Abstract
Gastroesophageal variceal hemorrhage is a medical emergency with high morbidity and mortality. Endoscopic therapy is the mainstay of management of bleeding varices. It requires attention to technique and the appropriate choice of therapy for a given patient at a given point in time. Subjects must be monitored continuously after initiation of therapy for control of bleeding and second line definitive therapies introduced quickly if endoscopic and pharmacologic treatment fails.
Keywords: variceal hemorrhage, esophageal varices, gastric varices, endoscopy, endoscopic band ligation, endoscopic sclerotherapy, endoscopic variceal obturation, cirrhosis, portal hypertension
1. INTRODUCTION
Gastroesophageal varices are present in approximately 50% of patients with cirrhosis, more so with Child C cirrhosis (up to 85%). Rupture of these varices constitutes a medical emergency and can be rapidly fatal unless quickly controlled. Acute variceal bleeding occurs in a yearly rate of about 5%-15% in subjects with varices and despite advancement in diagnostics and therapy the 6 week mortality rate from variceal bleeding can be as high as 20%.1 Prompt diagnosis is a key in effective and timely management of these patients. Focused history, directed physical examination and basic laboratory measurements are important part of the triage in order to plan resuscitative measures, timing of endoscopy, other therapies, and for prognostication. Below, we will discuss the role of endoscopy in the diagnosis and management of bleeding gastro-esophageal varices.
2. ENDOSCOPIC DIAGNOSIS OF VARICEAL HEMORRHAGE
The key objectives of the initial evaluation of a subject with suspected variceal bleed include assessment of the severity of bleeding, identification of the source of bleeding and risk assessment of prognosis including the presence of infection and complications. Once therapy is initiated, ongoing assessment of bleeding control is required to determine the need for second line interventions. Endoscopy plays a critical role in these processes and is central to the management of active variceal bleeding.
Any upper gastrointestinal bleeding in a patient with known cirrhosis or evidence of portal hypertension should be considered and managed as a case of variceal bleeding until proven otherwise by endoscopy. Esophagogastroduodenoscopy (EGD) is considered the gold standard for the diagnosis of gastroesophageal variceal bleeding. It can be performed at the bedside in the emergency department and therapy can be provided at the same time when diagnostic assessment is performed. In the setting of active bleeding, a diagnosis of variceal hemorrhage is based on demonstration of bleeding varices, stigmata of recent bleeding e.g. an adherent clot over a varix or a platelet plug (white nipple sign) or presence of varices and upper GI bleeding without other obvious identifiable sources of bleeding (Table 1).2 The location of the varices is also identifiable at the time of endoscopy along with assessment of the size of the varices. These data are needed both for the diagnosis and determination of the optimal approach for long term bleeding control.
Table 1.
Diagnosis of gastroesophageal variceal bleeding (adapted from Sarin et al Hepatol Int 2011)63
| Upper gastrointestinal endoscopy (EGD) is the gold standard for the diagnosis of acute variceal bleeding. A diagnosis of gastroesophageal variceal bleeding is made if any of the following criteria is satisfied. |
|---|
| 1. Direct visualization of blood (spurting or oozing) arising from an esophageal or gastric varix. |
| 2. Presence of gastroesophageal varix with signs of recent bleed (stigmata) such as white nipple sign or overlying clot. |
| 3. Presence of varix with red signs plus presence of blood in the stomach in the absence of another source of bleeding. |
| 4. Presence of varix with red signs (cherry red spots- small ~2 mm, red, spotty flat spot on the variceal surface, red wale signs- longitudinal read streaks on the variceal surface, hematocystic spots- large, >3 mm, round, discrete, red raised spots on the variceal surface) and clinical signs of upper gastrointestinal bleeding, without blood in the stomach. |
Timing of endoscopy
Ideally endoscopy should be performed as soon as the proper resuscitation has taken place and hemodynamics have been stabilized. AASLD guidelines suggest timing of endoscopy to be within 12 hours for acute variceal bleeding.3,4 In a retrospective study of patients who came with acute variceal bleeding but were hemodynamically stable, there was no significant difference in mortality in patients with endoscopy performed within 4 hours versus 8 hours or 12 hours.5 In contrast, another study which found delayed endoscopy (endoscopy time > 15 hours) as a risk factor for increase mortality in acute variceal bleeding.6 It is our opinion that the urgency is dictated by the severity of bleeding and the clinical setting. For example, a patient who is exsanguinating needs immediate therapy to stop bleeding whereas care could be delayed until hemodynamics are fully stabilized in those with less severe bleeding. Also, the presence of comorbidities such as cardiac disease etc. and the ability to tolerate hemorrhagic anemia must also be taken in to account when making the decision to proceed rapidly versus not so rapidly towards endoscopy.
Utility of endoscopy for diagnosis of variceal hemorrhage
Endoscopy provides direct visualization of varices and is the cornerstone of the diagnostic approach to confirm the presence of variceal hemorrhage. There are however occasional situations where it may be difficult to visualize bleeding varices. The most common situation is a large clot in the fundus of the stomach that prevents an adequate retroflexed view of the cardia and the gastro-esophageal junction. Several modalities can be attempted to improve the ability to diagnose variceal bleeding in this setting. If the blood pressure permits, one may raise the head end of the bed to allow the clot to pass to the antrum. There are only anecdotal reports of the utility of this maneuver. More commonly, a pro-kinetic agent such as erythromycin has been used for this purpose. A recent meta-analysis suggests that this may improve visualization of gastric varices.7 It must however be noted that none of the published trials are of very high quality.
Is airway protection required for urgent endoscopy for bleeding varices?
This is a frequently debated topic. Airway compromise can occur before endoscopy, during endoscopy and in the period after endoscopy when the subject may not have fully recovered from sedation. One retrospective study did not find any benefit for prophylactic airway intubation prior to endoscopy.8 However, this study did not address the expertise of the intubators and the potential for selection bias. In a previous uncontrolled study, prevention of aspiration was associated with a substantial improvement in mortality in a subset of patients with severe uncontrolled variceal bleeding despite first-line therapies.9 Based on these, we currently recommend airway protection in those subjects with severe active hematemesis and those who are unable to protect their airway and are at high risk in the peri-procedural period.
3. SPECIFIC THERAPIES FOR ESOPHAGEAL VARICEAL BLEEDING
Endoscopic variceal band ligation
Principles of band ligation
Endoscopic variceal band ligation (EVBL) is cornerstone for the management of acute variceal bleeding. The principle for band ligation is based on venous drainage system in esophagus. Vianna et al described four zones in esophagus (gastric, palisade, perforating, and truncal zones). The gastric zone extends 2-3 cm below gastroesophageal junction and drains in short gastric and left gastric veins. The palisade zone extends 2-3 cm superior to gastric zone and is a watershed area between portal and systemic circulation. The perforating zone extends 2 cm further above palisade zone and has perforating veins joining submucosal venous plexuses to paraesophageal venous plexuses. The truncal zone is 8-10 cm long and has perforating veins joining submucosal veins to extraesophageal veins. The palisade and perforating zones are important for esophageal varices ligation.10 The objective is to obliterate the submucosal veins in the palisade zone which is followed by thrombosis and obliteration of the perforating veins that connect the submucosal varices to extra-esophageal collaterals.
Consequences of EVBL
The pathological changes after EVBL have been evaluated in canine model and humans. Variceal ligation results in ischemic necrosis of banded tissue and thrombosis of varices (24-48 hours). The resultant mucosal ulceration takes 2-3 weeks for complete re-epithelialization.11,12 It has been reported that with complete variceal obliteration, the risk of portal vein thrombosis may be increased and the development of gastric varices may be facilitated.13 Portal hypertensive gastropathy may also worsen after successful esophageal variceal eradication by EVBL.13
Technique
Initially single band devices were used. It was cumbersome to use single band devices as it involved reloading and reintubating esophagus multiple times. To overcome this limitation, multiple band shooters including the Saeed Multiple Ligator (Wilson-Cook Medical, Inc, Winston-Salem, NC, USA) and the Speedband (Boston Scientific Corporation, Natick, MA, USA) were developed. Saeed six shooter is a safe and effective method to eradicate varices.14 The band ligator is attached to the shaft of endoscope. After advancing the endoscope towards the varix which needs to be banded, suction is applied till “red out” occurs and then the band is fired. It is important at this point not to release suction until after a band has been successfully applied. This is required to minimize the risk of iatrogenic bleeding. The bands are placed in distal 5 cm of esophagus in spiral fashion from the gastro-esophageal junction and moving upwards. This is dictated by the thickness of the overlying mucosa which is the least at the gastro-esophageal junction thereby making this region particularly prone to bleeding.
Efficacy
In 1991 Steigmann et al published landmark study on superiority of EVBL over endoscopic injection sclerotherapy (EIS) for active variceal bleeding. EVBL had fewer complications and rebleeding rates compared to EIS. The two techniques were equally effective (approximately 90%). EVBL required lesser mean number of sessions (3.5 sessions) compared to EIS (4 sessions).15 A recent meta-analysis showed better bleeding control and low mortality with EVBL compared to EIS.16 In another meta-analysis, combination of EVBL with EIS offered no advantage over EVBL alone in prevention of rebleeding or reducing mortality. On the other hand, stricture formation was higher after EIS compared to EVBL alone.17
Complications
Risk of complications after banding varies from 2-23%. Chest pain, infection, stricture and ulcers are complications seen. The incidence of post banding ulcer bleeding is 2.6-7.3%.18 and has been associated with Child B or C cirrhosis. In the absence of active bleeding, it can be managed conservatively. For actively bleeding ulcers, one need to consider alternate endoscopic therapies including sclerotherapy or TIPS. Pantoprazole given for 10 days has shown to decrease size of ulcers. It does not affect symptoms like chest pain and dysphagia, however.19
Combination endoscopic treatment with pharmacological treatment is better than either alone for active bleeding. This has been confirmed in numerous trials which have now been assessed by meta-analyses. Combination therapy was associated with improved bleeding related outcomes (RR=1.21, CI-1.13-1.30, p< 0.001) and survival advantage (RR 0.74, 95% CI 0.57-0.95, P=0.02) compared to EVBL alone.20
Endoscopic injection sclerotherapy
Sclerosants are chemical agents which are oily or aqueous solution when injected in or around the varices induces sclerosis. Several such agents have been used to induce phlebitis and thrombosis of varices with subsequent obliteration. Sodium tetradecyl sulfate, sodium morrhuate (5%), sodium ethanolamine (5%), polidocanol, absolute alcohol have all been used for control of variceal hemorrhage. Only sodium tetradecyl sulfate is FDA approved for this indication.
Technique
The type of needle used is usually 23 G or 25 G. Injections can be made in to the varices (intravariceal injection) or around the varices (paravariceal injection).21 For the intravariceal technique, the first injection is usually made just below bleeding site in the varix. Subsequent injections are made at all varices around gastroesophageal junction. Proximal injections are made at 2 cm intervals up to 5-6 cm from gastroesophageal junction. For paravariceal technique injection is made adjacent to the varix. There is no convincing evidence that one technique is better than the other. Also, even in expert hands, intravariceal injections often result in paravariceal spillover.
Efficacy
EIS is 60-100 % effective in controlling active esophageal variceal bleeding.22 Treatment is repeated at 1-3 week intervals until obliteration and then every 3 months. EIS is not recommended for primary prophylaxis. Effectiveness of different sclerosants has been studied. From currently available data one agent cannot be recommended over the others.23-25 Currently, EIS is generally restricted to the very uncommon situation where EVBL is not technically feasible mainly due to its adverse event profile noted below. None the less, EIS can and should be considered as a rescue therapy if EVBL is not successful or results into further bleeding. However, TIPS should be the preferred alternate whenever feasible as it has been shown to improve survival.
Complications
Chest pain is noted in about 10 % of patients after sclerotherapy. Ulcer formation is noted in 20-60% of cases. The volume of sclerosant and Child C cirrhosis has been associated with the risk of ulcer formation. When performed, the volume of sclerosant per site should not exceed the recommended amount (volume injected depends on sclerosant used) to avoid the risks associated with EIS. Ranitidine has been shown to hasten healing of ulcers but does not prevent ulcer formation.26 Stricture formation may occur in up to 40 % of cases. Mostly strictures are asymptomatic. Symptomatic strictures respond well to endoscopic dilation. Risk of rebleeding is 15-50% in first 24 hours. Other rare complications include perforation, mediastinitis, pericarditis, pneumothorax, spinal cord paralysis and mesenteric vein thrombosis. There are few case reports of esophageal squamous cell carcinoma after sclerotherapy.27
Esophageal stents
Endoscopic stent placement for control of active esophageal bleeding: Over the last 5 years, several studies have demonstrated the feasibility of controlling active bleeding from esophageal varices with an endoscopically placed stent in the esophagus. Initial bleeding control rates of 80-90% have been reported with minimal side effects.28 Also, the stent placement can occur at the bedside and can come handy as a rescue therapy and can buy time for those with severe bleeding who will need a more definitive treatment such as transjugular intrahepatic portosystemic shunt (TIPS). One of the stents evaluated in such settings is a fully covered self-expandable metal stent SX-Ella Danis stent (135 × 25 mm; ELLA-CS, Hradec Kralove, Czech Republic). It has atraumatic edges and is fully covered metal stent. The stent can be easily removed after 7 days.
Endoscopic therapy for secondary prophylaxis of esophageal variceal hemorrhage
Left untreated, survivors of an index bleed have a 70% probability of rebleeding within a year. This is associated with a high mortality as well. It is therefore imperative to plan treatment to prevent subsequent bleeds. TIPS is rapidly becoming a front line approach for secondary prophylaxis and should be considered especially among patients with high risks of treatment failure with EVBL. 29-31 In its absence, a combination of EVBL and nonselective beta blockers constitutes the standard of care of prevention of variceal rebleeding. Multiple trials have evaluated and demonstrated that combination therapy is superior to either EVBL alone or pharmacological therapy alone for secondary prophylaxis.4,32 Combination therapy reduces the risk of esophageal variceal rebleeding by over 20 %.32 EVBL is generally performed at 2-4 week intervals until varices are obliterated. Generally about 3-5 sessions are needed for complete obliteration.33 There are however rare instances where varices persist despite 5-6 sessions. In such cases, one should suspect underlying portal vein thrombosis (personal experience) and, in the absence thereof, move towards TIPS.
Once varices are obliterated, repeat endoscopy is indicated at 3-6 month intervals to detect recurrent varices. When present, EVBL should be used again to obliterate these varices. EUS was at one time advocated for early diagnosis of recurrent varices. However, currently its use is not supported by evidence from clinical trials. We typically perform endoscopy 2-4 week interval till obliteration of varices. Then surveillance endoscopy is performed at 3-6 months and then every 6- 12 months to check for variceal recurrence.
4. ENDOSCOPIC MANAGEMENT OF GASTRIC VARICEAL HEMORRHAGE
Anatomy of gastric varices
Gastric varices occur in approximately 20% of time among patients with cirrhotic portal hypertension. Unlike esophageal varices, gastric varices are rather heterogeneous group of disorders and their etiology and pathophysiology can be different. Gastric varices can also develop in the absence of cirrhosis, primarily due to splenic vein thrombosis or other thrombophilic conditions such as polycythemia vera and other hypercoaguable conditions 34,35.
Classification of gastric varices
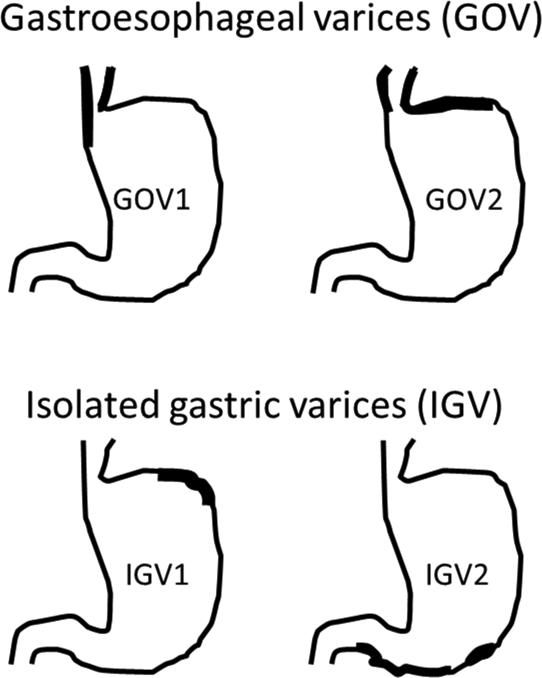
The most widely used classification system is the Sarin classification which categorizes gastric varices into 4 types based on location and in relation to esophageal varices.36 Gastric varices in the presence of esophageal varices are defined as gastroesophageal varices (GOVs). GOVs are believed to be extension of the esophageal varices. Type 1 GOVs (GOV1) are gastric varices that occur along the lesser curvature, whereas gastric varices present along the fundus are defined as type 2 GOVs (GOV2). Gastric varices with no concurrent esophageal varices as called isolated gastric varices (IGVs). IGVs are further classified into type 1 (IGV1) when they are present in the gastric fundus or type 2 (IGV2) if present elsewhere in the stomach or first portion of the duodenum. GOV2 and IGV1 are sometimes grouped together and referred to as ‘fundic varices’ (figure 1).
Figure 1. Classification of gastric varices (adapted from Sarin and Kumar)36.
Gastric varices in the presence of esophageal varices are defined as gastroesophageal varices (GOVs). Types 1 GOV (GOV1)are gastric varices that occur along the lesser curvature, whereas GOVs present along the fundus are defined as type 2 GOV (GOV2). Gastric varices with no concurrent esophageal varices as called isolated gastric varices (IGVs). IGVs are further classified into type 1 (IGV1) when they are present in the gastric fundus or type 2 (IGV2) if present elsewhere in the stomach or first portion of the duodenum. GOV2 and IGV1 are sometimes grouped together and referred to as ‘fundic varices’.
GOV, gastroesophageal varies, IGV, isolated gastric varices
Clinical correlates of gastric varices
Almost 70% of gastric varices are GOV1. Fundic varices (GOV2 and IGV1) account for the rest and IGV2 are quite rare. Although GOV1 are the most common type of varices present, fundic varices bleed more often, accounting for almost 80% of all gastric variceal bleeding. Gastric varices are often large and have numerous shunts present. While gastric varices tend to bleed less commonly than esophageal varices, such bleedings are more severe, are technically difficult and less amenable to therapy. They tend to bleed at a lower portal pressure than esophageal varices and bleeding can be massive owing to the increased blood flow from the gastric bed in these patients. The risks of rebleeding from gastric variceal bleeds are higher and mortality can be as high as 30%.33 Also, it is important to note that eradication of esophageal varices by variceal ligation or sclerotherapy can exacerbate gastric fundic varices.13,33
Diagnosis of gastric varices
The diagnosis of gastric varices by endoscopy can be difficult as gastric varices lie in the submucosa and are often indistinguishable from the gastric rugae. Examination of gastric varices is best done with full insufflation of the stomach in both direct and retroflexed view. When in doubt, a Doppler probe should be used to confirm presence of venous hum. The Doppler probe should be gently applied directly on top of the varix. A continuous venous hum will confirm presence of venous flow in the varix. Note should be made of the location of the varices, size (small <5 mm or large ≥5 mm), presence of high risk stigmata (cherry red spot, red wale sign, hematocystic spot), active or recent bleeding (white nipple sign, overlying clot), as well as presence or absence of esophageal varices.37
Specific endoscopic therapies for gastric variceal bleeding
Endoscopic therapies for gastric variceal bleeding include band ligation, sclerotherapy, and variceal obturation with cyanoacrylate glue. Novel approaches include thrombin/fibrin adhesives, detachable snares and hemostatic sprays. The choice of therapy is primarily determined by the location of the varix, and presence or absence of esophageal varices (summarized in Table 3). In case of torrential bleeding, salvage therapy with balloon tamponade (Sengstaken-Blakemore or Linton tubes) is used as a bridge to more definitive treatment such as placement of TIPS or balloon occluded retrograde transvenous obliteration (BRTO).
Table 3.
Summary of endoscopic therapies for esophagogastric variceal hemorrhage
| Treatment modality | Comment |
|---|---|
| Endoscopic variceal ligation (EVBL) | Therapy of choice for EV, and GOV1, alternate for non-GOV1 Primary hemostasis: 71-100% Rebleeding: 3-36% Complications: overall (2-23%), band ulcers (2.6-7.3%), stricture formation (2%), chest pain |
| Endoscopic variceal obturation (EVO) | Therapy of choice for fundic varices (GOV2 and IGV1) Primary hemostasis: > 90% Rebleeding: ~15% Complications: distant emboli (0.7%), sepsis (1.3%), ulcer formation (0.1%), fever, abdominal pain, chances of scope damage |
| Endoscopic injection sclerotherapy (EIS) | Second line therapy for both esophageal and gastric varices Primary hemostasis: 60-100% Rebleeding: 5-10% in EV, 37-89% in GV(half caused by therapy induced ulcers) Complications: chest pain (10%), ulcers and perforation (20-60%), strictures up to 40%, |
| Detachable snare | No controlled studies Small uncontrolled studies show as effective as band ligation |
| Thrombin/Fibrin injection |
Primary hemostasis: up to 92% Rebleeding: believed minimal as won't cause ulcers Complications: anaphylactic reactions, infection risk, high cost No controlled studies to date |
| Hemostatic spray | No controlled studies, Potential role as a rescue agent when primary modality fail Potential complications include allergic reactions, embolization, small bowel obstructions from foreign body impaction |
| Esophageal stent |
Hemostasis: 80-90% Potential role as a rescue agent when primary modality fail in place of balloon tamponade No controlled studies |
EVBL, endoscopic variceal ligation, EVO, endoscopic variceal obturation, EIS, endoscopic injection sclerotherapy, EV, esophageal varices, GV, gastric varices, GOV1, gastroesophageal varices type 1, GOV2, gastroesophageal varices type 2, IGV1, isolated gastric varices type 1
The quality of the literature on endoscopic management of gastric varices is not as robust as that for esophageal variceal hemorrhage. In general, GOV1 is regarded as extension of esophageal varices and is managed the same way as esophageal varices. EVBL not as effective for GOV2 and is not suitable for IGV1. Management of the ‘fundic varices’ is different and generally involves use of cyanoacrylate glue.3,4
Endoscopic variceal obturation
First described by Soehendra et al,38 endoscopic variceal obturation (EVO) using tissue adhesives such as n-butyl-2-cyanoacrylate (Histoacryl®, Brau Medical, Bethlehem PA; Indermil, Covedien, Mansfield, MA) or 2-octyl cyanoacrylate (Dermabond, Johnson and Johnson, New Brunswick, NJ) is an effective mean of hemostasis in bleeding gastric varices. Cyanoacrylate is a liquid polymer which upon coming in contact with plasma instantly polymerizes and can lead to obliteration of the varices. EVO with N-butyl-2-cyanoacrylate injections is widely performed across Europe and Asia with excellent outcomes but is not widely used in the U.S.
Technique
Cyanoacrylate (glue) injections are performed along the same principles of injection sclerotherapy, but extra precautions are required to protect the endoscope from glue damage. Use of personal protective devices including goggles are recommended while handling glue as this can lead to potential eye injury. Once a gastric varix to be glued is identified, the endoscope is withdrawn. The tip of the endoscope is coated with silicon oil and a few drops of the oil is applied to the channel and flushed to protect the scope from glue damage. The scope is reinserted. A 23- to 25 gauge needle with a metal hub is used for the injection. The needle is primed with sterile water (or saline if using octyl-cyanoacrylate). The needle is inserted directly onto the varix and 1-2 ml of the glue solution is rapidly injected directly in to the varix (intravariceal injection) followed by a sterile water flush (~1ml) to clear the glue remaining in the scope channel. The needle should be quickly withdrawn from the varix and continuously flushed to keep open for repeat use if necessary. Injection can be repeated until the varix is completely obliterated as evident by a feeling of “hardness” on probing. We routinely use a Doppler probe to confirm absence of the venous hum indicating complete variceal obliteration. We generally limit our treatment to 1-2 injections per varix. Larger volume of injection may increase risks of embolization. Treatment can be repeated to control recurrent bleeding. Once acute bleeding episode is controlled, follow up treatment should be performed every 3-4 weeks until complete eradication of the varices is achieved. The role of endoscopic ultrasound guidance for cyanoacrylate glue injection or placement of coils is evolving and remains to be defined.36
Efficacy
The reported success rate of EVO in fundic variceal bleeding is in the range of 90% with complication rate of about 15%.39,40 Kang et al performed EVO in 127 gastric varices (100 cases with active bleeding) and reported primary hemostasis rate of 98% and 1 year bleeding recurrence rate of 18%.41 There have been at least 3 controlled studies comparing EVO to EVBL or EIS with favorable outcomes of EVO in terms of primary hemostasis, rebleeding rates and complications (summarized in Table 2).42-44 In a recent meta-analysis involving 648 patients with bleeding gastric varices (all types), pooled analysis suggested better efficacy of EVO in achieving primary hemostasis, and lower rebleeding rates (OR 2.32; 95% CI 1.19-4.51).45 There was no difference between EVO and EVBL in terms of mortality, complications and number of treatment sessions required for complete variceal eradication. There were significant heterogeneity among the studies in terms of techniques used, type and dose of cyanoacrylate, number of injections, however. Most importantly, majority of the subjects in these trials were with GOV1 who could be managed by EVBL. It is important to note that EVBL is not suitable as a treatment for IGV1 and the response rates of EVBL for GOV2 are poorer than that for GOV1.
Table 2.
Randomized controlled studies comparing endoscopic variceal band ligation with endoscopic variceal obturation
| Study | Description | N (active bleeding) |
GOV1/GOV2/IGV | Primary hemostasis OR (95% CI) |
Rebleeding rate HR (95% CI) |
1yr Mortality | Comment |
|---|---|---|---|---|---|---|---|
| Lo et al, 200142 | RCT Taiwan Single center EVBL vs. EVO |
29 vs. 31 (11 vs. 15 with active bleeding) | 13/33/14 | 45% vs. 87% 7.8 (1.16,52.35) |
48% vs. 29% 0.44 (0.19, 1.00) |
62% vs. 42% | EVO more effective and safer than EVBL in gastric variceal bleeding |
| Tan et al, 200643 | RCT Taiwan Single center EVBL vs. EVO |
48 vs. 49 (15 vs. 15 with active bleeding) |
25/51/21 | 93% vs. 93% 1.00 (0.06, 17.62) |
42% vs.22% 0.41 (0.20, 0.82) |
44% vs. 42% | No difference between EVO and EVBL in efficacy, survival or severe complications. EVO associated with less rebreeding. |
| El Amin et al, 201044 | RCT Egypt Multicenter EVBL vs. EVO |
75 vs. 75 (All GOV1) |
150/0/0 | 81% vs. 91% 2.23 (0.84, 5.89) |
16% vs. 6% | 1.3% vs.7% mortality at 6 month, most died from HRS |
All pts with GOV1, EVBL performed better in junctional varices. EVO can be an alternate therapy |
EVBL, endoscopic variceal band ligation, EVO, endoscopic variceal obturation, GOV1, gastroesophageal varices type 1, GOV2, gastroesophageal varices type 2, IGV1, isolated gastric varices type 1, RCT, randomized controlled trial, NS, nonsignificant, PVT, portal vein thrombosis, HRS, hepatorenal syndrome, OR, odds ratio, HR, hazard ration, CI, confidence interval
Complications
Although safe and effective, severe complications related to embolization of cyanoacrylate glue have been reported including systemic embolization (pulmonary, cerebral, splenic 0.7%), recurrent sepsis (1.3%), recurrent bleeding from glue extrusions (4.4%), ulcer formation (0.1%).44,47 Most common ones are transient fever, chest and abdominal pain etc. In studies comparing EVO with EIS or EVBL, the overall complication rates were similar. Complications related to technical issues such as adherence of needle into the varix and scope damage due to glue adherence have been anecdotally reported. Of note, cyanoacrylate glue has not been approved by the Food and Drug Administration (FDA) for use in variceal bleeding. However, all other modalities for variceal hemostasis have never been FDA approved as well.
Endoscopic sclerotherapy
Similar to esophageal varices, sclerotherapy can be performed in gastric variceal bleeding but with a much lower initial hemostasis rates and higher rebleeding rates. A larger volume of injection is required as gastric varices are larger and consequently can induce more adverse events. In their 11 year experience with ethanol based sclerotherapy, Sarin et al reported a hemostatic rate of 66% in acute gastric variceal bleeding with sclerotherapy using absolute alcohol.48 Likewise, in a prospective nonrandomized trial, sclerotherapy with ethanolamine achieved initial hemostasis in 67% cases of fundic varices, far less than the with cyanoacrylate injections (93%).49 High rates of recurrent bleeding (up to 90%) have been reported with EIS for gastric varices.50,51 Moreover, sclerotherapy is also associated with increased complications such as fever, abdominal and chest pain, dysphagia, ulceration and rebleeding including ulcer related bleeding. Sclerotherapy has therefore fallen out of favor in management of gastric as well as esophageal varices.
Fibrin sealant/Thrombin
Thrombin promotes the conversion of fibrinogen to fibrin, producing a local fibrin clot. As a liquid preparation delivered topically via a catheter, thrombin has been shown to be effective in achieving primary hemostasis of 75% to 94% among patients with gastric variceal bleeding in few small uncontrolled studies.52-54 In an earlier randomized trial, however, thrombin plus ethanolamine was not superior to ethanolamine alone in controlling bleeding esophageal varices.55 Both bovine and human derived thrombin products are commercially available. Likewise, fibrin sealant has been anecdotally used with some success in gastric variceal bleeding, but their use at this point remains largely experimental.56,57 These agents are largely safe, but complications including anaphylaxis, antibody formations, transmission of infections and systemic embolization are possible. Of note, product labeling states that intravascular injection of fibrin glue is contraindicated because of the risk of embolization.
Detachable snares
Detachable snares (Endoloop, Olympus, Tokyo, Japan) in conjunction with a transparent endcap applied at the tip of the endoscope have been tried in bleeding esophageal varices with varied outcomes. Their application on gastric varices is limited. In an earlier study of 41 patients with large (>2 cm) gastric varices (12 with active bleeding, and 29 with red signs), endoscopic ligation using detachable snares and elastic bands reported an overall hemostasis of 82.9%.58 Repeated treatment resulted in eradication of varices in over 91%. Likewise, Naga et al compared detachable snares with EVBL in patients with bleeding esophageal varices (25 each group) and found a lower rate of recurrent bleeding (12% vs. 28%) with detachable snares. The difference was not however statistically significant. EVBL and detachable snares were comparable in terms of primary hemostasis (100% with both techniques), variceal eradication rate, and number of sessions required for eradication, and cost (Naga GIE 2004).59 Others have reported similar outcome.60 While the detachable snares along with endcap seems to have an appeal as it will give better visualization and can be applied without needing withdrawal and reinsertion of the endoscope, it has not yet gained popularity. Controlled trials are needed to demonstrate its utility for bleeding GOV2 and IGV1 varices.
Hemostatic sprays
Various mineral and plant based hemostatic granules or powders have been used for the control of external hemorrhages. These agents are now also incorporated in first aid kits. Their use in control of gastrointestinal bleedings including variceal bleeding remains experimental however. There have been a few case reports with successful use of Ankaferd Blood Stopper, (ABS, a plant alkaloid extract) in acute upper gastrointestinal bleedings including gastric variceal bleeding.61,62 Such agents may prove handy as a rescue agent in difficult cases that fail the standard approach of band ligation or glue injections. Potential complications include embolization, allergic reactions, and small bowel obstruction from foreign body impaction. Larger and controlled studies are required in order to establish their safety and efficacy.
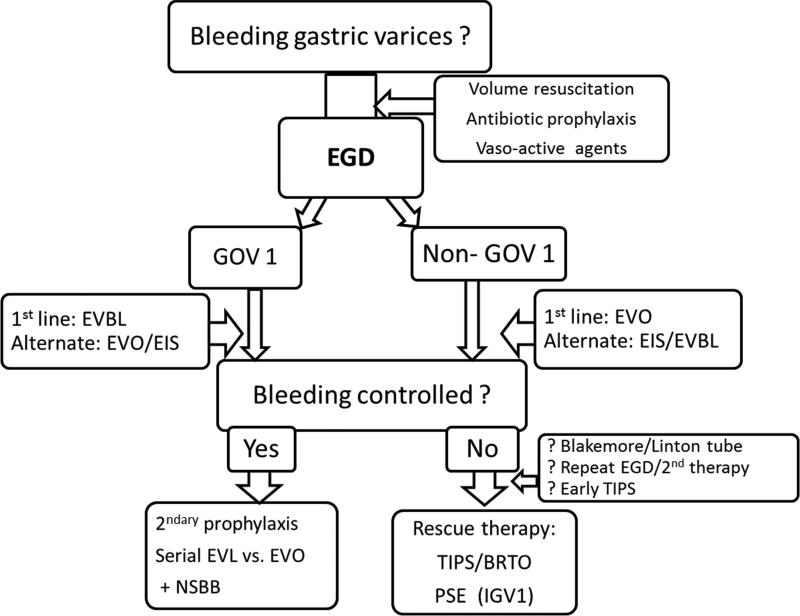
In summary, GOV1 are believed to be extension of esophageal varices and their management should follow the same principles as for the esophageal varices especially if they are limited within 3 cm of the gastroesophageal junction. As for bleeding esophageal varices, the preferred approach for GOV1 is EVL. Hemostasis and rebleeding rates for GOV1 are comparable to esophageal varices following EVBL. However, the efficacy of EVBL has not been proven for rest of the gastric variceal bleedings including GOV2, IGV1 and IGV2. The choice of endoscopic therapy for bleeding fundic varices (GOV2 and IGV1) is EVO with cyanoacrylate glue. TIPS should be considered in situations where EVO is not available. TIPS should also be considered in cases of treatment failures as a rescue therapy. Balloon retrograde transvenous obliteration is an alternate modality and is generally considered a second line treatment (Figure 2).
Figure 2. Suggested algorithm for management of gastric variceal hemorrhage.
Type 1 gastroesophageal varices (GOV1) are believed to be extension of esophageal varices and are best treated with band ligation. Cyanoacrylate glue can be applied as an alternate therapy. The choice of endoscopic therapy for bleeding fundic varices is EVO with cyanoacrylate glue. While EIS and thrombin may come as an alternate, TIPS should be considered in situations where EVO is not available. Alternately, balloon-occluded retrograde transvenous obliteration should be considered when TIPS is not suitable. If splenic vein thrombosis is identified as the cause of bleeding fundic varices, either splenectomy or partial splenic embolization should be considered.
EGD, esophagogastroduodenoscopy, EVBL, endoscopic variceal ligation, EVO, endoscopic variceal obturation, EIS, endoscopic injection sclerotherapy, GOV1, gastroesophageal varices type 1, GOV2,gastroesophageal varices type 2, IGV1, isolated gastric varices type 1, TIPS transjugular intrahepatic portosystemic shunt, BRTO, balloon-occluded retrograde transvenous obliteration, PSE, partial splenic embolization, NSBB, nonselective beta-blocker
Endoscopic therapy for secondary prophylaxis of gastric variceal hemorrhage
As noted above, gastric varices are at high risk for rebleeding. GOV1 are managed by EVBL of esophageal varices and the associated GOV1. They should be followed by repeat banding until eradication, followed by a periodic surveillance endoscopy as described above. In contrast, management of GOV2 and IGV1 can prove to be challenging. TIPS should be considered in situations where EVO is not available. Alternately, serial EVO should be performed to obliterate such varices. The role of beta blockers for prevention of gastric variceal rebleeding is controversial. Balloon retrograde transvenous obliteration is an alternate modality and is generally considered a second line treatment. If splenic vein thrombosis is identified as the cause of bleeding fundic varices, splenectomy should be considered.3,4
SUMMARY
In summary, endoscopic treatment of variceal hemorrhage is the mainstay of management of bleeding varices. It requires attention to technique and the appropriate choice of therapy for a given patient at a given point in time. Subjects must be monitored continuously after initiation of therapy for control of bleeding and second line definitive therapies introduced quickly if endoscopic and pharmacologic treatment fails.
Key points.
Gastroesophageal variceal hemorrhage is a medical emergency with high morbidity and mortality.
Endoscopic therapy is the mainstay of management of bleeding varices.
It requires attention to technique and the appropriate choice of therapy for a given patient at a given point in time.
Subjects must be monitored continuously after initiation of therapy for control of bleeding and second line definitive therapies introduced quickly if endoscopic and pharmacologic treatment fails.
Appropriate surveillance plan must be established for prevention of future bleedings.
Acknowledgments
Grant support: This manuscript was supported in part by NIH T32 DK 07150-038 to Dr. Sanyal
Affiliation: Div. of Gastroenterology, Dept. of Internal medicine, Virginia Commonwealth University School of Medicine, Richmond, VA
Abbreviations
- EVBL
Endoscopic variceal band ligation
- EIS
Endoscopic injection sclerotherapy
- EVO
Endoscopic variceal obturation
- GOV
Gastro-esophageal varices
- IGV
Isolated gastric varices
- EGD
Esophagogastroduodenoscopy
- BRTO
Balloon occluded retrograde transvenous obliteration
- TIPS
Transjugular intrahepatic portosystemic shunt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr. Kapoor: None to report
Dr. Dharel: None to report
Dr. Sanyal: Dr. Sanyal has stock options in Genfit. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Nimbus, Salix, Tobira, Takeda, Fibrogen, Immuron, Exhalenz and Genfit. He has been an unpaid consultant to Intercept and Echosens. His institution has received grant support from Gilead, Salix, Tobira and Novartis.
Contributor Information
Ashwani Kapoor, MCV Box 980341, Richmond, VA 23298-0341, akapoor@mcvh-vcu.edu.
Narayan Dharel, MCV Box 980341, Richmond, VA 23298-0341, ndharel@mcvh-vcu.edu.
Arun J. Sanyal, MCV Box 980341, Richmond, VA 23298-0341.
REFERENCES
- 1.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R, Pascal JP, Ancona E, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A consensus development workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol. 1992;15:256–261. doi: 10.1016/0168-8278(92)90044-p. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 5.Cheung J, Soo I, Bastiampillai R, Zhu Q, Ma M. Urgent vs. non-urgent endoscopy in stable acute variceal bleeding. Am J Gastroenterol. 2009;104:1125–1129. doi: 10.1038/ajg.2009.78. [DOI] [PubMed] [Google Scholar]
- 6.Hsu YC, Chen CC, Wang HP. Endoscopy timing in acute variceal hemorrhage: Perhaps not the sooner the better, but delay not justified. Am J Gastroenterol. 2009;104:2629–2630. doi: 10.1038/ajg.2009.432. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y, Guo JF, Li ZS. Meta-analysis: Erythromycin before endoscopy for acute upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;34:166–171. doi: 10.1111/j.1365-2036.2011.04708.x. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58–61. doi: 10.1067/mge.2003.46. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Freedman AM, Luketic VA, et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology. 1996;111:138–146. doi: 10.1053/gast.1996.v111.pm8698192. [DOI] [PubMed] [Google Scholar]
- 10.Vianna A, Hayes PC, Moscoso G, et al. Normal venous circulation of the gastroesophageal junction. A route to understanding varices. Gastroenterology. 1987;93:876–889. doi: 10.1016/0016-5085(87)90453-7. [DOI] [PubMed] [Google Scholar]
- 11.Polski JM, Brunt EM, Saeed ZA. Chronology of histological changes after band ligation of esophageal varices in humans. Endoscopy. 2001;33:443–447. doi: 10.1055/s-2001-14259. [DOI] [PubMed] [Google Scholar]
- 12.Stiegmann GV, Sun JH, Hammond WS. Results of experimental endoscopic esophageal varix ligation. Am Surg. 1988;54:105–108. [PubMed] [Google Scholar]
- 13.Yuksel O, Koklu S, Arhan M, et al. Effects of esophageal varices eradication on portal hypertensive gastropathy and fundal varices: A retrospective and comparative study. Dig Dis Sci. 2006;51:27–30. doi: 10.1007/s10620-006-3078-2. [DOI] [PubMed] [Google Scholar]
- 14.Saeed ZA. The saeed six-shooter: A prospective study of a new endoscopic multiple rubber-band ligator for the treatment of varices. Endoscopy. 1996;28:559–564. doi: 10.1055/s-2007-1005555. [DOI] [PubMed] [Google Scholar]
- 15.Stiegmann GV, Goff JS, Michaletz-Onody PA, et al. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527–1532. doi: 10.1056/NEJM199206043262304. [DOI] [PubMed] [Google Scholar]
- 16*.Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Ann Intern Med. 1995;123:280–287. doi: 10.7326/0003-4819-123-4-199508150-00007. [DOI] [PubMed] [Google Scholar]
- 17*.Singh P, Pooran N, Indaram A, Bank S. Combined ligation and sclerotherapy versus ligation alone for secondary prophylaxis of esophageal variceal bleeding: A meta-analysis. Am J Gastroenterol. 2002;97:623–629. doi: 10.1111/j.1572-0241.2002.05540.x. [DOI] [PubMed] [Google Scholar]
- 18.Tierney A, Toriz BE, Mian S, Brown KE. Interventions and outcomes of treatment of postbanding ulcer hemorrhage after endoscopic band ligation: A single-center case series. Gastrointest Endosc. 2013;77:136–140. doi: 10.1016/j.gie.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: A randomized, controlled trial. Hepatology. 2005;41:588–594. doi: 10.1002/hep.20593. [DOI] [PubMed] [Google Scholar]
- 20.Wells M, Chande N, Adams P, et al. Meta-analysis: Vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267–1278. doi: 10.1111/j.1365-2036.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarin SK, Nanda R, Sachdev G, Chari S, Anand BS, Broor SL. Intravariceal versus paravariceal sclerotherapy: A prospective, controlled, randomised trial. Gut. 1987;28:657–662. doi: 10.1136/gut.28.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memon MA, Jones WF. Injection therapy for variceal bleeding. Gastrointest Endosc Clin N Am. 1999;9:231–252. [PubMed] [Google Scholar]
- 23.Sarin SK, Kumar A. Sclerosants for variceal sclerotherapy: A critical appraisal. Am J Gastroenterol. 1990;85:641–649. [PubMed] [Google Scholar]
- 24.Andreani T, Poupon RE, Balkau BJ, et al. Preventive therapy of first gastrointestinal bleeding in patients with cirrhosis: Results of a controlled trial comparing propranolol, endoscopic sclerotherapy and placebo. Hepatology. 1990;12:1413–1419. doi: 10.1002/hep.1840120624. [DOI] [PubMed] [Google Scholar]
- 25.Paquet KJ. Prophylactic endoscopic sclerosing treatment of the esophageal wall in varices -- a prospective controlled randomized trial. Endoscopy. 1982;14:4–5. doi: 10.1055/s-2007-1021560. [DOI] [PubMed] [Google Scholar]
- 26.Tamura S, Shiozaki H, Kobayashi K, et al. Prospective randomized study on the effect of ranitidine against injection ulcer after endoscopic injection sclerotherapy for esophageal varices. Am J Gastroenterol. 1991;86:477–480. [PubMed] [Google Scholar]
- 27.Baillie J, Yudelman P. Complications of endoscopic sclerotherapy of esophageal varices. Endoscopy. 1992;24:284–291. doi: 10.1055/s-2007-1010483. [DOI] [PubMed] [Google Scholar]
- 28.Cardenas A, Fernandez-Simon A, Escorcell A. Endoscopic band ligation and esophageal stents for acute variceal bleeding. Clin Liver Dis. 2014;18:793–808. doi: 10.1016/j.cld.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Monescillo A1, Martínez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 30.García-Pagán JC1, Caca K, Bureau C, et al. Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 31*.Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006;(4):CD000553. doi: 10.1002/14651858.CD000553.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Franchis R. Somatostatin, somatostatin analogues and other vasoactive drugs in the treatment of bleeding oesophageal varices. Dig Liver Dis. 2004;36(Suppl 1):S93–100. doi: 10.1016/j.dld.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Stiegmann GV, Goff JS, Sun JH, Davis D, Bozdech J. Endoscopic variceal ligation: an alternative to sclerotherapy. Gastrointest Endosc. 1989;35:431–434. doi: 10.1016/s0016-5107(89)72850-9. [DOI] [PubMed] [Google Scholar]
- 34.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 35.Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach in the management of gastric varices. Gastroenterology. 2004;126:1175–1189. doi: 10.1053/j.gastro.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989;84:1244–1249. [PubMed] [Google Scholar]
- 37.Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307–312. doi: 10.1053/jhep.1997.v25.pm0009021939. [DOI] [PubMed] [Google Scholar]
- 38.Soehendra N, Griomm H, Nam V. N-butyl-2-cyanoacrylate: a supplement to endoscopic sclerotherapy. Endoscopy. 1986;19:221–224. doi: 10.1055/s-2007-1018288. [DOI] [PubMed] [Google Scholar]
- 39.Weilert F, Binmoeller KF. Endoscopic management of gastric variceal bleeding. Gastroenterol Clin North Am. 2014;43:807–818. doi: 10.1016/j.gtc.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Sarin SK, Kumar A. Endoscopic treatment of gastric varices. Clin Liver Dis. 2014;18:809–827. doi: 10.1016/j.cld.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Kang EJ, Jeong SW, Jang JY, et al. Long-term result of endoscopic Histoacryl (N-butyl-2-cyanoacrylate) injection for treatment of gastric varices. World J Gastroenterol. 2011;17:1494–1500. doi: 10.3748/wjg.v17.i11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060–1064. doi: 10.1053/jhep.2001.24116. [DOI] [PubMed] [Google Scholar]
- 43.Tan P-C, Hou M-C, Lin H-C, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-Butyl-2-Cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690–697. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]
- 44.El Amin H, Abdel Baky L, Sayed Z, et al. A randomized trial of endoscopic variceal ligation versus Cyanoacrylate injection for treatment of bleeding junctional varices. Trop Gastroenterol. 2010;31:279–284. [PubMed] [Google Scholar]
- 45*.Ye X, Huai J, Chen Y. Cyanoacrylate injection compared with band ligation for acute gastric variceal hemorrhage: a meta-analysis of randomized controlled trials and observational studies. Gastroenterol Res Pract. 2014;2014:806–586. doi: 10.1155/2014/806586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binmoeller KF, Borsatto R. Variceal bleeding and portal hypertension. Endoscopy. 2000;32:189–199. doi: 10.1055/s-2000-99. [DOI] [PubMed] [Google Scholar]
- 47.Cheng LF, Wang ZQ, Li CZ, et al. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clinical Gastroenterology and Hepatology. 2010;9:760–766. doi: 10.1016/j.cgh.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: an eleven-year experience. Gastrointest Endosc. 1997;46:8–14. doi: 10.1016/s0016-5107(97)70202-5. [DOI] [PubMed] [Google Scholar]
- 49.Oho K, Iwao T, Sumino M, Toyonaga A, Tanikawa K. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: a nonrandomized study. Endoscopy. 1995;27:349–354. doi: 10.1055/s-2007-1005712. [DOI] [PubMed] [Google Scholar]
- 50.Sarin SK, Sachdev G, Nanda R, Misra SP, Broor SL. Endoscopic sclerotherapy in the treatment of gastric varices. Br J Surg. 1988;75:747–750. doi: 10.1002/bjs.1800750809. [DOI] [PubMed] [Google Scholar]
- 51.Gimson AE, Westaby D, Williams R. Endoscopic sclerotherapy in the management of gastric variceal hemorrhage. J Hepatol. 1991;13:274–278. doi: 10.1016/0168-8278(91)90068-m. [DOI] [PubMed] [Google Scholar]
- 52.Yang WL, Tripathi D, Therapondos G, Todd A, Hayes PC. Endoscopic use of human thrombin in bleeding gastric varices. Am J Gastroenterol. 2002;97:1381–1385. doi: 10.1111/j.1572-0241.2002.05776.x. [DOI] [PubMed] [Google Scholar]
- 53.Przemioslo RT, McNair A, Williams R. Thrombin is effective in arresting bleeding from gastric variceal hemorrhage. Dig Dis Sci. 1999;44:778–781. doi: 10.1023/a:1026626212129. [DOI] [PubMed] [Google Scholar]
- 54.Ramesh J, Limdi JK, Sharma V, Makin AJ. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc. 2008;68:877–882. doi: 10.1016/j.gie.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 55.Kitano S, Hashizume M, Yamaga H, et al. Human thrombin plus 5 percent ethanolamine oleate injected to sclerose oesophageal varices: a prospective randomized trial. Br J Surg. 1989;76:715–718. doi: 10.1002/bjs.1800760721. [DOI] [PubMed] [Google Scholar]
- 56.Heneghan MA, Byrne A, Harrison PM. An open pilot study of the effects of human fibrin glue for endoscopic treatment of patients with acute bleeding from gastric varices. Gastrointest Endosc. 2002;56:422–426. doi: 10.1016/s0016-5107(02)70054-0. [DOI] [PubMed] [Google Scholar]
- 57.Datta D, Vlavianos P, Alisa A, et al. Use of fibrin glue (Beriplast) in the management of bleeding gastric varices. Endoscopy. 2003;35:675–678. doi: 10.1055/s-2003-41517. [DOI] [PubMed] [Google Scholar]
- 58.Lee MS, Cho JY, Cheon YK, et al. Use of detachable snares and elastic bands for endoscopic control of bleeding from large gastric varices. Gastrointest Endosc. 2002;56:83–88. doi: 10.1067/mge.2002.125104. [DOI] [PubMed] [Google Scholar]
- 59.Naga MI, Okasha HH, Foda AR, et al. Detachable endoloop vs. elastic band ligation for bleeding esophageal varices. Gastrointest Endosc. 2004;59:804–809. doi: 10.1016/s0016-5107(04)00361-x. [DOI] [PubMed] [Google Scholar]
- 60.Shim CS, Cho JY, Park YJ, et al. Mini-detachable snare ligation for the treatment of esophageal varices. Gastrointest Endosc. 1999;50:673–676. doi: 10.1016/s0016-5107(99)80019-4. [DOI] [PubMed] [Google Scholar]
- 61.Kurt M, Disibeyaz S, Akdogan M, Sasmaz N, Aksu S, Haznedaroglu IC. Endoscopic application of ankaferd blood stopper as a novel experimental treatment modality for upper gastrointestinal bleeding: a case report. Am J Gastroenterol. 2008;103:2156–2158. doi: 10.1111/j.1572-0241.2008.01982_15.x. [DOI] [PubMed] [Google Scholar]
- 62.Tuncer I, Doganay L, Ozturk O. Instant control of fundal variceal bleeding with a folkloric medicinal plant extract: Ankaferd Blood Stopper. Gastrointest Endosc. 2010;71:873–875. doi: 10.1016/j.gie.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Sarin SK, Kumar A, Angus PW, et al. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011;5:607–626. doi: 10.1007/s12072-010-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]