).
In cancer cells, telomere length maintenance occurs largely via the direct synthesis of TTAGGG repeats at chromosome ends by telomerase (for review, see Blackburn et al., 2006) or less frequently by the recombination-dependent alternative lengthening of telomeres (ALT) pathway (for review, see Cesare and Reddel, 2010). One of the several hallmarks of the ALT pathway is the atypical clustering of telomeres within promyeloctyic leukemia (PML) nuclear bodies (Yeager et al., 1999). These ALT-associated PML bodies (APBs) have been found to harbor a large and growing number of proteins that are linked with DNA repair and recombination activity (Cesare and Reddel, 2010). For this reason, it is speculated that APBs represent the sites of the recombination that maintains telomere length. Another feature of ALT is an increased frequency of sister chromatid exchange events between telomeres (T-SCEs) in individual cells that are thought to represent evidence of telomere recombination events in ALT cells.
The protocols described here can be employed for the routine investigation of the structural integrity of telomeres and the association of proteins at telomeres in normal cells, challenged cells (i.e., following knockdown of protein X, DNA damage, senescence, cancer), and archived formalin-fixed paraffin-embedded clinical tissue specimens that may have activated the ALT pathway. Basic Protocol 1 describes combined immunofluorescence and telomere fluorescence in situ hybridization (FISH) on adherent cells. Basic Protocol 2 details in vivo detection of telomeres in archived clinical specimens. Basic Protocol 3 describes detection of telomeric DNA sequence on mitotic chromosome spreads with FISH. Support protocols describe variations on these three basic protocols.
Strategic Planning
PNA probes
PNA probes bind with high affinity to complementary sequences, and PNA-DNA duplexes are highly stable (Egholm et al., 1993). Thus, the benefit of telomere PNA FISH is that the staining is generally of a very high quality, with minimal background. PNA probes are 18-mers consisting of three repeats of either TTAGGG or CCCTAA sequence, though miniPNA probes have been developed and applied to the study of telomeres (Pham et al., 2014). PNA probes can be purchased from several vendors, and thus should be used according to the provided instructions. Here, we indicate the source, concentrations, and dilutions of PNA probes used in our laboratories.
Imaging and controls
As with all experimental readouts, it is critical that the appropriate technical and experimental controls be employed at all times. Positive experimental controls are necessary to identify the appearance of expected co-localization events. In addition, image-acquisition thresholds need to be set to sub-saturating levels on positive experimental controls and maintained across all samples in a particular experimental replicate. This minimizes false positives that can occur when background noise is amplified by prolonged image acquisition times. Because of staining variability between experiments, it is critical that the appropriate controls be included in each data set. It is also appropriate to collect data in three dimensions and to confirm co-localization of IF and FISH signals within a single Z plane and not in maximum intensity projections.
BASIC PROTOCOL 1
COMBINED IMMUNOFLUORESCENCE AND TELOMERE FLUORESCENCE IN SITU HYBRIDIZATION (FISH) ON FIXED ADHERENT CELLS
This protocol describes how to combine antibody-based immunofluorescence (IF) and fluorescence in situ hybridization (FISH) with fluorescence-conjugated telomere peptide nucleic acid (PNA) probes to identify interactions between proteins of interest and telomeric DNA. In practical terms, by using telomere FISH, more options to multiplex IF staining using antibodies derived from different species are available (i.e., telomere PNA plus protein A plus protein B plus DAPI). This method can be co-opted for any IF target with the caveat that fixation and IF conditions need to be empirically determined for each antibody. The protocol that is detailed in this section is widely used to identify interactions between telomeres and DNA damage response factors at so-called “telomere-dysfunction induced foci (TIF)” (Takai et al., 2003) using antibodies that detect γH2A.X or α-53BP1 in conjunction with PNA FISH. It is also routinely used to identify ALT-associated PML nuclear bodies (APBs), a marker of ALT-positive cancers, using an α-PML primary antibody again in conjunction with PNA FISH (Yeager et al., 1999).
Materials
Adherent cells growing in culture
Appropriate tissue culture medium with serum (cell-line specific)
Alcian blue stain (optional, see recipe)
Phosphate-buffered saline (PBS; APPENDIX 2A)
Pre-extraction buffer (optional, see recipe)
2% paraformaldehyde (see recipe)
KCM (see recipe)
ABDIL (see recipe)
20 mg/ml DNase-free RNase A stock
Normal goat serum
Primary antibodies
PBST (see recipe)
Goat-derived, fluorophore-conjugated secondary antibodies (Molecular Probes)
Graded ethanol series (70%, 90%, 100% v/v ethanol)
0.3 µg/ml telomere PNA, Alexa Fluor 488–conjugated; see recipe)
DAPI (4′-6-diamidino-2-phenylindole)
PNA Wash A (see recipe)
PNA Wash B (see recipe)
Prolong Gold mounting medium (Life Technologies, cat. no. P10144)
Electron microscopy forceps (both sterile and unsterile)
12-mm round glass coverslips
24-well tissue culture plate
Bench top shaker
Menzel-Glaser Superfrost slides
Humidified chamber
Heat block with thermostat control
Fluorescence microscope
Cell culture
-
1.Using sterile electron microscopy forceps, place one sterile 12-mm round glass coverslip per well in a 24-well tissue culture plate. Culture adherent cells directly on the coverslip.Treating the coverslips with Alcian blue (by immersion in the Alcian blue staining solution as described in the recipe in described in Reagents and Solutions) prior to sterilization gives the glass a bluish tint, making it easier to see when submerged in solution. This treatment may also help with cell adherence.
Day 1: Fixation and primary antibody treatment
-
2.
Aspirate the culture medium and wash the cells twice with 1.5 ml of PBS. Continue with steps 3 to 7 while leaving the coverslip in the tissue culture plate.
-
3.Optional: Incubate with 500 µl diluted pre-extraction buffer on ice for up to 3 min.This removes proteins that are not chromatin-bound (Zhu et al., 2000). For some IF targets, particularly those that form foci, this step is necessary to identify the chromatin fraction of a protein population from the excess of protein that remains unbound to the chromatin. See Critical Parameters.
-
4.Fix in 500 µl 2% paraformaldehyde at room temperature for 10 min.This fixation method is effective for most primary antibodies. However, fixation conditions need to be determined empirically for each primary antibody.
-
5.
Rinse cells twice with 1.5 ml distilled, deionized water.
-
6.Permeabilize with 1 ml KCM at room temperature for 10 min.An alternative is to use a buffer containing 0.1% sodium citrate/0.1% Triton X-100 in distilled, deionized water for 10 min at room temperature.
-
7.Add 300 µl ABDIL containing 100 µg/ml DNase-free RNase A (added from 20 mg/ml stock) plus 2% (v/v) normal goat serum at room temperature for 1 hr.This serves as a blocking and RNase-treatment step.
-
8.Dilute primary antibody or antibodies in ABDIL containing 2% (v/v) goat serum.Two primary antibodies derived from different species can be used simultaneously (i.e., mouse α-γ H2A.X and rabbit α-53BP1). Determine primary antibody dilutions empirically.
-
9.Pipet 20 µl of diluted antibody (or antibodies) onto a clean slide placed in a humidified chamber. Remove coverslip from the 24-well plate with electron microscopy forceps and place the coverslip with the fixed cells down into the diluted antibody. Incubate overnight at 4°C.To easily make a humidified chamber, just place a lightly moistened small Kimwipe in a closed container to prevent any drying due to evaporation.
Day 2: Secondary antibody and telomere FISH
-
10.
Using electron microscopy forceps, return one coverslip per well, with the cell side up, to a well of an unused 24-well tissue culture plate. Perform steps 11 to 17 in the tissue culture plate.
-
11.
Wash the coverslip three times, each time with 1.5 ml PBST for 10 min at room temperature with shaking.
-
12.Dilute goat-derived, fluorophore-conjugated, secondary antibody (or antibodies) 1:1000 in ABDIL plus 2% (v/v) goat serum.Ensure fluorophore(s) do not overlap in the light spectrum with the Alexa Fluor 488– conjugated telomere PNA probe (see step 19). We typically use highly cross-adsorbed goat secondary antibodies from Molecular Probes (Life Technologies) conjugated to Alexa Fluor 564 and/or Alexa Fluor 647.
-
13.Add 250 µl of diluted secondary antibody per well. Incubate for 1 hr in a Nutator at room temperature in the dark. Alternatively, use a humidified chamber as described in step 9.From the step forward protect samples from light.
-
14.
Wash coverslips three times, each time 10 min in PBST as described in step 11.
-
15.Fix with 500 µl 2% paraformaldehyde for 10 min at room temperature.This is required to fix the antibodies in place for the subsequent PNA hybridization steps.
-
16.Rinse twice with 1.5 ml of distilled, deionized water.At this stage the coverslips can be stored in PBS at 4°C in the dark for up to 7 days.
-
17.Ethanol dehydrate the coverslips with a graded ethanol series: 1 ml 70% ethanol for 3 min, 1 ml 90% ethanol for 3 min, then 1 ml 100% ethanol, each time for 3 min.Do not aspirate the 100% ethanol. Doing so will cause the coverslip to adhere to the plate.
-
18.
Using electron microscopy forceps, remove the coverslip from the 100% ethanol and place with the cell side up on an absorbent paper towel. Air dry to completion (approximately 20 min).
-
19.
On a clean glass slide, pipet 8 µl of 0.3 µg/ml Alexa Fluor 488–conjugated C-strand telomere PNA probe and place the coverslip cell-side-down into the PNA solution.
-
20.
Denature for 5 min at 80°C and hybridize overnight at room temperature in the dark in a humidified chamber.
Day 3: PNA washing and mounting
-
21.
Return the coverslip to an unused 24-well plate as in step 10.
-
22.
Wash the coverslips twice, each time for 10 min with 1.5 ml PNA wash A with shaking.
-
23.Wash the coverslips three times, each time for 10 min with 1.5 ml PNA wash B with shaking. Add DAPI in the second wash to a concentration of 50 ng/ml.Adding DAPI to the second wash counterstains the DNA while minimizing background.
-
24.
Rinse twice with 1.5 ml distilled, deionized water.
-
25.
Dehydrate through a graded ethanol series and air dry the coverslip as described in steps 17 and 18.
-
26.
Mount with Prolong Gold and store overnight at room temperature before imaging the following day.
-
27.
Store slides at 4°C.
Day 4: Imaging
-
28.Visualize the slides and capture images using a fluorescent microscope equipped with the appropriate objectives, filter sets, and digital camera.For example, the Cesare laboratory uses a Zeiss AxioImager M.1 equipped with a Axio-Cam MRm digital camera, a Plan-Apochromat 63 × 1.40 NA objective or a Plan-Neofluar 40× objective, and the following Zeiss filter sets: DAPI (49), FITC (44), Texas Red (45) and Cy5 (50).
SUPPORT PROTOCOL 1
COMBINED IMMUNOFLUORESCENCE AND TELOMERE FLUORESCENT IN SITU HYBRIDIZATION (FISH) ON CYTOCENTRIFUGED CHROMOSOME SPREADS (METAPHASE-TIF ASSAY)
This is a variation on Basic Protocol 1 that enables a quantitative measurement of the number of telomeres that associate with an IF target in mitotic cells. As this technique has been applied to measure TIF in pro-metaphase and metaphase cells, it has been termed the “metaphase-TIF assay” (Cesare et al., 2009). However, this method can be applied to measure telomere interactions with diverse IF targets. The major benefit of this method is that proper analysis returns absolute numbers of DDR-positive telomeres. Moreover, this method can easily discern if the IF target in question localizes with one or both of the telomere sister-chromatids on a mitotic chromosome end, and if changes in telomere length as measured by telomere FISH intensity correlate with the binding of the protein of interest (Kaul et al., 2012). DDR factors downstream of MDC1 do not localize to mitotic chromatin due to cell-cycle-specific phosphorylation events (Giunta et al., 2010; Orthwein et al., 2014). As it is a component of chromatin itself, γH2A.X is the preferred IF target for metaphase-TIF assays.
Additional Materials (also see Basic Protocol 1)
10 µg/ml Colcemid (Karyomax) stock (Life Technologies, cat. no,. 15212-012)
Hypotonic solution: 0.2% trisodium citrate/0.2% KCl (see recipe)
Phosphate-buffered saline (PBS: APPENDIX 2A) containing 3.7% formaldehyde (see recipe)
α-γH2A.X primary antibody (Millipore 05–636, for metaphase-TIF assay)
Cytocentrifuge clips, funnel, and filter papers (Thermo Fisher, cat. no. 3120110)
Cytocentrifuge (e.g., Cytospin 4 Cytocentrifuge from Thermo Fisher, cat. no. A78300003)
Coplin jars
Parafilm
22 × 40–mm coverslips
DABCO antifade mountain medium (optional, see recipe)
Additional reagents and equipment for mammalian cell tissue culture including trypsinization and counting cells (APPENDIX 3B; Phelan, 2006)
Day 1: Cytocentrifugation, fixation, immunofluorescence and PNA hybridization
- Treat cultures with 20 ng/ml Colcemid (1:500 dilution of 10 µg/ml stock) by adding the drug directly to the culture medium and incubating under normal tissue culture conditions for no more than 1 hr.Please see the Critical Parameters, below, regarding Colcemid treatment.
- While the culture is incubating with Colcemid, assemble the appropriate number of cytocentrifuge clips, each containing a funnel, filter paper, and a Menzel-Gläser Superfrost Plus slide, and place in the cytocentrifuge.Steps 4 to 6 need to be completed in less than 10 min. It helps to have the cytocentrifuge prepared first, before cells are collected and resuspended in hypotonic solution.
Collect and pellet the cells by trypsinization (APPENDIX 3B; Phelan, 2006), quenching with growth medium, and centrifugation in a 15-ml conical tube for 3 min at 185 × g, room temperature.
- Resuspend the cells in fresh, room temperature 0.2% trisodium citrate/0.2% KCl. Incubate at room temperature for 5 min to swell the cells.This is a different hypotonic solution than that used in Basic Protocol 3. Longer swelling times may cause the cells to burst. It is critical at this step that the cells not be diluted past the ideal concentration for cytocentrifugation (see step 5).
- While the cells are swelling, measure the cell concentration (APPENDIX 3B; Phelan, 2006) in the hypotonic solution. Dilute to the desired cell concentration by adding 0.2% trisodium citrate/0.2% KCl as needed.The quality of metaphase morphology in cytocentrifuged samples is dependent on the cell concentration in hypotonic solution. The ideal cell concentration for most cell lines is around 1 × 105 cells/ml. However, this needs to be determined empirically for each cell line. This can be done by performing steps 1 to 9 in this protocol using cell concentrations ranging from 0.5 to 3.0 × 105 cells/ml. Stain these samples with 50 ng/ml DAPI in PBS and visualize chromosome morphology using a fluorescence microscope. Select the cell concentration which yields the best chromosome morphology.
Transfer 500 µl of diluted cells to the assembled funnels in the cytocentrifuge and centrifuge for 10 min at 2000 rpm with medium acceleration.
- Disassemble the cytocentrifuge clips and remove the slide.A “spot” corresponding to where the cells were deposited from the cytocentrifuge funnel onto the slide should be evident.Unlike cytogenetic chromosome dropping in Basic Protocol 3, chromosome morphology and metaphase spreads will not be visible at this point using a bright-field microscope.
- Fix in PBS containing 3.7% formaldehyde at room temperature for 10 min by placing the slides in a Coplin jar with fixative solution or by distributing a small amount of fixative onto the cell spots of slides while they are lying on a flat surface.PBS containing 4% formaldehyde is the fixative used for metaphase-TIF assays with the mouse α-γH2A.X antibody from Millipore. Fixation conditions need to be determined empirically for different antibodies.
- In a Coplin jar, rinse the slides with distilled, deionized water three times.This can be performed by sequentially moving slides through three Coplin jars containing water or simply in a single Coplin jar in which the water is drained and replaced.At this point the slides can be stored for up to 3 days in PBS at 4°C.
Permeabilize by soaking the slides in KCM in a Coplin jar at room temperature for 10 min.
Place the slides in a humidified chamber and add 200 µl ABDIL containing 100 µg/ml RNase A to each slide. Overlay with Parafilm. Incubate at 37°C for 15 min.
- Dilute mouse α-γH2A.X antibody 1:1000 in ABDIL.For other immunofluorescence targets, antibody concentrations and incubation times need to be determined empirically.
Remove Parafilm and distribute 200 µl diluted primary antibody to each slide. Overlay with Parafilm and incubate at room temperature for 1 hr.
Transfer slides to a Coplin jar and wash three times, each time for 5 min in PBST with shaking.
Dilute goat anti-mouse Alexa Fluor 564–conjugated secondary antibody 1:1000 in ABDIL.
- Return the slides to the humidified chamber, distribute 200 µl of diluted secondary antibody per slide, and overlay with Parafilm as described in step 13. Incubate at room temperature for 30 min.From this step onwards, protect slides from the light.
Wash the slides as in step 14.
- Fix the antibody-stained cells as in step 8.This is required to fix the antibodies in place during the subsequent PNA hybridization steps.
Rinse the slides as in step 9.
Dehydrate the slides in a Coplin jar using a graded ethanol series: 70% ethanol for 3 min, 90% ethanol for 2 min, and 100% ethanol for 2 min.
Remove the slides and allow them to air dry to completion.
- Distribute 20 µl of 0.3 µg/ml Alexa Fluor 488–conjugated C-strand telomere PNA probe to each cell spot and overlay with a coverslip.This is easily done by pipetting the PNA solution onto the slide just below, but not covering, the cell spot. Touch a 22 × 40–mm coverslip to the PNA solution and allow the solution to spread along the edge of the coverslip. Then, drop the coverslip so that it falls over the cell spot, distributing the solution evenly without introducing bubbles.
Denature for 5 min at 80°C.
Transfer the slides to humidified chamber and hybridize overnight at room temperature in the dark.
Day 2: PNA wash and mounting
-
25.
Gently remove the coverslips and return the slides to a Coplin jar.
-
24.
Wash slides in the Coplin jar twice, each time for 10 min in PNA Wash A with shaking.
-
25.
Wash slides in a Coplin jar three times, each time for 5 min in PNA Wash B with shaking. Add DAPI in the second wash to a concentration of 50 ng/ml
-
26.
Rinse the slides twice with distilled, deionized water then remove from the Coplin jar and air dry to completion.
-
27.
Mount in DABCO or Prolong Gold mounting medium and store overnight before imaging.
Day 3: Imaging
-
28.Visualize the slides and capture images using a fluorescent microscope equipped with the appropriate objectives, filter sets, and digital camera.This process can be expedited with automated metaphase finding and imaging software. For example, the Cesare laboratory uses Metasystems software to control automated imaging on a Zeiss AxioImager Z.2 fitted with a motorized 8-slide Maerzhaeuser stage, an AxioCam MRm digital camera, a Plan-Apochromat 63 × 1.40 NA objective, and the following Zeiss filter sets: DAPI (49), FITC (44), and TexasRed (45).
BASIC PROTOCOL 2
IN VIVO DETECTION OF TELOMERES IN ARCHIVED CLINICAL SPECIMENS
This protocol describes the use of a telomere-specific fluorescence in situ hybridization (FISH) assay in archived formalin-fixed paraffin-embedded clinical tissue specimens. This protocol provides single-cell resolution of telomere length while, importantly, maintaining the tissue architecture (Meeker et al., 2002). This protocol has been used successfully by numerous groups across a wide range of normal and tumor human tissue specimens for qualitative and quantitative telomere length assessment.
Materials
Archived formalin-fixed paraffin-embedded clinical tissue specimens (4 to 5 µm sections)Xylene
Graded ethanol series (70%, 95%, and 100%)
1% Tween 20 wash: 198 ml deionized distilled H2O plus 2 ml Tween 20 (store at 4°C)
Antigen unmasking solution (Vector Laboratories)
-
PNA probes:
Telomere-specific peptide nucleic acid (PNA) probe (CCCTAACCCTAACCCTAA with the N-terminal covalently linked to Cy3)
Centromere-specific PNA probe (ATTCGTTGGAAACGGGA with the N-terminal covalently linked to Alexa Fluor 488)
PNA wash buffer (see recipe)
PBST (see recipe)
500 ng/ml DAPI (4′-6-diamidino-2-phenylindole; Sigma)
Prolong antifade mounting medium (Molecular Probes)
Superfrost plus micro slides (VWR), or other positively charged slides
Slide warmer with thermostat control
Tissue-Tek Slide Staining Set (VWR) or set of Coplin jars
Plastic slide holder
Kitchen steamer
Coverslips
Heat block with thermostat control
Humidified slide hybridization chamber (e.g., empty pipet tip box containing moist paper towel, protected from light)
Forceps
-
Fluorescence microscope equipped with appropriate fluorescence filter set
- Obtain unstained tissue sections (4 to 5 µm) from tissue type of interest from archival formalin-fixed paraffin-embedded tissue blocks on Superfrost Plus Micro slides (VWR) or other positively charged slides.It is important not to use uncharged microscope slides, as the tissue may be lost or folded during this protocol, especially with tissues that have a high adipose content (e.g. normal breast tissue). In general, it is important to avoid heating tissue sections at high temperatures for prolonged periods because it may increase tissue autofluorescent background signals. For example, some reference laboratories bake the tissue sections at high temperatures after sectioning to dry the slides; if possible, this should be avoided.
To help melt the paraffin, place tissue slides on a slide warmer and heat at 65°C for 10 min.
- Wash tissue slides three times, each time for 5 min, in xylene.If available in your laboratory, steps 3 to 8 and 11 to 14 can be performed using the Tissue-Tek slide staining kit. If not, a set of Coplin jars or other appropriate containers may also be used.
- Wash tissue slides twice, each time for 3 min, in 100% ethanol.It is important not to allow the slides to air dry between steps 4 and 14.
Wash tissue slides twice, each time for 3 min, in 95% ethanol.
Wash tissue slides in 70% ethanol for 3 min.
Wash tissue slides in deionized distilled water for 3 min.
Wash tissue slides in 1% Tween 20 wash for 1 min.
- Incubate slides in a plastic slide holder with antigen unmasking solution in a preheated (boiling) kitchen steamer for 30 min.A general citrate buffer, pH6.0, may also be substituted for this specific antigen unmasking solution.
Remove container from kitchen steamer, open the lid of the container, and let cool at room temperature for 10 min.
Wash tissue slides in deionized distilled water for 1 min.
Wash tissue slides 2 times in 70% ethanol, each wash for 3 min.
Wash tissue slides 2 times in 95% ethanol, each wash for 3 min.
Wash tissue slides in 100% ethanol for 2 min.
Allow the slides to completely air dry at this point.
- Add 35 µl of PNA probe mix (0.33 µg/ml Cy3-conjugated telomere-specific PNA probe/0.33 µg/ml of the Alexa Fluor 488-conjugated centromere-specific PNA probe) to each slide.The centromere PNA probe is included in the hybridization mix to serve as a positive control for hybridization efficiency. However, if you plan to continue on to Support Protocol 2 to perform combined FISH and immunofluorescence staining, you may omit the centromere PNA probe (labeled with Alexa Fluor 488) or plan accordingly to use a fluorescent secondary antibody other than Alexa Fluor 488 (e.g., Alexa Fluor 647; Molecular Probes).
- Gently apply a coverslip to each tissue slide. Avoid air bubbles.There is no need to seal the coverslip with nail polish; however, handle the slides carefully so as not to let the coverslip slide off.
- Denature tissue slides at 84°C for 5 min.It is important that for all subsequent steps to minimize exposure of light to the tissue slides as much as possible.
- Hybridize tissue slides for at least 2 hr at room temperature in a dark, humidified slide hybridization chamber. Tissue slides can also be hybridized overnight at room temperature.To easily make a humidified chamber, just place a lightly moistened small Kimwipe in a closed container to prevent any drying due to evaporation.
After hybridization is complete, carefully remove the coverslip from the tissue slides with forceps.
- Wash tissue slides twice, each time for 15 min with gentle agitation, in PNA wash buffer.The PNA wash buffer contains formamide; dispose of appropriately.
Wash tissue slides three times in PBST, each time for 5 min.
If performing immunofluorescence antibody staining for APB (ALT-associatedPML bodies) detection, drain excess solution from the slides and proceed to Support Protocol 2. If not, continue directly with the next step.
Wash tissue slides in deionized distilled water for 5 min.
Incubate slides in 500 ng/ml 4′-6-diamidino-2-phenylindole (DAPI) solution for 10 min.
Wash tissue slides three times in deionized distilled water, each time for 5 min.
Drain tissue slides but do not allow to completely dry.
- Apply 40 µl of Prolong antifade mounting medium to each tissue slide.Slowly apply the Prolong, as it has a high viscosity; avoid air bubbles.
Carefully apply a coverslip to each tissue slide.
Store slides at room temperature (in the dark) for 24 hr to allow the Prolong to harden.
Store the slides at 4°C for at least 1 week before imaging.
- Visualize and image the slides using a fluorescence microscope equipped with an appropriate fluorescence filter set and a 40× objective (or 63× or 100× objective).For example, our laboratory uses a Nikon 50i epifluorescence microscope equipped with X-Cite series 120 illuminator (EXFO Photonics Solutions) and a 40×/0.95 NA PlanApo lens with correction collar. The fluorescence excitation/emission filters are: Cy3 excitation, 546 nm/10 nm BP; emission, 578 nm LP (Carl Zeiss); DAPI excitation, 330 nm; emission, 400 nm via an XF02 fluorescence set (Omega Optical); Alexa Fluor 488 excitation, 475 nm; emission, 535 via a B-2E/C filter set (Nikon).
SUPPORT PROTOCOL 2
IN VIVO DETECTION OF APBS IN ARCHIVED CLINICAL SPECIMENS
The protocol is a continuation of Support Protocol 1 (start this protocol after completing steps 1 to 23 of Support Protocol 1) and describes the use of an anti-PML antibody in conjunction with telomere-specific FISH to identify ALT-associated PML bodies (APBs) (Montgomery et al., 2004; Henson et al., 2005; Heaphy et al., 2011b). Although not described in detail, the same protocol has been used to identify numerous other proteins in different tissues specimens. For example, a basal-specific anti-pan-cytokeratin antibody has been used to distinguish normal basal epithelial cells from normal luminal epithelial cells in the prostate (Meeker et al., 2002; Heaphy et al., 2013). In addition, an anti-smooth-muscle antibody has been successfully used to delineate normal myoepithelial cells from normal luminal cells with terminal ductal lobular units within the breast (Meeker et al., 2004a). For successful staining, in conjunction with telomere-specific FISH, a primary antibody needs to be (i) validated using a citrate antigen retrieval buffer and (ii) already optimized for the appropriate concentration; (iii) the fluorescent secondary antibody needs to be from the appropriate species (Meeker et al., 2002).
Additional Materials (see Basic Protocol 1 and Support Protocol 1; start after step 23)
Anti-PML antibody (Dako, cat. no. PG-M3)
Antibody dilution buffer (Ventana)
Anti-mouse IgG fraction Alexa Fluor 488 (Molecular Probes)
Dilute the mouse anti-PML antibody in antibody dilution buffer at a 1:100 dilution.
- Apply 200 µl of diluted primary antibody onto each slide.Total volume can be adjusted as long as the entire tissue section is covered.
Incubate at room temperature for 45 min.
Wash tissue slides three times in PBST, each time for 5 min.
- Dilute the anti-mouse IgG fraction Alexa Fluor 488 (secondary antibody) in PBS at a 1:100 dilution.Other fluorescent dyes (e.g. Alexa Fluor 647) may be used as long as they do not interfere with the Cy3 fluorescent label from the telomere-specific PNA probe.
- Apply 200 µl of diluted secondary antibody onto each slide.Total volume can be adjusted as long as the entire tissue section is covered.
Incubate at room temperature for 30 min.
Wash tissue slides three times in PBST, each time for 5 min.
Wash tissue slides in deionized distilled water for 5 min.
Incubate tissue slides in 4′-6-diamidino-2-phenylindole (DAPI) solution (500 ng/ml in deionized distilled water) for 10 min.
Wash tissue slides three times in deionized, distilled water, each time for 5 min.
Drain tissue slides but do not allow to completely dry.
- Apply 40 µl of Prolong antifade mounting medium to each tissue slide.Slowly apply the Prolong, as it has a high viscosity; avoid air bubbles.
Carefully apply a coverslip to each tissue slide.
Store slides at room temperature (in the dark) for 24 hr to allow Prolong to harden.
Store the slides at 4°C for at least 1 week before imaging.
Visualized and image the slides using a fluorescence microscope equipped with appropriate fluorescence filter set and a 40× objective (or 63 × or 100 × objective).
BASIC PROTOCOL 3
DETECTION OF TELOMERIC DNA SEQUENCE ON MITOTIC CHROMOSOME SPREADS WITH FLUORESCENT IN SITU HYBRIDIZATION (FISH)
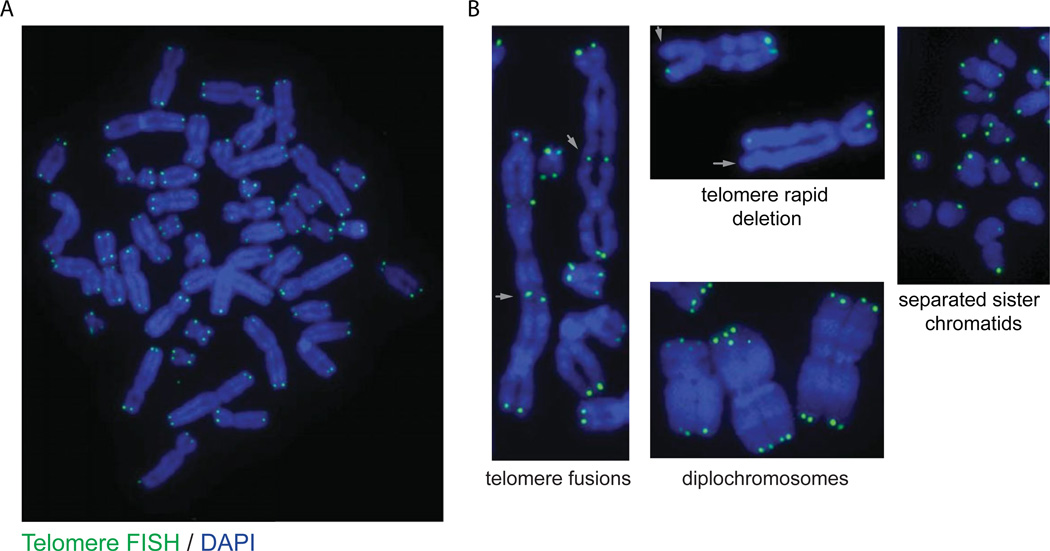
This protocol describes how to prepare cytogenetic chromosome spreads from cells growing in tissue culture and to identify telomeric DNA sequence using fluorescent in situ hybridization (FISH) with fluorescently labeled peptide nucleic acid (PNA) probe. This is a widely used technique in telomere biology with numerous applications, including: quantitative measurement of telomere fluorescent intensity as a relative measure of telomere length (Q-FISH; Poon and Lansdorp, 2001), visualization of covalent telomere-telomere ligation (i.e., end-to-end chromosome fusions; Smogorzewska et al., 2002; Denchi and de Lange, 2007), fragile telomeres (Sfeir et al., 2009), rapid telomere deletion (Wang et al., 2004), or the occurrence of chromosomal abnormalities such as diplochromosomes (i.e., endoreduplication; Hockemeyer et al., 2006) in relation to genetic alteration of telomere biology.
Materials
Adherent cells growing in culture
10 µg/ml Colcemid (Karyomax) stock (Life Technologies, cat. no,. 15212-012)
Fixative (see recipe)
Hypotonic solution: 0.075 M KCl (dissolve 0.56 g KCl in 100 ml H2O; prepare fresh and prewarm to 37°C)
100% ethanol
Phosphate-buffered saline (PBS; APPENDIX 2A)
Phosphate-buffered saline (PBS: APPENDIX 2A) containing 3.7% formaldehyde (see recipe)
1 mg/ml pepsin (Sigma, cat. no. P7000)
Phosphate-buffered saline (PBS) containing 250 µg/ml RNase A (see recipe)
Graded ethanol series (70%, 95%, and 100%)
0.3 µg/ml telomere PNA probe, Alexa Fluor 488–conjugated, see recipe)
PNA Wash A (see recipe)
PNA Wash B (see recipe)
500 ng/ml DAPI (4′-6-diamidino-2-phenylindole; Sigma)
Prolong Gold (Life Technologies, cat. no. P10144) or DABCO (see recipe) mounting medium
15-ml conical centrifuge tubes (e.g., BD Falcon)
Centrifuge
Coplin jars
Bright-field microscope
Bench top shaker
Humidified chamber
24 × 50 mm glass coverslips
Heat block with thermostatic control
Fluorescence microscope
Additional reagents and equipment for mammalian cell tissue culture including trypsinization and counting cells (APPENDIX 3B; Phelan, 2006)
Day 1: Cell harvesting and fixation
- Treat the cell culture with 100 ng/ml Colcemid (1:100 dilution of 10 µg/ml stock) by adding the drug directly to the culture medium and incubating under normal tissue culture conditions for 1 to 2 hr.Longer incubation times may result in poor chromosome morphology.
Prepare fresh fixative and hypotonic solution. Cool the fixative on ice and warm the hypotonic solution to 37°C.
Disperse cells from the tissue culture flask using trypsin (Phelan, 2006) and quench with serum-containing medium. Transfer the cell suspension to a 15-ml conical tube and pellet by centrifugation for 3 min at 185 × g, room temperature.
Aspirate the supernatant and resuspend the cell pellet in 10 ml of 37°C hypotonic solution. Incubate for 8 min at 37°C. Pellet the cells by centrifugation for 3 min at 185 × g, room temperature.
- Aspirate the supernatant, leaving ∼ 300 µl in the tube. Resuspend the cells by gently flicking the tube.If you have multiple samples to process, placing the samples on ice at this step will slow down the swelling process and maintain sample integrity.
Add 9 ml of fixative in the following manner. Add 1 ml of fixative dropwise and tap the tube after each drop to mix. Add the following 2 ml while consistently tapping or gently vortexing the tube to mix. Add the remaining 6 ml, cap the tube, and invert to mix.
Fix at 4°C overnight and then store indefinitely at −20°C.
Day 2: Chromosome dropping
-
8.
Clean slides by soaking in 100% ethanol in a Coplin jar for at least 1 hr. Remove the ethanol and allow slides to dry completely. Before dropping chromosomes, wipe the slide with a Kimwipe.
-
9.
Prepare fresh fixative and cool on ice.
-
10.Collect cells by centrifugation for 3 min at 290 × g, 4°C. Aspirate all the supernatant and gently resuspend the cell pellet in fresh fixative.Resuspension volume will vary depending on the size of the cell pellet. 2 × 106 cells/ml is a good estimate.
-
11.Drop chromosomes in the following manner. Thoroughly soak a stack of five paper towels with water and place a clean slide directly onto the wet paper towels. Drop one drop of the cells suspended in fresh fixative from a height of 2 cm onto three separate locations on the slide. Allow the solution to air dry (typically 3 to 5 min).There is no need to drop the cells from an elevated height onto an angled slide, as commonly described.
-
12.Observe metaphase quality under a bright-field light microscope. If the cells are too dense, dilute further and drop again. If they are too sparse, concentrate by centrifugation and resuspend in a smaller volume before dropping.Do not move on past this step until you have slides with nicely spread metaphases with good chromosome morphology.
-
13.
Allow the slides to cure overnight at room temperature.
Day 3: Telomere FISH
-
14.
Rehydrate the slides in PBS for 5 min in a Coplin jar.
-
15.
Decant the PBS and fix the slides in the Coplin jar with PBS containing 3.7% formaldehyde for 5 min at room temperature.
-
16.
Decant the formaldehyde solution and wash the slides in the Coplin jar three times, each time for 2 min in PBS at room temperature with gentle shaking.
-
17.Place the slides, cell side up, in a humidified chamber and add 200 µl of PBS containing 250 µg/ml RNase A to each slide. Overlay with Parafilm and incubate at 37°C for 15 min.To easily make a humidified chamber, just place a lightly moistened small Kimwipe in a closed container to prevent any drying due to evaporation.
-
18.Optional: Add 200 µl of 1 mg/ml pepsin solution and overlay with Parafilm as in step 17. Incubate at 37°C for 10 min.While pepsin treatment is commonly employed in DNA FISH protocols, for staining with small PNA probes, this step is typically unnecessary.
-
19.
Return the slides to a Coplin jar and fix again with PBS containing 3.7% formaldehyde and wash in PBS as described in steps 15 and 16.
-
20.
Decant the PBS and dehydrate the slides in the Coplin jar by passing them through a graded ethanol series as follows: 3 min in 70% ethanol, 3 min in 90% ethanol, and 3 min in 100% ethanol.
-
21.
Remove slides from the Coplin jar and air dry to completion (10 to 20 min).
-
22.
Distribute 50 µl of 0.3 µg/ml Alexa Fluor 488 conjugated-C strand telomere PNA probe to the surface of the slide with the adhered cells; this is easily done by placing a 24 × 50 mm glass coverslip on the bench top and pipetting 50 µl of PNA probe solution onto the coverslip. Touch the PNA probe with the slide, drawing up the coverslip and distributing the PNA probe across the sample without introducing air bubbles.
-
23.
Denature by placing slides on a heat block at 70°C for 10 min.
-
24.Transfer the slides to a humidified chamber and hybridize overnight in the dark at room temperature.From the step forward protect the slides from light.
Day 4: Washing and mounting
-
25.
Gently remove the coverslip and return the slides to a Coplin jar.
-
26.
Wash in the Coplin jar twice, each time for 10 min in PNA Wash A with shaking.
-
27.
Wash three times, each time for 5 min in PNA Wash B with shaking. Add 50 ng/ml DAPI (from 500 ng/ml stock) to the second wash as a DNA counter stain.
-
28.
Rinse the slides twice with distilled, deionized water.
-
29.
Remove the slides from the Coplin jar and air dry to completion.
-
30.
Mount in DABCO or Prolong Gold antifade mounting medium and store overnight before imaging.
Day 5: Imaging
- 31. Visualize the slides and capture images using a fluorescent microscope equipped with the appropriate objectives, filter sets, and digital camera.This process can be expedited with automated metaphase finding and imaging software. For example, the Cesare laboratory uses Metasystems Software to control automated imaging on a Zeiss AxioImager Z.2 fitted with a motorized 8-slide Maerzhaeuser stage, an AxioCam MRm digital camera, a Plan-Apochromat 63 × 1.40 NA objective, and the following Zeiss filter sets: DAPI (49), FITC (44).
SUPPORT PROTOCOL 3
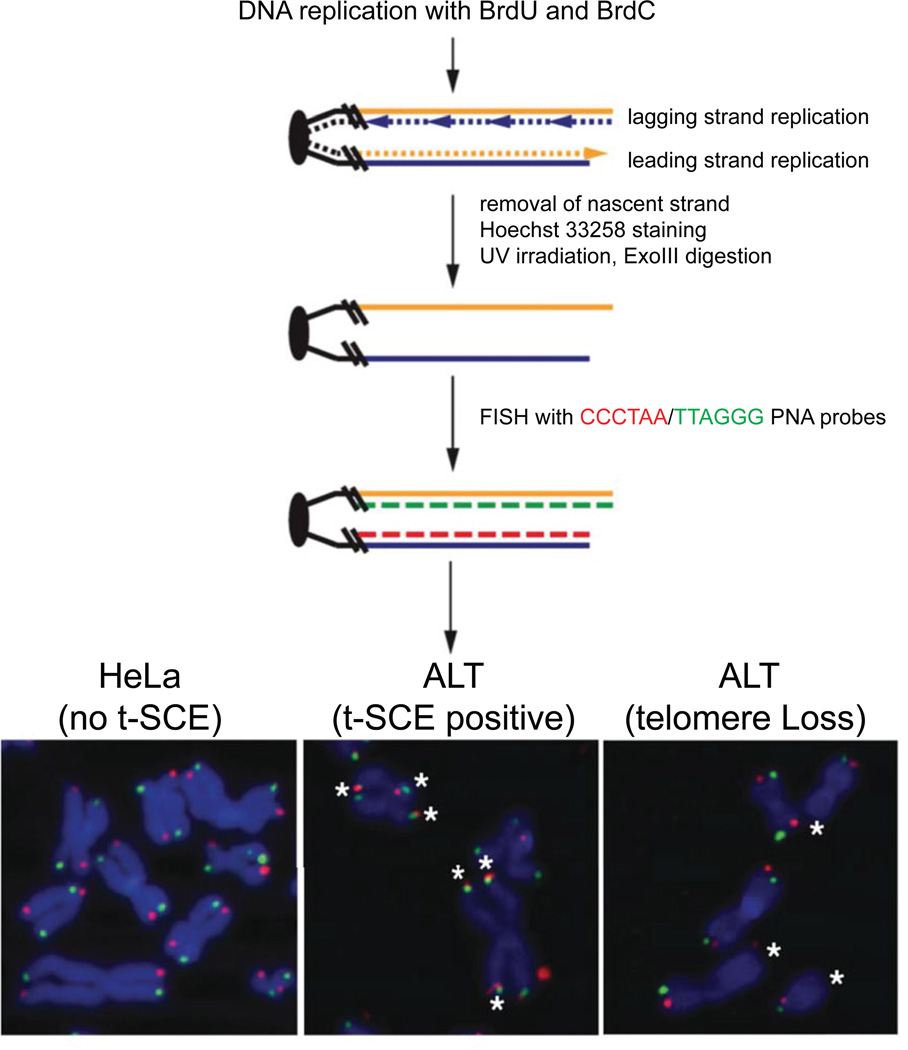
DETECTION OF TELOMERE SISTER CHROMATID EXCHANGE (T-SCE) ON MITOTIC CHROMOSOME SPREADS BY CHROMOSOME ORIENTATION FISH (CO-FISH)
Chromosome Orientation FISH (CO-FISH) is a powerful technique that has been used to delineate DNA strands that are replicated by the leading and lagging replicative machinery and to assess the exchange of telomeric DNA between sister chromatids (Bailey et al., 2001). The latter is frequently associated with elevated recombination that generates “cross-over” events as part of the ALT pathway of telomere maintenance (Londoño-Vallejo et al., 2004), but can also occur as a result of aberrant replicative processing of telomeres as in premature aging syndromes like Werner Syndrome (Crabbe et al., 2004).
CO-FISH involves dual labeling of cells with the thymidine analog 5′-bromo-2′deoxyuridine (BrdU) and 5′-bromo-2′deoxyuridine (BrdC) for a single round of DNA replication. The newly replicated strands can be degraded by UV-irradiation and exonucleolytic cleavage to generate extended stretches of TTAGGG/CCCTAA DNA strands. These DNA strands are then visualized following hybridization with complementary fluorescently coupled CCCTAA/TTAGGG PNA probes.
Additional Materials (also see Basic Protocol 3)
10 mM 3:1 BrdU/BrdC mixture (1000× stock; see recipe)
20 × SSC (see recipe)
2× SSC with 2.5 mg/ml Hoechst 33258 (see recipe)
Exonuclease III (ExoIII) and corresponding buffer (NEB or Promega)
70% deionized formamide:30% 2× SSC mixture
TexasRed-G-rich telomere PNA probe (see recipe)
Stratalinker 1800 UV irradiator (Stratagene)
Day 1: Cell culture
- Seed cells in normal growth medium at 40% to 50% density.Cells should be ∼50% confluent at the time of BrdU/BrdC addition.
Day 2: BrdU/C labeling
-
2.Incubate cells for ∼ 12 to 16 hr in growth medium containing 10 µM 3:1 BrdU/BrdC, a 1:1000 dilution of the stock.See Critical Parameters regarding duration of BrdU/BrdC labeling.
Day 3: Cell harvesting and fixation
-
3.
Replace medium containing BrdU/BrdC with normal growth medium.
-
4.Treat the cell culture with 100 ng/ml Colecemid (1:100 dilution of 10 µg/ml stock) by adding the drug directly to the culture media and incubating under normal tissue culture conditions for 1 to 2 hr.Inadequate Colcemid treatment time will yield low numbers of metaphases. Longer incubation times may result in poor chromosome morphology. See Troubleshooting.
-
5.
Prepare fresh fixative and hypotonic solution. Cool the fixative on ice and warm the hypotonic solution to 37°C.
-
6.
Disperse cells from the tissue culture flask using trypsin and quench with serum-containing medium (APPENDIX 3B; Phelan, 2006). Transfer the cell suspension to a 15-ml conical tube and pellet by centrifugation for 3 min at 185 × g, room temperature.
-
7.
Aspirate the supernatant and resuspend the cell pellet in 10 ml of 37°C hypotonic solution. Incubate for 8 min at 37°C. Pellet the cells by centrifugation for 3 min at 185 × g, room temperature.
-
8.Aspirate the supernatant, leaving ∼ 300 µl in the tube. Resuspend the cells by gently flicking the tube.If you have multiple samples to process, placing the samples on ice at this step will slow down the swelling process and maintain sample integrity.
-
9.
Add 9 ml of fixative in the following manner. Add 1 ml of fixative dropwise and tap the tube after each drop to mix. Add the following 2 ml while consistently tapping or gently vortexing the tube to mix. Add the remaining 6 ml, cap the tube, and invert to mix.
-
10.
Fix at 4°C overnight and then store indefinitely at −20°C.
Day 4: Chromosome dropping
-
11.
Clean slides by soaking in 100% ethanol in a Coplin jar for at least 1 hr. Remove the ethanol and allow slides to dry completely. Before dropping chromosomes wipe the slide with a Kimwipe.
-
12.
Prepare fresh fixative and cool on ice.
-
13.Collect cells by centrifugation for 3 min at 290 × g, 4°C. Aspirate all the supernatant and gently resuspend the cell pellet in fresh fixative.Resuspension volume will vary depending on the size of the cell pellet. 2 × 106 cells/ml is a good estimate.
-
14.Drop chromosomes in the following manner. Thoroughly soak a stack of five paper towels with water and place a clean slide directly onto the wet paper towels. Drop one drop of the cells suspended in fresh fixative from a height of 2 cm onto three separate locations on the slide. Allow the solution to air dry (typically 3 to 5 min).There is no need to drop the cells from an elevated height onto an angled slide, as commonly described.
-
15.Observe metaphase quality under a bright-field light microscope. If the cells are too dense, dilute further and drop again. If they are too sparse, concentrate by centrifugation and re-suspend in a smaller volume before dropping.Do not move on past this step until you have slides with nicely spread metaphases with good chromosome morphology.
-
16.
Allow the slides to cure overnight at room temperature.
-
17.
Store slides at room temperature for days or keep at −20°C and use as necessary.
Day 5: Degradation of newly synthesized DNA strands
-
18.
Rehydrate the slides in PBS for 5 min in a Coplin jar.
-
19.
Decant the PBS and fix the slides in the Coplin jar with PBS containing 2% formaldehyde for 5 min at room temperature.
-
20.
Decant the formaldehyde solution and wash the slides in the Coplin jar three times, each time for 2 min in PBS at room temperature with gentle shaking.
-
21.Place the slides, cell side up, in a humidified chamber and add 200 µl of PBS containing 250 µg/ml RNase A to each slide. Overlay with Parafilm and incubate at 37°C for 15 min.To easily make a humidified chamber, just place a lightly moistened small Kimwipe in a closed container to prevent any drying due to evaporation.
-
22.Optional: Add 200 µl of 1 mg/ml pepsin solution and overlay with Parafilm as in step 17 of Basic Protocol 3. Incubate at 37°C for 10 min.While pepsin treatment is commonly employed in DNA FISH protocols, for staining with small PNA probes, this step is typically unnecessary.
-
23.
Wash twice in PBS followed by a rinse in 2× SSC (diluted 10× in distilled water from 20 × SSC stock solution).
-
24.
Incubate slides in 2× SSC containing 0.5 µg/ml Hoechst 33258 for 15 min in a Coplin jar in the dark.
-
25.
Expose slides to ∼365-nm UV light in a Stratalinker 1800 UV irradiator for 30 min as follows:
Cover the bottom of the Stratalinker in Saran wrap
Place 300 µl of 2× SSC on a coverslip and pick up the coverslip with the slide.
- Gently turn over holding the coverslip in place and place on the bottom of the Stratalinker.Cells need to be facing up.
Move the slides to the Stratalinker in a plastic dish that is covered in Saran wrap to prevent evaporation and desiccation of cells.
Set time on the Stratalinker and begin irradiation. After 30 min irradiation the plate will be warm.
-
26.
Rinse the slides twice in distilled, deionized water to remove excess 2× SSC.
-
27.
Digest the newly synthesized (BrdU/BrdC-containing) DNA strands with 80 µl of 10 U/µl of Exonuclease III (1:10 dilution of enzyme) in the supplied buffer at 37°C for 15 to 30 min. Place enzyme mixture on coverslips and pick up coverslip with slide as described in step 25b.
-
28.
Wash slides in PBS for 5 min
-
29.
Further denature the newly synthesized DNA strands by incubation in solution of 70% deionized formamide/30% 2× SSC on a heating block set to 70°C for 10 min. Place ∼100 µl deionized formamide/2 × SSC solution on coverslips and pick up coverslip with the slide as described above.
-
30.
Remove the coverslips, rinse them twice with water, and dehydrate the slides in a Coplin jar by soaking through a graded ethanol series as follows: 3 min in 70% ethanol, 3 min in 90% ethanol, and 3 min in 100% ethanol. Proceed directly to hybridization with telomere stand-specific probes or store the slides until ready for hybridization.
Day 5: Telomere FISH with strand-specific probes
-
31.
Place 40 to 60 µl of 0.06 µg/ml TexasRed-G-rich Telomere PNA probe onto a coverslip and pick up with the slide.
-
32.Denature at 70°C for 10 min then hybridize for 2 hr at room temperature in a humidified chamber in the darkFrom the step forward, protect the slides from light.
-
33.
Gently remove the coverslip and return the slides to the Coplin jar.
-
34.
Wash twice, each time for 5 min in PNA Wash A in a Coplin jar with shaking in the dark.
-
35.
Place 40 to 60 µl of 0.3 µg/ml Alexa 488-conjugated C-strand telomere PNA probe onto a coverslip and pick up with the slide. Hybridize for 2 hr at room temperature in a humidified chamber in the dark.
-
36.
Gently remove the coverslips and return the slides to the Coplin jar.
-
37.
Wash in the Coplin jar twice, each time for 10 min in PNA Wash A with shaking.
-
38.
Wash in the Coplin jar three times, each time for 5 min in PNA Wash B with shaking.
-
39.
Rinse the slides twice with distilled and deionized water then remove from the Coplin jar and air dry to completion.
-
40.
Mount in DABCO or Prolong Gold antifade, with DAPI if necessary, and store overnight before imaging.
Day 6: Imaging
-
41.Visualize the slides and capture images using a fluorescent microscope equipped with the appropriate objectives, filter sets and digital camera.For example, the Cesare laboratory uses a Zeiss AxioImager M.1 equipped with a Axio-Cam MRm digital camera, a Plan-Apochromat 63 × 1.40 NA objective, or a Plan-Neofluar and 40× objective, and the following Zeiss filter sets: DAPI (49), FITC (44), TexasRed (45), Cy5 (50).
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps.
ABDIL
20 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
2% (w/v) bovine serum albumin (BSA)
0.2% (w/v) fish gelatin (e.g., Sigma)
150 mM NaCl
0.1% (v/v) Triton X-100
0.1% (w/v) sodium azide
Store up to 1 year at 4°C
Alcian blue stain
-
Dilute Alcian blue 8GX (Sigma, cat. no. A5268) to 1 mg/ml in distilled, deionized water. Store indefinitely at room temperature.
Can be used multiple times.
Alcian blue–stained coverslips
In an appropriately sized beaker, immerse glass coverslips in Alcian blue stain (see recipe). Gently agitate using a benchtop shaker on low speed for 1 hr. Wash with deionized water until no more dye comes off. Rinse thoroughly with distilled, deionized water. Wash several times with 100% ethanol. Place coverslips on Whatman paper and allow them to air dry to completion. Sterilize in a covered 100-mm glass petri dish by baking at 400°C for 4 hr.
Blocking reagent
Make a 10% stock of Blocking Reagent (Roche Life Science, cat. no. 11096176001) in maleic acid buffer (100 mM maleic acid, 150 mM NaCl, Adjust pH to 7.5 with NaOH). Store up to 1 year at 4°C.
BrdU/BrdC mixture, 3:1, 10 mM (1000 × Stock)
Mix 3 parts of 10 mM bromodeoxyuridine (BrdU) with 1 part of 10 mM bromod-eoxycytidine (BrdC) to get final stock solution of 7.5 mM BrdU and 2.5 mM BrdC. Store up to 1 year at −20°C or indefinitely at −80°C.
Fixative (3:1 methanol:acetic acid)
75% (v/v) methanol
25% (v/v) glacial acetic acid
Prepare fresh each time and cool in freezer or in ice-bucket
KCM
120 mM KCl
20 mM NaCl
10 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
0.1% (v/v) Triton X-100
Store up to 1 year at room temperature
Paraformaldehyde (PFA), 2%
Prepare 4% paraformaldehyde: High-purity (methanol-free) 16% paraformaldehyde can be purchased from vendors such as Pierce or Thermo Fisher. Alternatively 4%
PFA can be prepared as follows:
Weigh out 40 g paraformaldehyde (Sigma; use respiratory protection)
Add 800 ml distilled, deionized water
Add 5 M NaOH (∼ 500 µl) dropwise
Stir and heat on a hot plate (let paraformaldehyde dissolve; up to 60°C, do not boil!)
Add 100 ml 10× PBS (see recipe for 1 × PBS in APPENDIX 2A)
Cool down to room temperature
Adjust pH to precisely 7.4
Make up to 1 liter with distilled, deionized water
-
Store up to 1 year at −20°C in aliquots as necessary
- Prepare 2% paraformaldehyde: Prior to use, dilute 4% paraformaldehyde 1:1 in PBS (APPENDIX 2A) for a final concentration of 2%.Use of low-purity paraformaldehyde powder or incorrect measurement and setting of the pH of the final solution will make the cells/chromosomes appear fuzzy when visualized. All buffers should be made fresh for daily use.
PBST (PBS with Tween 20)
Phosphate-buffered saline (PBS; APPENDIX 2A) containing:
0.1% (v/v) Tween 20
Store up to 1 year at room temperature
Phosphate-buffered saline (PBS) containing 3.7% formaldehyde
Per 100 ml:
10 ml 10× PBS (see recipe for PBS in APPENDIX 2A)
10 ml 37% formaldehyde solution stabilized with methanol (Sigma)
80 ml distilled, deionized water
Prepare fresh
Phosphate-buffered saline containing 250 µg/ml RNase A
10 ml phosphate-buffered saline (PBS)
125 µl 20 mg/ml RNase A (Sigma, cat. no. R4875)
Store at 4°C
PNA hybridization solution
70% (v/v) formamide (deionized)
0.25% (w/v) Blocking Reagent (see recipe)
10 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
Store up to 6 months at −20°C
PNA wash A
70% (v/v) formamide (deionized)
10 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
Prepare fresh
PNA wash B
50 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
150 mM NaCl
0.8% (v/v) Tween 20
Store at room temperature
PNA wash buffer
140 ml formamide (deionized)
58 ml deionized distilled water
2 ml 1 M Tris·Cl, pH 7.5 (APPENDIX 2A)
Store up to 1 year at room temperature
Pre-extraction buffer (10×)
0.5% Triton X-100
20 mM HEPES-KOH, pH 7.9
50 mM NaCl
3 mM MgCl2
300 mM sucrose
Sterilize by autoclaving and store indefinitely
Dilute to 1 × with distilled, deionized water prior to use
SSC, 20×
3 M NaCl
300 mM trisodium citrate dihydrate
For 1 liter, dissolve 175.3 g NaCl and 88.2 g trisodium citrate dihydrate in 1800 ml water. Adjust pH to 7.0 with a few drops of concentrated HCl. Adjust final volume to 1 liter. Aliquot as necessary. Sterilize by autoclaving
Store indefinitely at room temperature
Telomere PNA, 0.3 µg/ml, Alexa Fluor 488–conjugated
Resuspend lyophilized Alexa Fluor 488-OO-CCCTAACCCTAACCCTAA 3′ PNA (Panagene) at 0.2 µg/ml in 0.1% (w/v) trifluoroacetic acid. Heat to 60°C while vortexing frequently. Divide into 5-µg aliquots (25 µl) and re-lyophilize. Store aliquots indefinitely in the dark at −20°C.
Make a 25 µg/ml concentrated stock by dissolving a 5-µg PNA aliquot in 200 µl of 0.1% (w/v) trifluoroacetic acid. Heat to 60°C while vortexing frequently. Store solution at 4°C in the dark for up to 1 year.
Prepare 0.3 µg/ml Alexa Fluor 488-OO-CCCTAACCCTAACCCTAA 3′ PNA working solution by diluting 12 µl of the 25 µg/ml concentrated stock in 1 ml of PNA hybridization solution (see recipe). Heat to 60°C while vortexing frequently. Store solution at −20°C in the dark for up to 1 year. Heat and vortex before each use.
Texas Red-G-rich telomere PNA probe
Resuspend lyophilized Texas Red/Alexa Fluor-555/568/594-OO-CCCTAACCC TAACCCTAA 3′ PNA (Panagene) at 0.2 µg/ml in 0.1% (w/v) trifluoroacetic acid. Heat to 60°C while vortexing frequently.
Distribute 5 µg aliquots (25 µl) and re-lyophilize. Store aliquots indefinitely in the dark at −20°C.
Make a 25 µg/ml concentrated stock by dissolving a 5-µg PNA aliquot in 200 µl 0.1% trifluoroacetic acid. Heat to 60°C while vortexing frequently. Store solution at 4°C in the dark for up to 1 year.
Prepare 0.06 µg/ml Texas Red/Alexa Fluor-555/568/594-OO-CCCTAACCC TAACCCTAA 3′ PNA working solution by diluting 2.4 µl of the 25 µg/ml concentrated stock in 1 ml of PNA hybridization solution (see recipe). Heat to 60°C while vortexing frequently. Store solution at −20°C in the dark for up to 1 year. Heat and vortex before each use.
Trisodium citrate, 0.2%/KCl, 0.2% (hypotonic solution for Support Protocol 1)
0.2 g trisodium citrate
0.2 g KCl
100 ml distilled, deionized water
Prepare fresh
COMMENTARY
Background Information
Basic Protocol 1
Microscopic identification of putative co-localization between telomeric DNA and a protein, or proteins, of interest is a common assay in telomere biology laboratories. Major benefits of this assay are that it can be modified to accommodate essentially any immunofluorescence (IF) target and that it can be combined with almost any molecular biology– or cell-based treatment.
Support Protocol 1
Combined IF and telomere FISH on mitotic chromosome spreads provides the opportunity to gather quantitative data related to the number of chromosome ends in a mitotic cell that interact with an IF target of interest. This has proven to be immensely valuable in measuring the number of DNA damage response (DDR)– positive telomeres, or “metaphase-TIF.” While this method is commonly employed to measure a telomere DDR, it can be used to visualize other IF targets of interest.
Basic Protocol 2 and Support Protocol 2
These protocols detail the utilization of a telomere-specific fluorescence in situ hybridization (FISH) assay that allows for single-cell telomere length determination in archived formalin-fixed paraffin-embedded clinical tissue specimens, while preserving the inherent architecture of the tissue (Meeker et al., 2002). The method in Basic Protocol 2 can be combined with fluorescent immunostaining as in Support Protocol 2, for example with an anti-PML antibody to identify ALT-associated PML bodies (APBs; Heaphy et al., 2011a). These protocols have been used successfully employed by a number of investigators in a wide range of tumor tissues and normal and/or benign control tissues (Montgomery et al., 2004; Meeker et al., 2004b; Heaphy et al., 2011b; Hurwitz et al., 2014).
Basic Protocol 3
Telomere FISH on mitotic chromosome spreads provides the opportunity to visualize individual telomeres in the context of chromosome morphology. It allows a quick assessment of the quantities of telomere DNA on individual chromosomes. It also permits investigation of telomere structure whereby one can observe and count the presence of aberrant features that reflect the status of telomeric integrity.
Support Protocol 3
Chromosome Orientation FISH or CO-FISH is an extension of conventional detection of telomeres on metaphase chromosome spreads. The procedure involves several technical steps that must be performed with care and stringency, and the analysis of CO-FISH should be approached with care and rigor. If performed successfully, CO-FISH offers the potential to ascertain data regarding the replicative history of telomeres in a single cell (Bailey et al., 2001). It allows the clear assessment of telomere recombination (Londoño-Vallejo et al., 2004).
Critical Parameters
Basic Protocol 1
Controls
For the TIF assay, siRNA depletion of TRF2 provides a strong readout—i.e., high percent co-localization of telomeres with γH2AX and/or 53BP1. For the APB assay, a known ALT cell line will contain −5% of cells with positive co-localization of PML with telomere FISH.
Antibody conditions
It is necessary to empirically determine the fixation conditions and dilution for each primary antibody.
Pre-extraction
Although not always necessary, pre-extraction can be extremely useful to visualize foci of chromatin bound proteins (e.g., γH2AX, 53BP1, RPA2, RAD51), as it removes soluble non-chromatin-associated proteins. However, since it involves the use of Triton detergent, which removes the cell membranes and cytosol, the final working dilution and duration of pre-extraction should be tested for each cell type.
Support Protocol 1
Meta-TIF origin
It is critical to understand that metaphase-TIF can arise from two distinct mechanisms. Unlike the DDR resulting from genomic DNA breaks, a telomere DDR does not activate the G2/M checkpoint (Cesare et al., 2013). As a result, TIF present in G2-phase are passed into mitosis, where they can be visualized and quantified as metaphase-TIF. In addition, metaphase-TIF can arise due to a prolonged mitotic arrest (Hayashi et al., 2012). After cells are arrested in mitosis for around 6 hr, this activates an Aurora B kinase dependent mechanism where TRF2 is partially dissociated from chromosome ends, resulting in a telomere DDR. Treatment of cell cultures with 20 ng/ml Colcemid for less than 1 hr has been shown to be insufficient to induce the prolonged mitotic arrest telomere DDR (Kaul et al., 2012; Hayashi et al., 2012).
The metaphase-TIF resulting from passage of DDR-positive telomeres from G2 into mitosis and those originating from prolonged mitotic arrest will appear no different when imaged. However, it is possible to suppress prolonged mitotic arrest–dependent metaphase-TIF through treatment with 40 mM hesperadin, an Aurora B kinase inhibitor (Hayashi et al., 2012). This allows the researcher to identify what percentage of induced metaphase-TIF are due to a prolonged mitotic arrest and what percentage have been passed into mitosis from the previous G2 phase (Cesare et al., 2013). Keep in mind that if a genetic or pharmacological treatment induces metaphase-TIF, it might not be through a direct effect on the telomeres per se, but may instead represent the manifestation of a prolonged mitotic arrest.
Basic Protocol 2 and Support Protocol 2
The quality of the tissue specimen is often a limiting factor in determining telomere length by FISH. As outlined above, it is important to only use charged microscope slides. Additionally, it is desirable to ask the reference laboratory preparing tissue sections to not bake the tissue sections at high temperatures to dry the slides after sectioning.
Basic Protocol 3
Cell culture
Cells should be growing exponentially and not confluent at any time.
Chromosome morphology
The duration of Colcemid treatment must be optimized for each cell line. In general, cells with a high mitotic index (i.e., cells that are rapidly proliferating and enter and exit mitosis without delay or death) will require less time with Colcemid to generate adequate numbers of mitotic cells from which metaphase spreads can be derived.
Scoring
Having captured the necessary images, the telomeres and chromosomes must be counted. This should be done in “double-blinded” manner. Have a lab colleague who is unfamiliar with the experiment do it (and reward them later) or have a lab colleague re-name the samples (keeping a record of course) so that the images can be assessed without bias.
Support Protocol 3
Controls
If the goal of the experiment is to assess for telomere sister chromatid exchange events, it is essential to treat and process a negative control, i.e., a cell line in which T-SCE should not be present, such as HeLa cells.
Cell culture
Cells should be growing exponentially and not be confluent at any time.
BrdU/BrdC labeling
To avoid incorporation of BrdU and BrdC onto the same strand and consequently the introduction of artifacts, labeling of DNA strands should only occur once. Thus, it is essential that the cell-cycle duration of the cell line used for CO-FISH be determined. In addition, standard doses of BrdU (10 µM) have been shown to have cytotoxic effects in some cell types (mouse embryonic stem cells; Sauer et al., 2013). Thus, potentially detrimental effects of BrdU should be assessed before performing CO-FISH.
Also, it is not essential to use 10 mM 3:1 BrdU/BrdC mix. This is only necessary if hybridization will be performed with TelG and TelC PNAs probes to determine if telomere sister chromatid exchange (t-SCE) events have occurred. Labeling with 10 mM BrdU and hybridization with the TelC PNA probe is sufficient to determine sister-telomere loss.
Fixation
It is critical to fix the cells as described here. Rapid addition of fixative will negatively impact chromosome spreading
Degradation of newly synthesized strands
This is a particularly key step in the protocol. It is absolutely essential to include a sample/cell type in which sister chromatid exchange events are not expected (e.g., HeLa cells), and thus TelC and TelG signals will be observed on separate chromatids.
Image capture
It is very difficult to accurately gauge and compare red and green intensity during CO-FISH. The probes are of different colors and different fluorescent intensities, used at different concentrations, with different background staining on the chromosomes. Often, though not always, the red G-rich probe will be of greater intensity than the green C-rich probe. The best way around the imaging problem is probably to allow the microscope and software to image each channel based on automated settings. If the automated settings gather light until a certain percentage of total pixels are saturated, than imaging both red and green with the same automated settings should provide some normalization between red and green signals. DAPI staining on properly prepared CO-FISH metaphase chromosomes will be less bright than on normal chromosomes due to the removal of one DNA strand, and imaging times will need to be increased accordingly.
Scoring
Having captured the necessary images, the telomeres and chromosomes must be counted. This should be done in “double-blinded” manner. Have a lab colleague who is unfamiliar with the experiment do it (and reward them later) or have a lab colleague re-name the samples (keeping a record of course) so that the images can be assessed without bias.
Troubleshooting
Basic Protocol 1
It frequently occurs that no signal for the target protein is observed. This can be for several technical reasons including:
The dilution/concentration of the antibody needs to be empirically determined
PNA probes should be made and stored as indicated
The fixation should be done with good quality PFA (see recipe for PFA in Reagents and Solutions).
Ensure that the IF staining is fixed with PFA prior to FISH (see Basic Protocol 1, step 15). The denaturation step of the hybridization will destroy the immunofluorescence signals.
Re-check the wash buffer recipes
Ensure that a positive control is used in conjunction with your experiment
Support Protocol 1
Chromosome morphology
As described in step 5, chromosome morphology is dependent on cell concentration during centrifugation. Unlike cytogenetic chromosome spreading, described in Basic Protocol 3, it is not possible to visualize chromosome morphology after cytocentrifugation before continuing with the staining protocol. Therefore, it is critical to determine the ideal cell concentration for centrifugation, as described in step 5, prior to endeavoring a critical experiment.
Hypotonic swelling
The duration of hypotonic swelling is another critical factor that impacts sample quality. Ideal swell time is typically around 5 min. Increased swelling times may cause cells to burst and disrupt chromosome morphology. Because of the rather rapid transition necessary from resuspension in 0.2% trisodium citrate/0.2% KCl to cytocentrifugation, it is recommended that the centrifuge funnels be prepared in advance and the number of samples prepared simultaneously be minimized to ensure that all steps can be completed on time.
Basic Protocol 2 and Support Protocol 2
The telomere FISH signals can be visualized using a 40× objective, but usually only when a correction collar is present (e.g. 40×/0.95 NA PlanApo lens with correction collar). If this setup is not available in your laboratory, then the use of a 63× or 100× oil objective is preferable.
Basic Protocol 3
Insufficient mitotic/metaphase cells
Generating slides with an abundance of mitotic cells increases the probability that a number of useful chromosome spreads will be present on each slide for further analysis. If too few mitotic cells are observed, then the duration of Colcemid treatment was inadequate. In general terms, transformed cells have a higher mitotic index than primary cells and therefore require shorter exposure to Colcemid. Mitotic cells can be seen under a light microscope as circular cells with a halo surrounding their surface. Cells are ready for collection, fixation, and processing when several hundred of such round cells are observable within a culture.
Chromosome morphology
A major difficulty encountered with this protocol is the generation of properly spread mitotic chromosomes in adequate numbers. Ideal chromosome spreads have well-spaced chromosomes that are not overlapping or huddled together but also not dispersed too broadly. To some extent, this relies on the stochastic distribution of chromosomes within each mitotic cell as the cells adhere to the slide. Increasing the duration of hypotonic swelling may help disperse chromosomes more effectively, but may come at the cost of poor chromosome morphology and an increased risk of burst cells.
Chromosome dropping
There has been a rich tradition of elaborate methods to prepare optimally spread chromosomes. This typically involves dropping chromosomes from an extended distance onto an angled slide. In reality, chromosome distribution is a function of relative humidity and drying conditions (Spurbeck et al., 1996). Thus, in the absence of a controlled atmospheric environment, the simple method of chromosome dropping described here produces reliable and consistent chromosome spreading.
Weak telomere FISH signal and high background
PNA probes should be made fresh before use. Ensure washing steps are correctly followed and buffers are correctly made.
Scoring
Having captured the necessary images, the telomeres and chromosomes must be counted. This should be done in “double-blinded” manner. Have a lab colleague who is unfamiliar with the experiment do it (and reward them later) or have a lab colleague re-name the samples (keeping a record of course) so that the images can be assessed without bias.
Support Protocol 3
Also see Basic Protocol 3 troubleshooting.
Dual PNA signals on sister chromatids
This can result if BrdU and BrdC incorporation into the same strand has taken place. The duration of BrdU/BrdC labeling should be reduced to avoid this. Alternatively, dual FISH signals on telomere on each chromatid may be the result of incomplete digestion of the newly synthesized strands. To solve this and eradicate potential artifacts, several steps can be taken. The duration of UV irradiation and ExoIII digestion can be extended. Since t-SCEs can occur in ALT-positive cells, it is absolutely essential to include a sample/cell type in which sister chromatid exchange events are not expected (e.g., HeLa cells) and thus TelC and TelG PNA signals will be observed on separate chromatids.
Anticipated Results
Basic Protocol 1
The anticipated result is the determination of whether an IF target, or targets, of interest co-localize with telomeric DNA in three-dimensional space. Examples of telomere co-localization are shown in Figures 12.40.1 and 12.40.2. Typically, at least 100 cells are assessed and counted with several clear examples of co-localization being present so that significance can be statistically assessed. For example, although only −5% of ALT cell lines contain APBs at a given time, the consistency of this observation indicates that although infrequent, APBs are a significant hallmark of ALT activity within a given cell line.
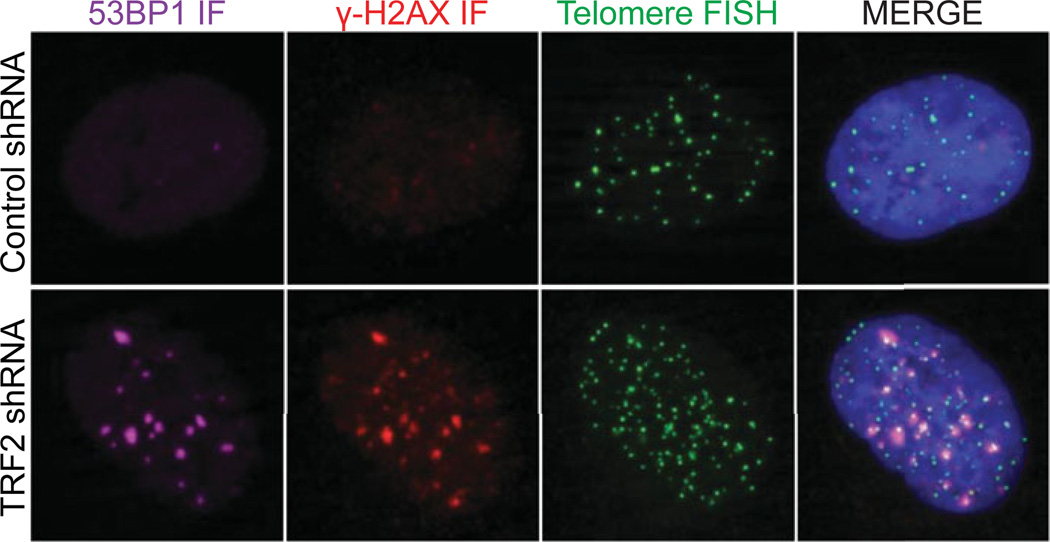
Figure 12.40.1.
In this example, HT1080 6TG cells that were untreated or transduced with a TRF2 shRNA are stained with a telomere PNA (green), γ-H2A.X (red), and 53BP1 IF (magenta), and DAPI (blue). Telomere dysfunction induced foci (i.e., TIF) are evident as co-localized IF and FISH signals in the TRF2 shRNA treated cell.
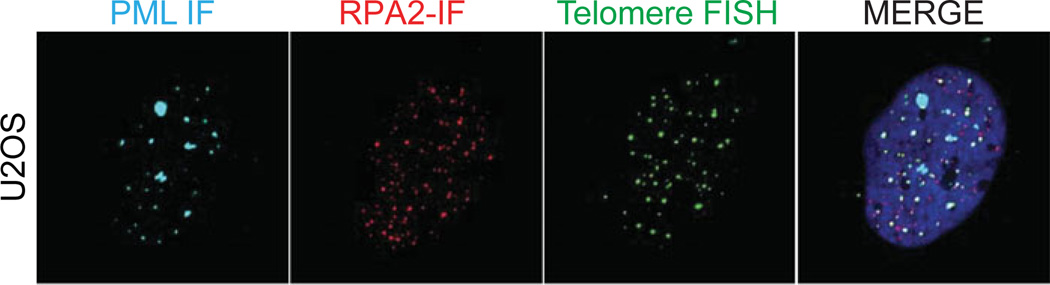
Figure 12.40.2.
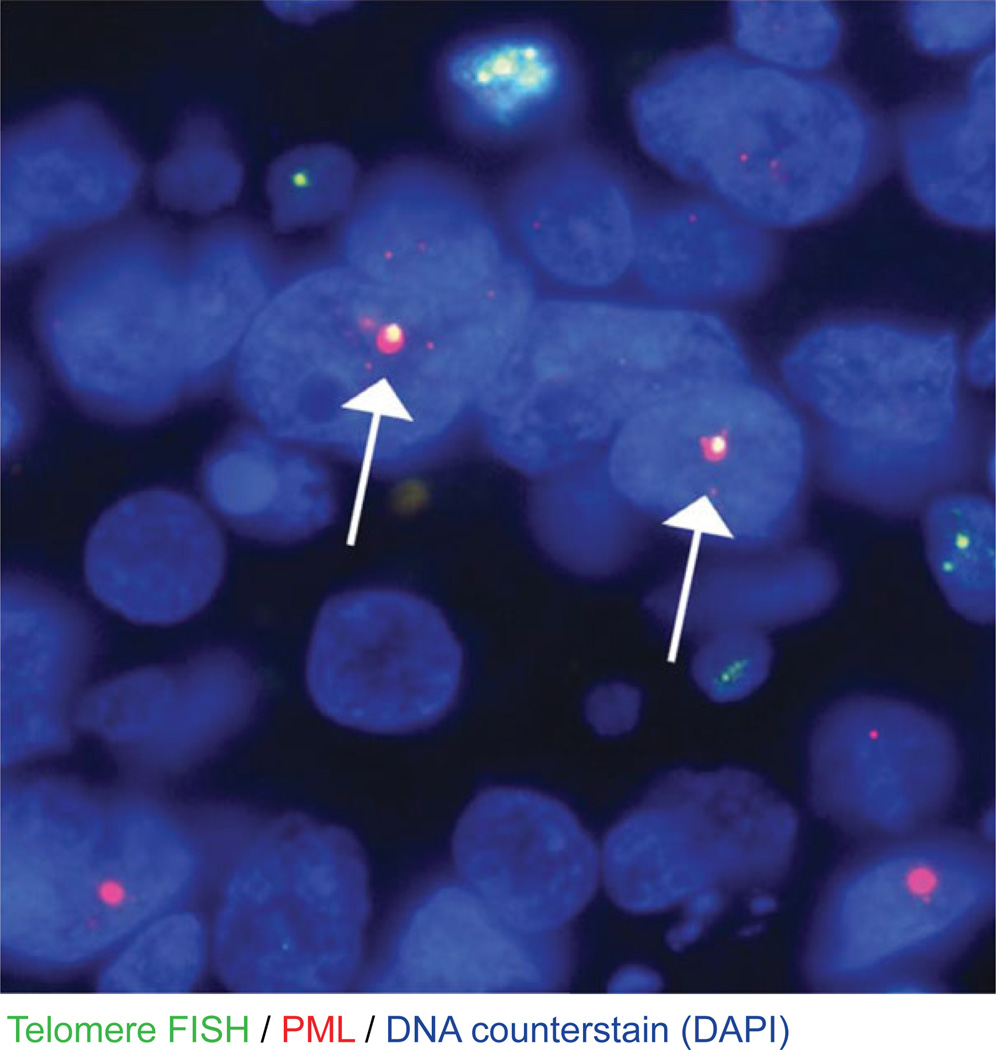
Example of ALT associated PML bodies in U-2-OS (osteosarcoma) cells. The image shows co-localization of Telomere PNA (green), RPA2 (red), and PML (cyan), and DAPI (blue). The cropped enlarged section displays clustering of telomeres with PML bodies that is typical of ALT.
Support Protocol 1
The anticipated result is that one can easily determine the number of telomeres in a metaphase or prometaphase cell that interact with an IF target of interest. It may also be possible to determine if the IF target localizes with one or both sister chromatid telomeres at a chromosome end and if co-localization is dependent on fluctuations in telomere length. See Figure 12.40.3 for example results.
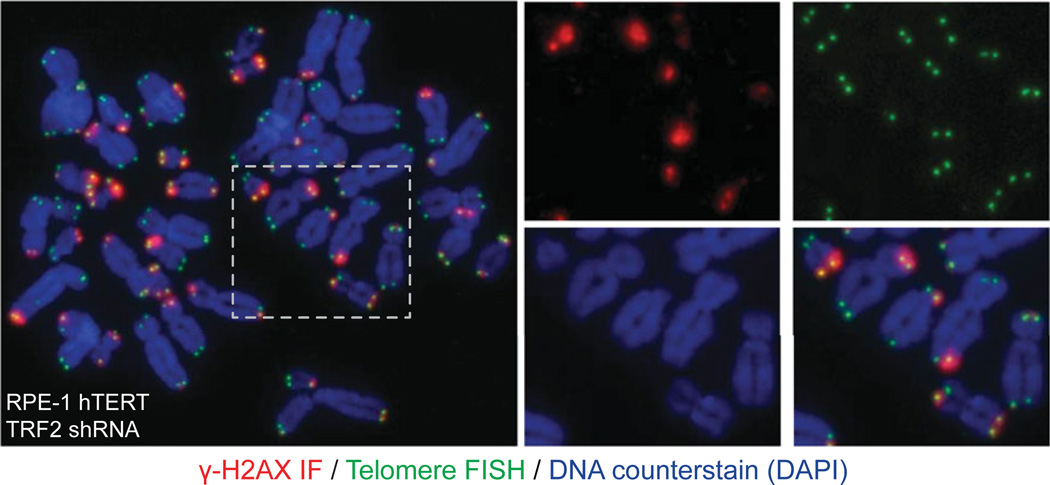
Figure 12.40.3.
Example of the metaphase-TIF assay. In this example, RPE-1 hTERT cells were transduced with a TRF2 shRNA to induce TIF, chromosome spreading was prepared using cytocentrifugation, and the sample stained with telomere PNA (green), γ-H2A.X (red) IF, and DAPI (blue). Individual and merged colors of an expanded region are shown on the right.
Basic Protocol 2 and Support Protocol 2
In Basic Protocol 1, the user should expect to see punctate dots (Cy3) in the nucleus (DAPI) of the cells of interest in the tissue. Depending on the tumor type that will be assessed, many cancer cells display drastic telomere shortening, and thus these telomere FISH signals will be absent in the cancer cells. However, the centromere PNA signals (Alexa Fluor 488), also present as punctate dots in the nucleus, should be present in all cells. Additionally, adjacent benign cells (either normal epithelium, cancer-associated stromal cells, or invading inflammatory cells) should display fairly robust telomere signals. Within the tissue, areas of necrosis should be avoided. For Support Protocol 2, large, ultra-bright, telomere FISH foci located inside the nucleus can be observed in cancers classified as ALT positive. In the vast majority of cases, these large foci co-localize with the PML protein; however, it appears that co-localization is not an absolute requirement. Previous studies have proposed the following criteria for classifying an ALT-positive cancer: (1) the presence of ultra-bright, intra-nuclear foci of telomere FISH signals (ALT-associated telomeric foci); (2) the telomere FISH signals have an integrated total signal intensities for individual foci being > 10 fold that of the per cell mean integrated signal intensities for all telomeric signals in individual benign stromal cells within the same case; and (3) ≥1% of tumor cells displaying these ALT-associated telomeric foci (Heaphy et al., 2011b, 2011a). Cases lacking ALT-associated telomeric foci in which at least 500 cells are assessed can be considered ALT-negative. See Figures 12.40.4 and 12.40.5 for example results.
Figure 12.40.4.
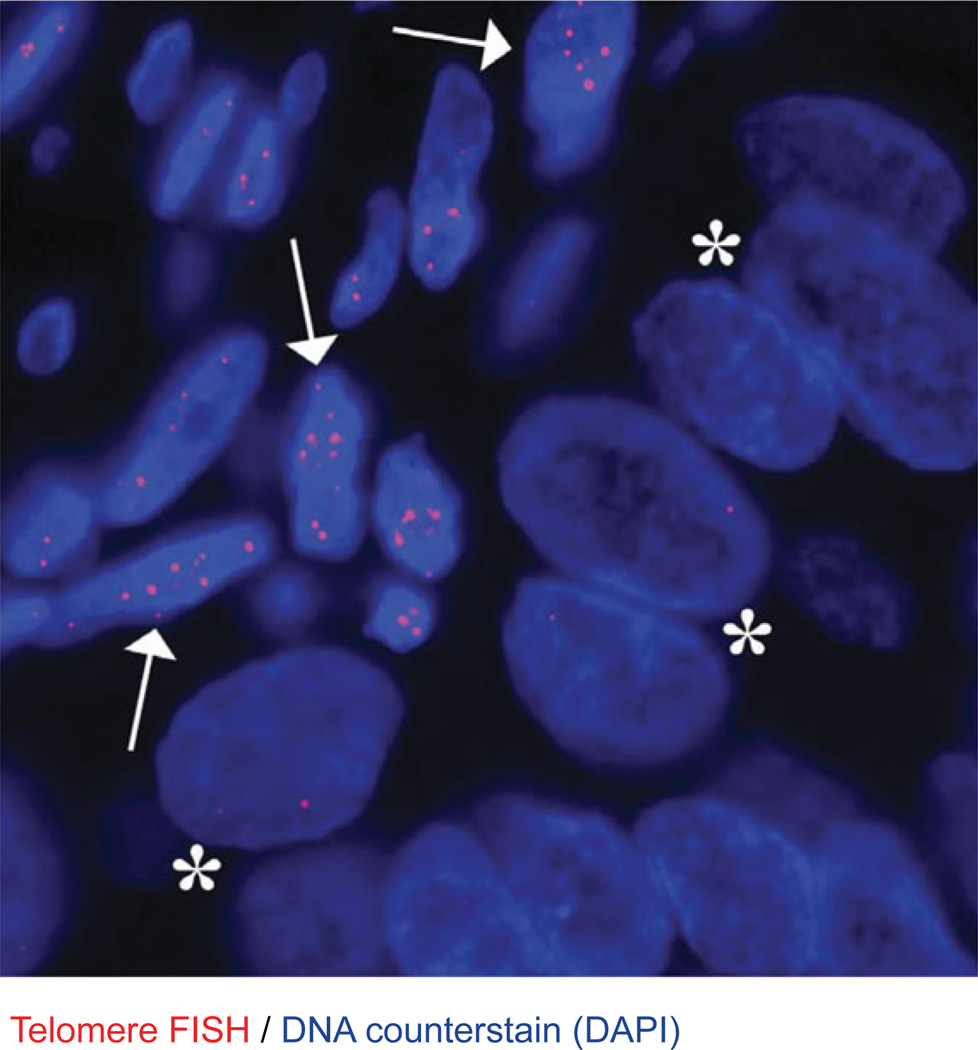
Telomere-specific FISH in a prostate adenocarcinoma. This representative case demonstrates dramatically diminished telomere FISH signals in the cancer cells (*) as compared to the presence of robust telomere FISH signals in the surrounding benign stromal cells (arrows). The DNA is stained with DAPI (blue; 80 msec exposure time), and telomere DNA is stained with the Cy3-labeled telomere-specific PNA probe (red; 500 msec exposure time). Original magnification, 400×.
Figure 12.40.5.
Representative example of an ALT-positive pancreatic neuroendocrine tumor (PanNET). This PanNET case highlights the distinctive large, very bright, intra-nuclear foci of telomere FISH signals marking ALT-associate telomeric foci throughout the tumor cells that also tend to, but not always, co-localize with the PML protein (arrows). The DNA is stained with DAPI (blue; 80 msec exposure time), telomere DNA is stained with the Cy3-labeled telomere-specific PNA probe (red; 200 msec exposure time), and PML protein is stained with an anti-PML antibody that is detected with an Alexa Fluor 488 secondary antibody (green; 500 msec exposure time). Note that the exposure time for the Cy3 channel is greatly reduced compared to the image in Figure 12.40.1 to allow for accurate detection of the large telomeric foci. Original magnification 400×.
Basic Protocol 3
The anticipated result is to identify telomeric DNA sequence on individual mitotic chromosome spreads. This can be used to ascertain the state of telomere structure and associated phenotypes: i.e., telomere fusions and telomere loss. See Figure 12.40.6 for example results.
Figure 12.40.6.
Examples of telomere FISH on cytogenetic chromosome spreads. (A) Example of a typical metaphase from human HT1080 cells stained with telomere FISH (green) and DAPI (blue). (B) Examples of telomere fusions, telomere rapid deletions, diplochromsomes, and separated sister chromatids from human cells lines as visualized using Basic Protocol 3.
Support Protocol 3
If using CO-FISH to assess the frequency of T-SCEs, such as in ALT cells, it is essential that a control sample such as HeLa cells be processed in parallel. HeLa, which use telomerase to maintain their telomeres, show virtually nonexistent T-SCEs. In HeLa, the expectation is that each sister chromatid should have a single FISH signal corresponding to the leading and lagging strand. If this is the case, the procedure has worked efficiently and other samples can be assessed with confidence that experimental artifacts have been minimized.
For scoring and enumeration of T-SCE events, here are some considerations:
Score good metaphases with clearly separated chromosomes
Score clearly distinguishable sister chromatids
For mouse cells, only score telomeres on long arm chromosomes
Score cross-over events in which the new signal is significantly above background and matches the intensity on other chromatids
Score your slides in a “double-blinded” manner to avoid bias
Assess events from at least 10 metaphases in three independent experiments (total 30 metaphases, at least)
See Figure 12.40.7 for example results.
Figure 12.40.7.
This figure shows a schematic of the CO-FISH procedure and some examples of the anticipated results. From left to right, in the negative control (HeLa cells), reciprocal single PNA FISH signals are observed on individual sister chromatids. This indicates that the duration of BrdU/C labeling and degradation of the newly replicated strands has been efficiently performed. Examples of telomere sister chromatid exchange events and loss of the leading and/or lagging strand such as occurs in ALT-positive cells are also shown.
Time Considerations
Basic Protocol 1
Sample preparation is best performed over 3 days. This is because IF and FISH benefit from overnight incubation with primary antibody and overnight PNA hybridization. It is possible to reduce incubation with primary antibody to 1 hr at room temperature for some antibodies, and PNA hybridization to 3 hr, but this may come at the cost of weaker signal and poor signal-to-noise ratio. Experimental procedures are anticipated to take 2, 3.5, and 1.5 hr on days 1, 2, and 3, respectively.
Support Protocol 1
Sample preparation is best performed over 2 or 3 days. Sample collection to overnight PNA hybridization as written here requires 6.5 hr to complete. If necessary, this can be broken up into sample collection through fixation (steps 1 to 9) on day 1 and permeabilization to PNA hybridization on day 2, which will take 2 and 4.5 hr respectively. PNA washing and mounting on the final day require 1.5 hr.
Basic Protocol 2 and Support Protocol 2
Basic Protocol 1 can be completed in 1 day (∼6 hr). Support Protocol 2 can also be completed in 1 day (∼9 hr). However, for both protocols, the tissue slides may be hybridized with the PNA probes in a dark humidified chamber overnight, thus making for a 2-day experiment. As outlined in the protocol, slides should be allowed to harden or “cure” at room temperature for 24 hr and then stored in a refrigerator for 1 week to ensure optimal imaging with a fluorescent microscope.
Basic Protocol 3
Due to several required overnight steps, this protocol requires a minimum of 4 days to prepare samples. Anticipate sample preparation requiring 2, 1, 2, and 1 hr on days 1, 2, 3, and 4 respectively.
Support Protocol 3
This protocol requires 6 days to prepare and process samples. Days 4 and 5 can be combined into a single long day. Scoring and analysis can take several days.
Literature Cited
- Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH. Strand-specific postreplicative processing of mammalian telomeres. Science. 2001;293:2462–2465. doi: 10.1126/science.1062560. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Hayashi MT, Crabbe L, Karlseder J. The telomere deprotection response is functionally distinct from the genomic DNA damage response. Mol. Cell. 2013;51:141–155. doi: 10.1016/j.molcel.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat. Struct. Mol. Biol. 2009;16:1244–1251. doi: 10.1038/nsmb.1725. [DOI] [PubMed] [Google Scholar]
- Crabbe L, Verdun RE, Haggblom CI, Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J. Cell Biol. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Hayashi MT, Cesare AJ, Fitzpatrick JAJ, Lazzerini Denchi E, Karlseder J. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat. Struct. Mol. Biol. 2012;19:387–394. doi: 10.1038/nsmb.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannucci E, Mucci LA, Kenfield SA, Stampfer MJ, Hicks JL, De Marzo AM, Platz EA, Meeker AK. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 2013;3:1130–1141. doi: 10.1158/2159-8290.CD-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL, Westra WH, Shih IeM, Iacobuzio-Donahue CA, Maitra A, Li QK, Eberhart CG, Taube JM, Rakheja D, Kurman RJ, Wu TC, Roden RB, Argani P, De Marzo AM, Terracciano L, Torbenson M, Meeker AK. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011b;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, Offerhaus GJ, McLendon R, Rasheed BA, He Y, Yan H, Bigner DD, Oba-Shinjo SM, Marie SK, Riggins GJ, Kinzler KW, Vogelstein B, Hruban RH, Maitra A, Papadopoulos N, Meeker AK. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011a;333:425–425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson JD, Hannay JA, McCarthy SW, Royds JA, Yeager TR, Robinson RA, Wharton SB, Jellinek DA, Arbuckle SM, Yoo J, Robinson BG, Learoyd DL, Stalley PD, Bonar SF, Yu D, Pollock RE, Reddel RR. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin. Cancer Res. 2005;11:217–225. [PubMed] [Google Scholar]
- Hockemeyer D, Daniels J-P, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Hurwitz LM, Heaphy CM, Joshu CE, Isaacs WB, Konishi Y, DeMarzo AM, Isaacs SD, Wiley KE, Platz EA, Meeker AK. Telomere length as a risk factor for hereditary prostate cancer. Prostate. 2014;74:359–364. doi: 10.1002/pros.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- Kaul Z, Cesare AJ, Huschtscha LI, Neumann AA, Reddel RR. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2012;13:52–59. doi: 10.1038/embor.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64:2324–2327. doi: 10.1158/0008-5472.can-03-4035. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am. J. Pathol. 2004a;164:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. Telomere length assessment in human archival tissues: Combined telomere fluorescence in situ hybridization and immunostaining. Am. J. Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, Ronnett BM, DeMarzo AM. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. 2004b;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- Montgomery E, Argani P, Hicks JL, De-Marzo AM, Meeker AK. Telomere lengths of translocation-associated and nontranslocation-associated sarcomas differ dramatically. Am. J. Pathol. 2004;164:1523–1529. doi: 10.1016/S0002-9440(10)63710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthwein A, Fradet-Turcotte A, Noordermeer SM, Canny MD, Brun CM, Strecker J, Escribano-Diaz C, Durocher D. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science. 2014;344:189–193. doi: 10.1126/science.1248024. [DOI] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Pham HH, Murphy CT, Sureshkumar G, Ly DH, Opresko PL, Armitage BA. Cooperative hybridization of γPNA miniprobes to a repeating sequence motif and application to telomere analysis. Org. Biomol. Chem. 2014;12:7345–7354. doi: 10.1039/c4ob00953c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC. Techniques for mammalian cell tissue culture. Curr. Protoc. Cytom. 2006;36:A.3B.1–A.3B.18. doi: 10.1002/0471142956.cya03bs36. [DOI] [PubMed] [Google Scholar]
- Poon SS, Lansdorp PM. Quantitative fluorescence in situ hybridization (Q-FISH) Curr. Protoc. Cell Biol. 2001;12:18.4.1–18.4.21. doi: 10.1002/0471143030.cb1804s12. [DOI] [PubMed] [Google Scholar]
- Sauer S, Burkett SS, Lewandoski M, Klar AJS. A CO-FISH assay to assess sister chromatid segregation patterns in mitosis of mouse embryonic stem cells. Chromosome Res. 2013;21:311–328. doi: 10.1007/s10577-013-9358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, Karlseder J, Holtgreve-Grez H, Jauch A, de Lange T. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 2002;12:1635–1644. doi: 10.1016/s0960-9822(02)01179-x. [DOI] [PubMed] [Google Scholar]
- Spurbeck JL, Zinsmeister AR, Meyer KJ, Jalal SM. Dynamics of chromosome spreading. Am. J. Med. Genet. 1996;61:387–393. doi: 10.1002/(SICI)1096-8628(19960202)61:4<387::AID-AJMG15>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Wang RC, Smogorzewska A, de Lange T. Homologous recombination generates T-loopsized deletions at human telomeres. Cell. 2004;119:355–368. doi: 10.1016/j.cell.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Yeager TR, Neumann AA, Englezou A, Huschtscha LI, Noble JR, Reddel RR. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 1999;59:4175–4179. [PubMed] [Google Scholar]
- Zhu XD, Küster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]