Abstract
Objective
To understand the spread of HIV among and between age and racial/ethnic groups of men who engage in male-to-male sexual contact (men who have sex with men, MSM) in the United States.
Design
Analysis of HIV-1 pol sequences for MSM collected through the U.S. National HIV Surveillance System (NHSS) during 2001–2012.
Methods
Pairwise genetic distance was calculated to determine potential transmission partners (those with very closely related nucleotide sequences, i.e., distance ≤1.5%). We described race/ethnicity and age of potential transmission partners of MSM.
Results
Of 23,048 MSM with HIV sequences submitted to NHSS during 2000–2012, we identified potential transmission partners for 8,880 (39%). Most potential transmission partners were of the same race/ethnicity (78% for blacks/African Americans, 64% for whites, and 49% for Hispanics/Latinos). This assortative mixing was even more pronounced in the youngest age groups. Significantly fewer young black/African American and Hispanic/Latino MSM had older potential transmission partners compared with young white MSM.
Conclusion
Black/African American MSM, who are more profoundly affected by HIV, were more likely to have potential HIV transmission partners who were of the same race/ethnicity and similar in age, suggesting that disparities in HIV infections are in large part not due to age-disassortative relationships. Concerted efforts to increase access to pre-exposure prophylaxis, quality HIV care, and effective treatment are needed to interrupt transmission chains among young, black/African American MSM.
Keywords: HIV, homosexuality, male, transmission, African Americans, Hispanic Americans, adolescent, young adult
INTRODUCTION
In the United States, persons with HIV infection attributed to male-to-male sexual contact (men who have sex with men; MSM) account for nearly two-thirds of HIV diagnoses [1]. Young MSM, particularly those who are black/African American or Hispanic/Latino, bear a disproportionate burden of HIV infections.
A common hypothesis explaining the high number of HIV infections in young MSM is sex with older partners, who are more likely to have HIV infection [2-8]. The selection of an older sexual partner has been found to be significantly associated with HIV infection among samples of young MSM in North Carolina and Mississippi [8,9].
Understanding the characteristics of HIV transmission among subpopulations of young MSM provides insight into disparities. Sexual network mixing patterns, such as age and racial/ethnic assortativity (preferential connections between persons with similar characteristics) or dissassortativity (preferential connections between persons with different characteristics), may contribute to elevated risk for HIV infection among young, black/African American and Hispanic/Latino MSM by exposing them to a pool of partners with higher HIV prevalence. Previous research examining the role of age and racial dynamics in the sexual networks of young MSM relied on small sample sizes [2,3,4,5,7,8] or survey data [4,8]. Whereas survey data can provide information about social and sexual networks, identifying genetically highly similar HIV sequences can allow us to infer HIV transmission networks and describe transmission between the persons within these networks [10-19]. In an effort to better understand the role that age and race/ethnicity of sexual partners may play in HIV acquisition for young MSM, we used HIV sequence data reported to U.S. National HIV Surveillance System (NHSS) to investigate HIV transmission dynamics for young MSM.
METHODS
Sources of Sequence Data
HIV-1 pol region sequences (protease and partial reverse transcriptase) reported through NHSS during 2001–2012 were used for the analysis. These sequences were collected in 21 U.S. states and the District of Columbia [20, 21]. Data collection methods have been previously described in detail [21]. Briefly, through mid-2011, Sanger sequencing was performed by a centralized CDC-funded laboratory. From mid-2011 through 2012, sequence data generated from genotypic drug resistance testing conducted as part of routine HIV care were instead collected by participating jurisdictions from commercial, private, and public laboratories.
Inclusion Criteria
The analysis was limited to men aged ≥13 years at HIV diagnosis with reported risk factor information categorizing them as MSM (i.e., with HIV infection attributed to male-to-male sexual contact) or MSM who also injected drugs (MSM/IDU). Persons with missing risk information were excluded from the analysis. We included only one sequence per person; when more than one sequence was available, we selected the longest sequence. Sequences less than 500 nucleotides in length were excluded, because the transmission network approach has not been validated for very short sequences [18].
Transmission Network Analysis
We used a local version of HIV-TRACE (hivtrace.org), following the protocol outlined by Wertheim et al [18, 21] for transmission network construction. Each sequence was aligned to an HIV reference sequence (HXB2, positions 2253 to 2869). We then conducted pairwise comparison of all sequences. Tamura-Nei 93 genetic distance ≤1.5% was considered evidence of possible linkage; those meeting this criterion were considered potential transmission partners.
Measures
Data on age, sex, race/ethnicity, transmission category, and area of residence at diagnosis were collected through the NHSS using standard CDC classification schemes. Age differences were calculated using differences in year of birth. Race/ethnicity was categorized as white; black or African American; Hispanic or Latino; or other (including American Indian/Alaska Native, Asian, Native Hawaiian and Other Pacific Islander, and multiple races). U.S. census regions included Northeast, Midwest, South, and West [1].
Analysis of Race/Ethnicity and Age of Potential Transmission Partners
We analyzed the transmission network to understand the race/ethnicity and age of potential transmission partners. In many cases, one person had more than one potential transmission partner identified. We assumed that each person acquired HIV from only one person. Therefore, each potential transmission partner for a given person was assigned a weight based on k (the number of potential transmission partners associated with the person). The weight was simply 1/k for each of the k links.
For each racial/ethnic group, we described the race/ethnicity of potential transmission partners, both overall and by age group. We then calculated the percentage of MSM aged 13–24 years at diagnosis who had potential transmission partners who were more than 5 years older, stratified by race/ethnicity, U.S. census region, transmission category (MSM vs. MSM/IDU), age at diagnosis, and potential transmission partner’s race/ethnicity (same vs. different). We used multivariable logistic regression including all of these variables and controlled for year of diagnosis. We calculated adjusted prevalence ratios (APRs) and 95% confidence intervals (CIs) to compare the proportion with potential transmission partners who were more than 5 years older.
RESULTS
There were 23,048 MSM with HIV sequences that met the inclusion criteria. Of these MSM, 36% were black/African American, 35% were white, and 24% were Hispanic/Latino; 6% were aged 13–19 years and 21% aged 20–24 years when diagnosed with HIV. Few (6%) men included were MSM/IDU. We identified potential transmission partners for 8,880 (39%) MSM.
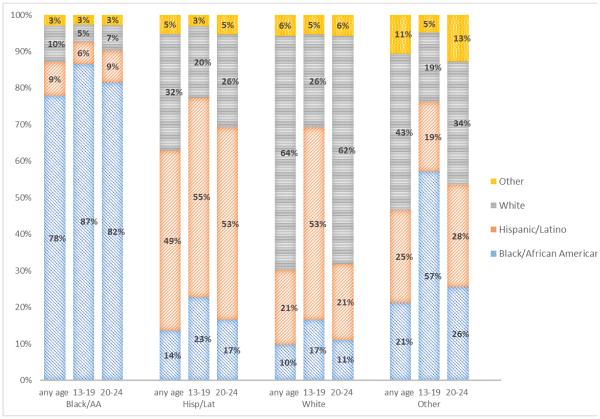
Most MSM had potential transmission partners of their race/ethnicity (Figure 1); however, the extent of this assortative mixing varied by race/ethnicity (i.e., 78% for blacks/African Americans, 64% for whites, and 49% for Hispanics/Latinos). The proportion of blacks/African Americans and Hispanics/Latinos with potential transmission partners of the same race/ethnicity was even higher for MSM aged 13–19 years and aged 20–24 years (Figure 1).
Figure 1. Estimated proportion of potential transmission partners of different racial/ethnic groups, 2001–2012—21 U.S. states and the District of Columbia.
Bar chart depicts the proportion of potential HIV transmission partners of men who have sex with men from different racial/ethnic groups for any age at HIV diagnosis and for those 13-19 years and 20-24 years of age, by race/ethnicity.
Of the 3,322 MSM aged 13–24 years, 1,186 (35.7%) had a potential transmission partner that was more than 5 years older (Table 2). Multivariable modeling showed that having a potential transmission partner that was older was less common for black/African MSM (APR=0.65; CI=0.57–0.73) and Hispanics/Latino MSM (APR=0.86; CI=0.77–0.97) compared with white MSM. The likelihood of having an older potential transmission partner was also associated with age, region, and potential transmission partner race/ethnicity.
Table 2.
Estimated percentage of infections from older partners among men who have sex with men who are aged 13–24 years at HIV diagnosis, 2001–2012—21 U.S. states and the District of Columbia.
| Number of Young MSM |
Number Linked with Older Partner |
Percentage Linked with Older Partner |
Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| % | 95% CI | PR | 95% CI | PR | 95% CI | |||
| Race/ethnicity | ||||||||
| Black/African | 1,862 | 520 | 27.9 | (26.0-30.0) | 0.58 | (0.52-0.65) | 0.65 | (0.57-0.73) |
| American | ||||||||
| Hispanic/Latino | 677 | 296 | 43.7 | (40.2-47.7) | 0.91 | (0.81-1.03) | 0.86 | (0.77-0.97) |
| Other | 107 | 46 | 43.0 | (35.0-53.9) | 0.90 | (0.72-1.14) | 0.82 | (0.65-1.03) |
| White | 676 | 324 | 47.9 | (44.2-51.8) | Ref | Ref | ||
| Region | ||||||||
| Northeast | 668 | 260 | 38.9 | (35.4-42.8) | 1.21 | (1.04-1.39) | 1.04 | (0.90-1.21) |
| South | 1,301 | 392 | 30.2 | (27.8-32.8) | 0.94 | (0.82-1.07) | 0.96 | (0.84-1.10) |
| West | 638 | 303 | 47.5 | (43.8-51.6) | 1.47 | (1.29-1.68) | 1.23 | (1.07-1.41) |
| Midwest | 713 | 230 | 32.3 | (29.1-35.9) | Ref | Ref | ||
|
Transmission
category |
||||||||
| MSM & IDU | 111 | 53 | 47.7 | (39.0-57.7) | 1.35 | (1.10-1.64) | 1.11 | (0.92-1.34) |
| MSM only | 3,211 | 11,333 | 35.3 | (33.7-37.0) | Ref | Ref | ||
| Age at diagnosis | ||||||||
| 20-24 | 2,507 | 851 | 33.9 | (32.2-35.9) | 0.83 | (0.75-0.91) | 0.72 | (0.66-0.79) |
| 13-19 | 815 | 335 | 41.1 | (37.8-44.6) | Ref | Ref | ||
| Partner‘s race | ||||||||
| Different | 978 | 447 | 45.7 | (42.7-49.0) | 1.45 | (1.33-1.59) | 1.25 | (1.14-1.38) |
| Same | 2,344 | 739 | 31.5 | (29.7-33.5) | Ref | Ref | ||
| Total | 3,322 | 1186 | 35.7 | |||||
The multivariable model included all variables listed and also controlled for year of diagnosis.
DISCUSSION
We found that, as has been documented for HIV transmission overall in the United States [21], HIV transmission among MSM was highly assortative by race/ethnicity. Racial/ethnic-assortative mixing was especially evident for black/African American MSM. We found that disassortativity by age varied by race/ethnicity, as young black/African American and Hispanic/Latino MSM were significantly less likely to have potential transmission partners more than 5 years older compared with young white MSM.
These results indicate that most black/African American MSM are acquiring HIV from other black/African American MSM, which may be the result of two related factors. First, the sexual networks of black/African American MSM are more likely to be exclusively or predominantly other black/African American MSM [4,9,22-25]. Second, because black/African American MSM may have higher levels of HIV prevalence and incidence and lower levels of HIV care and viral suppression than other MSM, black/African American MSM may be at increased risk for HIV transmission with their black/African American partners than with other partners [26-28].
We found that Hispanic/Latino MSM, for whom approximately half of potential transmission partners were Hispanic/Latino, had lower assortativity by race/ethnicity than black/African American and white MSM. As a result, HIV infections among Hispanic/Latino MSM may originate from a variety of subgroups of MSM. Efforts to reduce HIV acquisition among Hispanic/Latino MSM may need to be delivered broadly, as interventions directed solely at HIV-infected Hispanic/Latino MSM may not sufficiently interrupt acquisition among Hispanic/Latino MSM.
Our findings regarding mixing of different age groups in HIV transmission varied by racial/ethnic group. We found that young black/African American and Hispanic/Latino MSM were less likely to have older potential transmission partners than young white MSM. This observation suggests that those young white MSM who became infected with HIV were more commonly infected through an age-disassortative relationship. Due to increased HIV incidence and prevalence, first introduced through sexual relationships with older MSM, sex between young black/African American and Hispanic/Latino MSM and their peers may carry higher risk for HIV infection beyond that experienced by young white MSM. These results may begin to explain why young black/African American and Hispanic/Latino MSM acquire HIV at higher numbers than young whites [29].
These findings have implications for prevention. We found that young black/African American and Hispanic/Latino MSM are largely acquiring HIV from other young and minority MSM, who have been demonstrated to be less commonly engaged in HIV care and virally suppressed [27]. Focused efforts to increase access to and consistent engagement in HIV care among black/African American and Hispanic/Latino MSM may help to decrease HIV transmission in this group [27]. In addition, increasing efforts to make black/African American MSM and Hispanic/Latino MSM aware of the benefits of pre-exposure prophylaxis (PrEP) and increasing consistent use of PrEP in these communities could substantially decrease HIV acquisition [30]. These targeted HIV prevention interventions should address the unique risks for HIV infection for young black/African American and Hispanic/Latino MSM.
This study is the first to use HIV nucleotide sequence data from NHSS to explore HIV transmission networks among MSM. The availability of these data enabled us to identify potential transmission links between MSM, explore transmission networks instead of sexual networks, and prevent problems associated with recall bias [4,23,24,31-33].
Despite its strengths, this study also has limitations. First, although we identified potential transmission partners, we could not draw a definitive link between any two individuals. Nonetheless, we were still able to draw inferences from the indirect links. Second, although we are not able to establish directionality of transmission from these data, evidence indicates that older MSM have higher HIV prevalence, and we can therefore infer that the majority of these links likely represent transmissions from older MSM to younger MSM. Third, we limited our sample to men whose transmission category was either MSM or MSM/IDU. Men who acquired HIV through male-to-male sexual contact but whose records were missing the information needed to categorize them as such were excluded; we estimate that the persons excluded represent a small portion (<15%) of MSM. Finally, we only had HIV-1 sequences from 21 US states and the District of Columbia. Differences in data completeness and other characteristics from these 21 areas may preclude them from being representative of the rest of the country.
The use of molecular HIV surveillance data provided us an opportunity to make inferences about the spread of HIV among young MSM. Our results indicate that racial/ethnic and age assortativity vary by race/ethnicity and are important factors in HIV acquisition and transmission among young MSM. Molecular transmission network analysis is an important tool than can be used to help understand disparities in HIV infection. The insight acquired from this analysis can be used to focus efforts to increase access to and consistent engagement in HIV care and treatment among young MSM.
Acknowledgments
We acknowledge the local and state health department staff instrumental in collecting HIV sequence and other surveillance data. We also acknowledge Angela Hernandez, M. Cheryl Bañez Ocfemia, and H. Irene Hall for their contributions to the collection, analysis, and interpretation of these data. JOW was funded in part by an NIH K01 Career Development Award (K01AI110181). YOW, RS, JOW, and AMO designed the study. RS, AMO, and YOW conducted the analysis. YOW wrote the manuscript. All authors interpreted the findings and critically revised the manuscript.
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest or sources of funding to disclose.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention HIV Surveillance Report. 2013;25 http://www.cdc.gov/hiv/library/reports/surveillance/. Published February 2015. Accessed March 2, 2015. [Google Scholar]
- 2.Morris M, Zavisca J, Dean L. Social and sexual networks: their role in the spread of HIV/AIDS among young gay men. AIDS Educ Prev. 1995;7(Suppl 5):24–35. [PubMed] [Google Scholar]
- 3.Service S, Blower SM. HIV transmission in sexual networks: an empirical analysis. Proc Biol Sci. 1995;260:237–244. doi: 10.1098/rspb.1995.0086. [DOI] [PubMed] [Google Scholar]
- 4.Bingham TA, Harawa NT, Johnson DF, Secura GM, MacKellar DA, Valleroy LA. The effect of partner characteristics on HIV infection among African American men who have sex with men in the Young Men’s Survey, Los Angeles, 1999-2000. AIDS Educ Prev. 2003;1(Suppl A):39–52. doi: 10.1521/aeap.15.1.5.39.23613. [DOI] [PubMed] [Google Scholar]
- 5.Blower SM, Service SK, Osmond DH. Calculating the odds of HIV infection due to sexual partner selection. AIDS Behav. 1997;1:273–274. [Google Scholar]
- 6.Service SK, Blower SM. Linked HIV epidemics in San Francisco. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:311–313. doi: 10.1097/00042560-199603010-00016. [DOI] [PubMed] [Google Scholar]
- 7.Coburn BJ, Blower S. A major HIV risk factor for young men who have sex with men is sex with older partners. J Acquir Immune Defic Syndr. 2010;54:113–114. doi: 10.1097/QAI.0b013e3181d43999. [DOI] [PubMed] [Google Scholar]
- 8.Hurt CB, Matthews DD, Calabria BM, Green KA, Adimora AA, Golin CE, et al. Sex with older partner is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr. 2010;54:185–190. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oster AM, Dorell CG, Mena LA, Thomas PE, Toledo CA, Heffelfinger JD. HIV risk among young African American men who have sex with men: a case-control study in Mississippi. Am J Public Health. 2011;101:137–143. doi: 10.2105/AJPH.2009.185850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oster AM, Pieniazek D, Zhang X, Switzer WM, Ziebell RA, Mena LA, et al. Demographic but not geographic insularity in HIV transmission among young black MSM. AIDS. 2011;25:2157–2165. doi: 10.1097/QAD.0b013e32834bfde9. [DOI] [PubMed] [Google Scholar]
- 11.Holmes EC, Zhang LQ, Robertson P, Cleland A, Harvey E, Simmonds P, et al. The molecular epidemiology of human immunodeficiency virus type 1 in Edinburgh. J Infect Dis. 1995;171:45–53. doi: 10.1093/infdis/171.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Kouyos RD, von Wyl V, Yerly S, Böni J, Taffé P, Shah C, et al. Molecular epidemiology reveals long-term changes in HIV type 1 subtype B transmission in Switzerland. J Infect Dis. 2010;201:1488–1497. doi: 10.1086/651951. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 14.Smith DM, May SJ, Tweeten S, Drumright L, Pacold ME, Kosakovsky Pond SL, et al. A public health model for the molecular surveillance of HIV transmission in San Diego, California. AIDS. 2009;23:225–232. doi: 10.1097/QAD.0b013e32831d2a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yerly S, Junier T, Gayet-Ageron A, Amari EB, von Wyl V, Günthard HF, et al. The impact of transmission clusters on primary drug resistance in newly diagnosed HIV-1 infection. AIDS. 2009;23:1415–1423. doi: 10.1097/QAD.0b013e32832d40ad. [DOI] [PubMed] [Google Scholar]
- 16.Yerly S, Vora S, Rizzardi P, Chave JP, Vernazza PL, Flepp M, et al. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. AIDS. 2001;15:2287–2292. doi: 10.1097/00002030-200111230-00010. [DOI] [PubMed] [Google Scholar]
- 17.Leigh Brown AJ, Lycett SJ, Weinert L, Hughes GJ, Fearnhill E, Dunn DT. Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J Infect Dis. 2011;204:1463–9. doi: 10.1093/infdis/jir550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wertheim JO, Leigh Brown AJ, Hepler NL, et al. The global transmission network of HIV-1. J Infect Dis. 2014;209:304–13. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little SJ, Kosakovsky Pond SL, Anderson CM, Young JA, Wertheim JO, Mehta SR, et al. Using HIV Networks to Inform Real Time Prevention Interventions. PLoS One. 2014;9:e98443. doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–1212. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 21.Oster AM, Wertheim JO, Hernandez AL, Ocfemia MCB, Saduvala N, Hall HI. Using Molecular HIV Surveillance Data to Understand Transmission between Subpopulations in the United States. JAIDS. doi: 10.1097/QAI.0000000000000809. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry M, Raymond HF, McFarland W. Same race and older partner selection may explain higher HIV prevalence among black men who have sex with men. AIDS. 2007;21:2349–50. doi: 10.1097/QAD.0b013e3282f12f41. [DOI] [PubMed] [Google Scholar]
- 23.Tieu HV, Murril C, Xu G, Koblin BA. Sexual partnering and HIV risk among black men who have sex with men. J Urban Health. 2010;87:113–121. doi: 10.1007/s11524-009-9416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS Behav. 2009;13:630–637. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maulsby C, Sifakis F, German D, Flynn CP, Holtgrave D. Partner characteristics and undiagnosed HIV seropositivity among men who have sex with men only (MSMO) and men who have sex with men and women (MSMW) in Baltimore. AIDS Behav. 2012;16:543–553. doi: 10.1007/s10461-011-0046-4. [DOI] [PubMed] [Google Scholar]
- 26.Kelley CF, Rosenberg ES, O’Hara BM, Frew PM, Sanchez T, del Rio C, et al. Measuring population transmission risk for HIV: an alternative metric of exposure risk in men who have sex with men (MSM) in the US. PLoS One. 2012;7:e53284. doi: 10.1371/journal.pone.0053284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Bradley H, Hu X, et al. Men living with diagnosed HIV who have sex with men: Progress along the continuum of HIV care — United States, 2010. MMWR. 2014;63:829–833. [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention HIV Surveillance Report. 2013;25 http://www.cdc.gov/hiv/library/reports/surveillance/. Published February 2015. Accessed March 2, 2015. [Google Scholar]
- 30.Centers for Disease Control and Prevention Pre-exposure Prophylaxis (PrEP) for HIV Prevention. http://www.cdc.gov/hiv/pdf/PrEP_fact_sheet_final.pdf. May 2014. Accessed March 17, 2015.
- 31.Joseph H, Marks G, Belcher L, Millet GA, Stueve A, Bingham TA, et al. Older partner selection, sexual risk behaviour and unrecognised HIV infection among black and Latino men who have sex with men. Sex Transm Infect. 2011;87:442–447. doi: 10.1136/sextrans-2011-050010. [DOI] [PubMed] [Google Scholar]
- 32.Bocour A, Renaud TC, Wong MR, Udeagu CCN, Shepard CW. Differences in risk behaviors and partnership patterns between younger and older men who have sex with men in New York City. J Acquir Immune Defic Syndr. 2011;58:417–423. doi: 10.1097/QAI.0b013e318230e6d9. [DOI] [PubMed] [Google Scholar]
- 33.Prestage G, Jin F, Bavinton B, Scott SA, Hurley M. Do differences in age between sexual partners affect sexual risk behaviour among Australian gay and bisexual men? Sex Transm Infect. 2013;89:653–658. doi: 10.1136/sextrans-2012-050947. [DOI] [PubMed] [Google Scholar]