Abstract
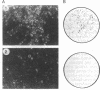
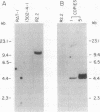
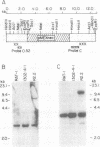
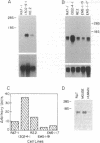
Revertants were isolated from v-fos-transformed rat-1 cells cotransfected with a human cDNA expression library and a selectable marker (pMEX-neo). Molecular analysis of one clone, R2.2, suggested that the revertant phenotype resulted from the disruption of a transformation effector gene by the integration of the pMEX-neo plasmid. Genomic sequences flanking the plasmid integration site were cloned and used as probes in Northern blot analyses. A probe derived from sequences 5' to the integration site hybridized to a unique 1.2-kilobase mRNA and was used to isolate a 0.9-kilobase cDNA clone (fte-1). The open reading frame of the fte-1 cDNA predicts a highly basic protein that shows a remarkable level of similarity with two genes from Saccharomyces cerevisiae. One of these yeast genes contains an unidentified open reading frame and the other, MFT1, is a gene isolated from a yeast mutant that fails to import a fusion protein into mitochondria [Garrett, J. M., Singh, K. K., Vonder Haar, R. A. & Emr, S. D. (1991) Mol. Gen. Genet. 225, 483-491]. Expression of the fte-1 gene was induced approximately 5-fold in v-fos-transformed fibroblasts, but expression was reduced in clone R2.2 and in several independent revertant clones. Transfection of R2.2 cells with fte-1 expression vectors resulted in the reacquisition of a transformed phenotype. These results demonstrate that the mammalian homologue of a gene implicated in protein import into yeast mitochondria is a v-fos transformation effector gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auwerx J., Sassone-Corsi P. IP-1: a dominant inhibitor of Fos/Jun whose activity is modulated by phosphorylation. Cell. 1991 Mar 8;64(5):983–993. doi: 10.1016/0092-8674(91)90322-p. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M. O., Zarbl H. Transformation effector and suppressor genes. J Cell Biochem. 1991 Jul;46(3):199–205. doi: 10.1002/jcb.240460303. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Garrett J. M., Singh K. K., Vonder Haar R. A., Emr S. D. Mitochondrial protein import: isolation and characterization of the Saccharomyces cerevisiae MFT1 gene. Mol Gen Genet. 1991 Mar;225(3):483–491. doi: 10.1007/BF00261691. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kho C. J., Zarbl H. A rapid and efficient protocol for sequencing plasmid DNA. Biotechniques. 1992 Feb;12(2):228–230. [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Kodama H., Ito M., Hattori T., Nakamura K., Komamine A. Isolation of Genes that Are Preferentially Expressed at the G(1)/S Boundary during the Cell Cycle in Synchronized Cultures of Catharanthus roseus Cells. Plant Physiol. 1991 Feb;95(2):406–411. doi: 10.1104/pp.95.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Pey N. K., Herrnstadt C., Marcil R. A., Smith L. M. A universal method for the direct cloning of PCR amplified nucleic acid. Biotechnology (N Y) 1991 Jul;9(7):657–663. doi: 10.1038/nbt0791-657. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Noda M., Kitayama H., Matsuzaki T., Sugimoto Y., Okayama H., Bassin R. H., Ikawa Y. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):162–166. doi: 10.1073/pnas.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Schaefer R., Iyer J., Iten E., Nirkko A. C. Partial reversion of the transformed phenotype in HRAS-transfected tumorigenic cells by transfer of a human gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1590–1594. doi: 10.1073/pnas.85.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann M., Neuberg M., Hunter J. B., Jenuwein T., Ryseck R. P., Bravo R., Müller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989 Feb 10;56(3):507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Squire M., Nasmyth K. A. Characterization of two genes required for the position-effect control of yeast mating-type genes. EMBO J. 1984 Dec 1;3(12):2817–2823. doi: 10.1002/j.1460-2075.1984.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Stepien G., Hodge J. A., Wallace D. C. Neoplastic transformation is associated with coordinate induction of nuclear and cytoplasmic oxidative phosphorylation genes. J Biol Chem. 1990 Nov 25;265(33):20589–20593. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wisdom R., Verma I. M. Revertants of v-fos-transformed rat fibroblasts: suppression of transformation is dominant. Mol Cell Biol. 1990 Nov;10(11):5626–5633. doi: 10.1128/mcb.10.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]