Abstract
Background:
Previous Costa Rica Vaccine Trial (CVT) reports separately demonstrated vaccine efficacy against HPV16 and HPV18 (HPV16/18) infections at the cervical, anal, and oral regions; however, the combined overall multisite efficacy (protection at all three sites) and vaccine efficacy among women infected with HPV16 or HPV18 prior to vaccination are less known.
Methods:
Women age 18 to 25 years from the CVT were randomly assigned to the HPV16/18 vaccine (Cervarix) or a hepatitis A vaccine. Cervical, oral, and anal specimens were collected at the four-year follow-up visit from 4186 women. Multisite and single-site vaccine efficacies (VEs) and 95% confidence intervals (CIs) were computed for one-time detection of point prevalent HPV16/18 in the cervical, anal, and oral regions four years after vaccination. All statistical tests were two-sided.
Results:
The multisite woman-level vaccine efficacy was highest among “naïve” women (HPV16/18 seronegative and cervical HPV high-risk DNA negative at vaccination) (vaccine efficacy = 83.5%, 95% CI = 72.1% to 90.8%). Multisite woman-level vaccine efficacy was also demonstrated among women with evidence of a pre-enrollment HPV16 or HPV18 infection (seropositive for HPV16 and/or HPV18 but cervical HPV16/18 DNA negative at vaccination) (vaccine efficacy = 57.8%, 95% CI = 34.4% to 73.4%), but not in those with cervical HPV16 and/or HPV18 DNA at vaccination (anal/oral HPV16/18 VE = 25.3%, 95% CI = -40.4% to 61.1%). Concordant HPV16/18 infections at two or three sites were also less common in HPV16/18-infected women in the HPV vaccine vs control arm (7.4% vs 30.4%, P < .001).
Conclusions:
This study found high multisite vaccine efficacy among “naïve” women and also suggests the vaccine may provide protection against HPV16/18 infections at one or more anatomic sites among some women infected with these types prior to HPV16/18 vaccination.
Consistent with other randomized trials (1,2), the National Cancer Institute (NCI)–sponsored, community-based human papillomavirus (HPV) 16/18 Costa Rica Vaccine Trial (CVT) demonstrated strong prophylactic vaccine efficacy against persistent cervical HPV16 and HPV18 (HPV16/18) infection (3) and cervical intraepithelial neoplasia 2 or more severe disease (CIN2+) associated with those types (4). While HPV vaccination has the potential to substantially reduce the cervical cancer burden, several countries have documented recent increases in HPV-associated anal and oropharyngeal cancer (5,6). In fact, these HPV-associated noncervical cancers now account for over half of the HPV-associated cancer burden in the United States (5,7).
Less is known about the vaccine efficacy (VE) at noncervical sites in women, as the vaccines were licensed on the basis of cervical clinical outcomes (CIN2+). The quadrivalent HPV vaccine subsequently received an indication for prevention of anal cancer after efficacy was demonstrated against anal intraepithelial neoplasia in men (8). However, the vaccines do not have an indication for prevention of oropharyngeal cancer in men or women, partially because of the lack of a detectable precancerous lesion (9). However, more recent reports have focused on virologic endpoints (10), given that HPV infection is the etiologic agent in these cancers. Indeed, recent reports from the CVT have suggested that the VE against a single measurement of anal and oral HPV16/18 is high and similar to that against cervical HPV16/18 (11,12).
While the HPV16/18 vaccine appears efficacious at multiple sites in women “naïve” to HPV16/18 infection, the single-site VEs and combined multisite VE (protection at all three: cervical, anal, and oral) sites are less understood among women with prior infection. Previous reports suggest the HPV16/18 vaccine has no therapeutic effect on current cervical HPV16/18 infection (13), but it is possible that the vaccine may prevent HPV16/18 infection in at-risk (noninfected) anatomical sites and/or reduce re-infection in women previously or currently exposed to HPV16/18 at the time of vaccination. Thus, we examined the combined multisite and single-site VEs in sexually active women, including those who were never, formerly, or currently exposed to HPV16 or HPV18 infection at the time of vaccination.
Methods
Study Design and Laboratory Procedures
In the CVT, women age 18 to 25 years were randomly assigned at enrollment from 2004 to 2005 to be vaccinated with the bivalent HPV16/18 vaccine (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) or a Hepatitis A vaccine (modified Havrix, GSK Biologicals) (3,14). This trial included three-dose vaccination (provided over six months) and active annual follow-up. At the enrollment and follow-up visits, risk-factor questionnaires were administered and a pelvic examination was performed on sexually active women. Exfoliated cervical cells were collected in PreservCyt medium (Cytyc Corp, now Hologic, Marlborough, MA) for liquid-based cytology and HPV DNA testing. In addition, blood was collected at enrollment to evaluate HPV16/18 serologic status. Further design and methods for the CVT have been previously described (3,14). The institutional review boards of the NCI and the Costa Rican Instituto Costarricense de Investigación y Ensenanza en Nutrición y Salud (INCIENSA) approved this trial, and all study participants signed institutional review board–approved consent forms giving written informed consent. The trial is registered at clinicaltrials.gov, identifier NCT00128661.
Anal and oral specimens were also collected at the four-year visit, the final blinded visit of the CVT. The anal specimen was collected prior to the pelvic exam among sexually active women (defined by a history of vaginal intercourse) by inserting a dry swab 3 to 4 centimeters into the anal canal, rotating it one time, and then removing the swab while rotation continued using pressure against the wall of the anal canal. The swab was placed in 1mL of PreservCyt and frozen immediately in liquid nitrogen. The oral rinse specimen was collected by use of a 30-second oral rinse and gargle (15-second rinse/15-second gargle) with 15mL of Scope mouthwash (Procter and Gamble Company, Cincinnati, OH) and was kept between 2° and 8° Celsius until same-day processing at the local laboratory (15,16).
DNA was extracted from the cervical, oral, and anal samples through the MagNAPure LC DNA isolation procedure (Roche Diagnostics, Indianapolis, IN), and the extracted DNA was used for each polymerase chain reaction. Specimens were then tested for HPV DNA types utilizing the SPF10 PCR-DEIA (DNA enzyme immunoassay)-LiPA25 version 1 method (Labo Biomedical Products, Rijswijk, the Netherlands) (17,18). The LiPA25 system detects 25 HPV genotypes, including 12 high-risk (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) types. To increase the sensitivity of HPV16/18 detection, all positive specimens on SPF10 PCR/DEIA that were negative for HPV16 or HPV18 by LiPA25 were also tested using HPV16 and 18 type-specific primers (19).
Baseline HPV16 and HPV18 serological status was determined using a virus-like particle-based direct enzyme-linked immunosorbent assay (VLP-ELISA), a standard assay that measures polyclonal antibodies (GlaxoSmithKline Biologicals) (20,21). Serologic results were dichotomized utilizing standard cutoffs calculated as antibody titer values three standard deviations above the geometric mean titers obtained from HPV-negative self-reported virgins (20). Individuals with an optical density of at least 8 ELISA units/mL for anti-HPV16 and at least 7 ELISA units/mL for anti-HPV18 were considered seropositive (20–22).
Statistical Analyses
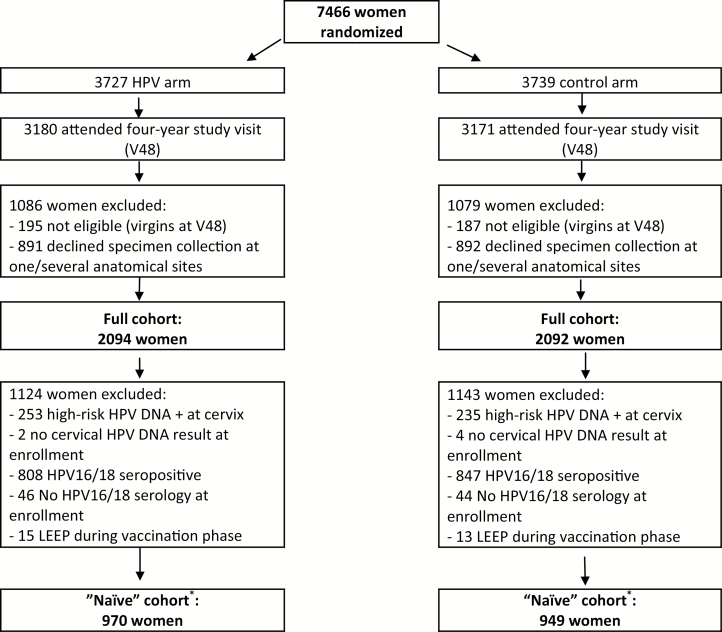
The full study population included all women who consented to cervical, anal, and oral samples at the four-year CVT visit and had HPV DNA test results available (Figure 1). Previous CVT reports have compared the characteristics of women who accepted or declined the oral rinse and/or anal swab samples and have demonstrated that the acceptance was similar across arms but higher among women reporting more lifetime sex partners and those reporting anal and oral sex (11,12). There were no oral/anal specimens collected prior to the four-year follow-up visit, which would have allowed for a more restricted naive population with exclusion of women with prevalent oral or anal HPV16 or HPV18 DNA at enrollment. Given the age ranges of this study population (age 18–25 years at time of vaccination), we divided women into three distinct categories based on their HPV16/18 status at the time of vaccination. These categories included: 1) the “naïve” cohort that had no evidence of prior infection (negative for cervical high-risk HPV DNA and HPV16/18 antibodies at enrollment and did not receive cervical excision treatment [loop electrosurgical excision procedure {LEEP}] during the vaccination phase), 2) evidence of previous exposure (serologically HPV16 or HPV18 positive but cervical HPV16/18 DNA-negative), and 3) currently exposed (cervical HPV16 or HPV18 DNA positive regardless of serologic status).
Figure 1.
Consort diagram for this Costa Rica Vaccine trial–based study. Asterisks indicate no baseline anal or oral sampling, so a true “According-to-Protocol” analysis could not be included. HPV = human papillomavirus; LEEP = loop electrosurgical excision procedure.
VEs and 95% confidence intervals (CIs) were computed for one-time detection of HPV in the cervical, anal, and oral regions in full and subpopulations. VE represents the percent change (reduction or increase) in outcome observed when the HPV arm is compared against the control arm. We calculated the combined multisite woman-level VE, where an event (among n = 4186 women) was defined as a woman with HPV16 and/or HPV18 DNA at any of the cervical, anal, or oral regions at the four-year visit. We also calculated combined multisite infection level analyses, which can be considered as weighted averages of the cervical, anal, and oral HPV16/18 VE results. An event in the multisite infection-level VE analysis (n = 12 558 sites) was defined as a woman’s anatomical site having HPV16 and/or HPV18 DNA (ie, each woman contributed three times). The VEs for the infection-level analysis were calculated utilizing the generalized estimating equation (GEE) method to adjust for the correlation within an individual (23). Exact confidence intervals for single-site VEs were calculated based on the binomial distribution of the number of events, while the multisite VEs that included GEE methods utilized mid-p corrected exact confidence limits because of their incompatibility with binomial conditioning. A concordant HPV infection was defined as a type-specific infection being present at two or three anatomic sites examined in this study. HPV concordance between different anatomic sites in the vaccine and control arms was compared while examining whether the vaccine may protect at least one or two of the three anatomic sites among women who may have been previously exposed to HPV16/18 infection. Type-specific concordance was also evaluated in pair-wise comparisons of sites within women using Kappa statistics with 95% confidence intervals. Chi-square tests were utilized to calculate P values. A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided.
Results
Among the 7466 women randomly assigned to the two arms in the CVT, 6351 (85.1%) attended the four-year study visit (Figure 1). Among these women, 2165 were excluded from the full cohort because they were virgins at the four-year follow-up visit (n = 382) or declined an anal, oral, or cervical specimen collection at this visit (n = 1783). The full analytical cohort therefore included 4186 women (2094 in the HPV vaccine arm and 2092 in the control arm). There were 1919 women in the restricted (naïve) cohort (970 in the HPV arm, 949 in the control arm), who were HPV16/18 seronegative at enrollment, high-risk HPV DNA-negative at enrollment, and did not receive LEEP therapy during the vaccination phase. Overall, 52.9% of the study population was both seronegative and cervical DNA negative for HPV16/18 infection at the time of vaccination, while 35.9% of participants were HPV16 or HPV18 seropositive but cervical HPV16/18 DNA negative, and 11.2% had a current cervical HPV16 or HPV18 infection (DNA) at vaccination.
The distributions of prerandomization characteristics were similar in the HPV and control arms, including age, cervical cytology, and cervical low-risk and high-risk HPV DNA positivity at enrollment (P > .05) (Supplementary Table 1, available online). In addition, postrandomization characteristics among the women were similar between the HPV and control arms, including self-reported oral, vaginal, and anal sex at the four-year follow-up visit. The rate of acceptance to provide an anal and an oral sample was also similar across arms (Figure 1).
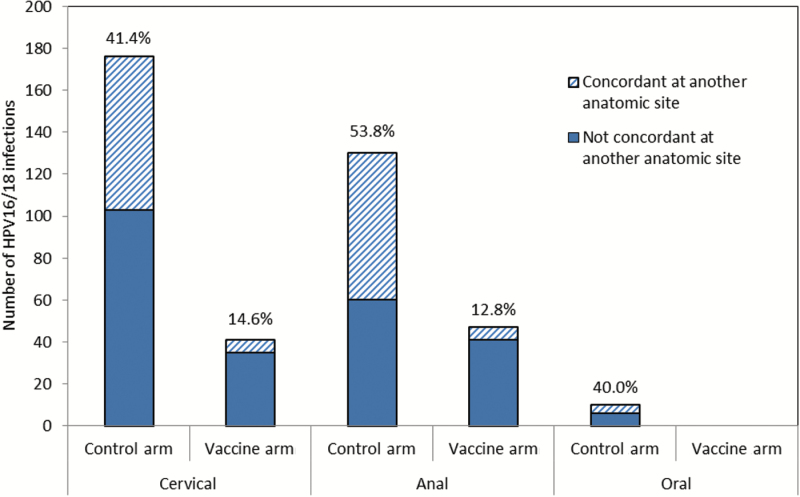
In the full cohort, the combined multisite woman-level HPV16/18 vaccine efficacy (VE) at the four-year follow-up visit was 64.8% (95% CI = 54.8% to 72.8%) (Table 1). Among the 2094 women in the vaccine arm, 81 had an HPV16 or HPV18 infection at one or more anatomic sites (3.9%), while 230 of the 2092 women in the control arm (11.0%) had an HPV16 or HPV18 infection at one or more anatomic sites (P < .001). While multisite woman-level HPV16/18 VE (for infection at one or more site) was evident in this population, it was higher for the combined multisite infection-level HPV16/18 VE of 71.2% (95% CI = 62.8% to 77.7%), although the difference was not statistically significant (Table 1). The difference between these two multisite measures was accounted for by a difference in HPV concordance (ie, type-specific HPV16/18 at two or three anatomic sites) in the two arms. Seventy of the 230 (30.4%) HPV16/18-infected women in the control arm had the same type-specific HPV16/18 infection at two or three anatomic sites, compared with only six of the 81 (7.4%) HPV16/18-infected women in the HPV vaccine arm (P < .001). Indeed, the VE against type-specific concordant HPV16/18 infection at two or three anatomic sites was statistically significantly higher (VE = 91.4%, 95% CI = 81.4% to 96.6%) (Table 1) than the multisite woman-level estimates. HPV concordance was most often found between the cervix and anus (75 of 79 concordant infections), and the percent agreement between cervical and anal HPV16/18 was considerably lower in the HPV vaccine arm than the control arm (kappa = 0.13 vs 0.44, P < .001) (Figure 2).
Table 1.
HPV16/18 Vaccine efficacy against prevalent cervical, anal, and oral HPV16/18 DNA four years after vaccination in the full cohort
| Anatomic site | Arm | Women or sites | Events | HPV16/18 prevalence, % | Efficacy (95% CI), % |
|---|---|---|---|---|---|
| Cervical, anal, or oral HPV16/18 multisite woman-level* |
HPV vaccine | 2094 | 81 | 3.9 | 64.8 (54.8 to 72.8) |
| Control | 2092 | 230 | 11.0 | ||
| Cervical HPV16/18 | HPV vaccine | 2094 | 40 | 1.9 | 76.4 (66.9 to 83.4) |
| Control | 2092 | 169 | 8.1 | ||
| Anal HPV16/18 | HPV vaccine | 2094 | 47 | 2.2 | 62.1 (47.3 to 73.1) |
| Control | 2092 | 124 | 5.9 | ||
| Oral HPV16/18 | HPV vaccine | 2094 | 0 | 0.0 | 100.0 (60.5 to 100.0) |
| Control | 2092 | 9 | 0.4 | ||
| Cervical, anal, oral HPV16/18 multisite infection level† |
HPV vaccine | 6282 | 87 | 1.4 | 71.2 (62.8 to 77.7) |
| Control | 6276 | 302 | 4.8 | ||
| HPV16/18 at two or three sites | HPV vaccine | 2094 | 6 | 0.3 | 91.4 (81.4 to 96.6) |
| Control | 2092 | 70 | 3.3 |
* A woman-level event is defined as a woman who is positive for cervical, anal, and/or oral human papillomavirus DNA at the four-year visit. CI = confidence interval; HPV = human papillomavirus; VE = vaccine efficacy. † An infection-level event is defined as a woman’s anatomical site having HPV16/18 DNA (women contribute three times for each anatomic site); the VEs for this analysis utilized generalized estimating equations to adjust for the correlation within an individual.
Figure 2.
Type-specific concordance of human papillomavirus (HPV) 16/18 infections at the four-year follow-up visit among 2092 women in the control arm and 2094 women in the HPV vaccine arm. The kappa for anal/cervical HPV16/18 agreement was 0.44 (95% confidence interval [CI] = 0.37 to 0.51) for the control arm and 0.13 (95% CI = 0.03 to 0.23) for the vaccine arm. The percentages on the figure indicate the amount of HPV16/18 infections that were concordant at another anatomic site. Seventy-five of the 79 concordant infections were anal/cervical infections. One individual was infected at all three sites. HPV = human papillomavirus.
Compared with the HPV16/18 VE demonstrated in the full cohort, the combined multisite woman-level HPV16/18 VE was higher (VE = 83.5%, 95% CI = 72.1% to 90.8%) (Table 2) among the 1919 women in the “naïve” cohort representing women less likely to be exposed to HPV16/18 prior to vaccination. Similar to the full cohort, there was a higher multisite infection-level VE (VE = 100.0%, 95% CI = 91.0% to 100.0%) (Table 2) than multisite woman-level VE because of a high VE against HPV16/18 concordance.
Table 2.
HPV16/18 vaccine efficacy against prevalent cervical, anal, and oral HPV16/18 DNA four years after vaccination in the “Naïve” cohort*,†
| Study population | Arm | Women or sites | No. events | HPV16/18 prevalence, % | Efficacy (95% CI), % |
|---|---|---|---|---|---|
| Cervical, anal, or oral HPV16/18 | HPV vaccine | 970 | 15 | 1.5 | 83.5 (72.1 to 90.8) |
| Multisite woman level‡ | Control | 949 | 89 | 9.4 | |
| Cervical HPV16/18 | HPV vaccine | 970 | 8 | 0.8 | 89.4 (79.0 to 95.2) |
| Control | 949 | 74 | 7.8 | ||
| Anal HPV16/18 | HPV vaccine | 970 | 7 | 0.7 | 85.1 (68.4 to 93.8) |
| Control | 949 | 46 | 4.8 | ||
| Cervical, anal, oral HPV16/18 | HPV vaccine | 2910 | 15 | 0.5 | 88.2 (79.6 to 93.1) |
| Multisite infection level§ | Control | 2847 | 124 | 4.4 | |
| HPV16/18 at two or three sites | HPV vaccine | 970 | 0 | 0.0 | 100.0 (91.0 to 100.0) |
| Control | 949 | 34 | 3.6 |
* The naïve cohort excluded those who were human papillomavirus (HPV) 16/18 seropositive at baseline or high-risk cervical HPV DNA+ at baseline or had loop electrosurgical excision procedure during the vaccination phase. CI = confidence interval; HPV = human papillomavirus. † The vaccine efficacy for oral HPV16/18 alone was excluded given low number of events. These results are presented in Supplementary Table 2 (available online).
‡ A woman-level event is defined as a woman who is positive for cervical, anal, and/or oral HPV DNA at the four-year visit.§ An infection-level event is defined as a woman’s anatomical site having HPV16/18 DNA (women contribute three times for each anatomic site); the vaccine efficacies for this analysis utilized generalized estimating equations to adjust for the correlation within an individual.
While HPV16/18 VE was highest in the naïve cohort, there was evidence of VE against cervical (VE = 76.5%, 95% CI = 54.6% to 88.8%) and anal HPV16/18 (VE = 54.4%, 95% CI = 22.4% to 73.9%) in the 1384 women with evidence of HPV16/18 infection prior to vaccination (HPV16 and/or HPV18 seropositive, but cervical HPV16/18 DNA negative at enrollment) (Table 3). Additionally, there was evidence of vaccine efficacy against the composite outcome of infection at the cervical, anal, or oral sites (multisite woman-level HPV16/18 VE = 57.8%, 95% CI = 34.4% to 73.4%) and even higher efficacy against HPV16/18 at two or three sites (VE = 90.6%, 95% CI = 65.8% to 98.5%) (Table 3). The multisite VE was not limited to the HPV type women were never exposed to, as there was evidence of multisite VE against HPV16 specifically among women who were HPV16 seropositive and cervical HPV16 DNA negative at baseline (VE = 47.7%, 95% CI = 3.2% to 72.7%) (Table 4; Supplementary Table 3, available online) and VE against HPV18 among those who were HPV18 seropositive and cervical HPV18 DNA negative at baseline (VE = 47.8%, 95% CI = -22.3% to 79.0%) (Table 4; Supplementary Table 3, available online). However, this VE was restricted to women with lower enrollment antibody titers, as there was no statistically significant multisite or cervical VE in those with a titer higher than the median titer (32 EU/mL for HPV16, 17 EU/mL for HPV18) (Table 4).
Table 3.
Multisite HPV16/18 vaccine efficacy among individuals with evidence of a previous* HPV16 or HPV18 infection prior to vaccination†
| Study population | Arm | Women or sites | No. events | HPV16/18 prevalence, % | Efficacy (95% CI), % |
|---|---|---|---|---|---|
| Cervical, anal, or oral HPV16/18 | HPV vaccine | 681 | 27 | 4.0 | 57.8 (34.4 to 73.4) |
| Multisite woman level‡ | Control | 703 | 66 | 9.4 | |
| Cervical HPV16/18 | HPV vaccine | 681 | 10 | 1.5 | 76.5 (54.6 to 88.8) |
| Control | 703 | 44 | 6.3 | ||
| Anal HPV16/18 | HPV vaccine | 681 | 19 | 2.8 | 54.4 (22.4 to 73.9) |
| Control | 703 | 43 | 6.1 | ||
| Cervical, anal, oral HPV16/18 | HPV vaccine | 2043 | 29 | 1.4 | 66.4 (47.0 to 78.6) |
| Multisite infection level§ | Control | 2109 | 89 | 4.2 | |
| HPV16/18 at two or three sites | HPV vaccine | 681 | 2|| | 0.3 | 90.6 (65.8 to 98.5) |
| Control | 703 | 22|| | 3.1 |
* Defined as women who were human papillomavirus (HPV) 16 and/or HPV18 seropositive at baseline and cervical HPV16/18 DNA negative at baseline. CI = confidence interval; HPV = human papillomavirus.
† The vaccine efficacy for oral HPV16/18 alone was excluded given low number of events. These results are presented in Supplemental Table 2 (available online).
‡ A woman-level event is defined as a woman who is positive for cervical, anal, and/or oral HPV DNA at the four-year visit.§An infection-level event is defined as a woman’s anatomical site having HPV16/18 DNA (women contribute three times for each anatomic site); the vaccine efficacies for this analysis utilized generalized estimating equations to adjust for the correlation within an individual.|| Statistically significantly lower HPV16/18 concordance at two or more anatomic sites in HPV16/18-infected women in the HPV vaccine arm (7.4% [2/27] of HPV16/18-infected women) than the control arm (33.3% [22/66] of HPV16/18-infected women), P = .01.
Table 4.
HPV16 and HPV18 vaccine efficacies by antibody titer among individuals with evidence of a previous* HPV16 or HPV18 infection prior to vaccination
| Population | Arm | No. women | No. events* | HPV16/18 prev., % | Efficacy (95% CI), % |
|---|---|---|---|---|---|
| HPV16 seropositive and cervical HPV16 DNA negative at baseline | |||||
| Cervical, anal, or oral HPV16 | HPV vaccine | 477 | 15 | 3.1 | 47.7 (3.2 to 72.7) |
| Multisite woman level† | Control | 482 | 29 | 6.0 | |
| Cervical HPV16 | HPV vaccine | 477 | 5 | 1.0 | 71.9 (27.4 to 90.7) |
| Control | 482 | 18 | 3.7 | ||
| HPV16 titer below the median titer (<32 EU/mL) | |||||
| Cervical, anal, or oral HPV16 | HPV vaccine | 247 | 4 | 1.6 | 73.4 (25.3 to 92.3) |
| Multisite woman level‡ | Control | 279 | 17 | 6.1 | |
| Cervical HPV16 | HPV vaccine | 247 | 3 | 1.2 | 75.8 (21.9 to 94.4) |
| Control | 279 | 14 | 5.0 | ||
| HPV16 titer at or above the median titer (≥32 EU/mL) | |||||
| Cervical, anal, or oral HPV16 | HPV vaccine | 230 | 11 | 4.8 | 19.1 (-86.5 to 65.2) |
| Multisite woman level‡ | Control | 203 | 12 | 5.9 | |
| Cervical HPV16 | HPV vaccine | 230 | 2 | 0.9 | 55.9 (-148.8 to 94.3) |
| Control | 203 | 4 | 2.0§ | ||
| HPV18 seropositive and cervical HPV18 DNA negative at baseline | |||||
| Cervical, anal, or oral HPV18 | HPV vaccine | 509 | 8 | 1.6 | 47.8 (-22.3 to 79.0) |
| Multisite woman level‡ | Control | 498 | 15 | 3.0 | |
| Cervical HPV18 | HPV vaccine | 509 | 2 | 0.4 | 78.3 (8.7 to 96.8) |
| Control | 498 | 9 | 1.8 | ||
| HPV18 titer below the median titer (<17 EU/mL) | |||||
| Cervical, anal, or oral HPV18 | HPV vaccine | 249 | 2 | 0.8 | 81.0 (23.7 to 97.1) |
| Multisite woman level‡ | Control | 260 | 11 | 4.2 | |
| Cervical HPV18 | HPV vaccine | 249 | 0 | 0.0 | 100.0 (52.6 to 100.0) |
| Control | 260 | 8 | 3.1 | ||
| HPV18 titer at or above the median titer (≥17 EU/mL) | |||||
| Cervical, anal, or oral HPV18 | HPV vaccine | 260 | 6 | 2.3 | -37.3 (-451.8 to 62.4) |
| Multisite woman level‡ | Control | 238 | 4 | 1.7 | |
| Cervical HPV18 | HPV vaccine | 260 | 2 | 0.8 | -83.1 (-5300.3 to 86.1) |
| Control | 238 | 1 | 0.4|| | ||
* Defined as women who were human papillomavirus (HPV) 16 seropositive and cervical HPV16 DNA negative at baseline for the HPV16 analysis, and HPV18 seropositive and cervical HPV18 DNA negative at baseline for the HPV18 analysis. CI = confidence interval; HPV = human papillomavirus.
† For this analysis, events were defined as detection of type-specific HPV16 or HPV18 DNA at the four-year visit among women who were HPV16 or HPV18 seropositive for that same HPV type at baseline (ie, the analysis was restricted to examining the potential protection against the HPV type that the women previously had prior to vaccination).
‡ A woman-level event is defined as a woman who is positive for cervical, anal, and/or oral HPV DNA at the four-year visit. § Cervical HPV16 prevalence at the four-year visit among high-titer HPV16 seropositive/HPV16 DNA-negative individuals was not statistically significantly lower than the cervical HPV16 prevalence in the control arm of the naïve cohort (2.0% vs 5.1%, P = .06).
|| Cervical HPV18 prevalence at the four-year visit among high-titer HPV18 seropositive/HPV18 DNA-negative individuals was statistically significantly lower than the cervical HPV18 prevalence in the control arm of the naïve cohort (0.4% vs 3.2%, P = .02).
In contrast to the protection seen in women exposed to HPV16 or HPV18 prior to vaccination, there was no statistically significant evidence of HPV16/18 VE in the 407 women with a current cervical HPV16/18 infection at the time of vaccination against anal/oral HPV16/18 (VE = 25.3%, 95% CI = -40.4% to 61.1%) (Table 5) or against cervical HPV16/18 re-infection (Table 5; Supplemental Table 4, available online).
Table 5.
HPV16/18 vaccine efficacy among individuals with cervical HPV16 or HPV18 DNA detected at enrollment
| Study population | Arm | Women or sites | No. events | HPV prevalence, % | Efficacy (95% CI), % |
|---|---|---|---|---|---|
| Anal or oral HPV16/18 at the four-year visit | HPV vaccine | 192 | 16 | 8.3 | 25.3 (-40.4 to 61.1) |
| Control | 215 | 24* | 11.2 | ||
| Redetection of cervical HPV16/18 (among women with HPV16/18 at enrollment who subsequently cleared their infection)† | HPV vaccine | 180 | 10 | 5.6 | 38.9 (-31.7 to 72.9) |
| Control | 198 | 18 | 9.1 | ||
| HPV16/18 at two or three sites at the four-year visit | HPV vaccine | 192 | 3 | 1.6 | 44.0 (-124.9 to 88.6) |
| Control | 215 | 6 | 2.8 |
* Only two of the 40 total anal/oral events were oral HPV16/18 infections. These two oral HPV16/18 infections were both detected in the control arm, but oral HPV16/18-specific vaccine efficacy did not approach statistical significance given its small sample size. CI = confidence interval; HPV = human papillomavirus. † Clearance defined as one negative cervical HPV16/18 DNA test after the positive cervical HPV16/18 DNA test.
Discussion
This randomized trial demonstrated multisite vaccine efficacy against cervical, anal, and oral HPV16/18 at the four-year follow-up visit among women with no evidence of HPV16 or HPV18 exposure and among some women who may have cleared/controlled their HPV16/18 infection prior to vaccination. In addition, HPV16/18-infected women in the vaccine arm were less likely to have an HPV16 or an HPV18 infection at two or more anatomic sites than HPV16/18-infected women in the control arm. These findings suggest that while the HPV16/18 vaccine is most efficacious among women prior to sexual debut, the vaccine may provide protection against HPV at some or all at-risk anatomic sites in many sexually active women.
The lower but statistically significant multisite VE in formerly HPV16/18-infected women is important given their abundance in sexually active populations, as they represented over one-third of this study population. The multisite vaccine efficacy among women exposed to HPV16/18 prior to vaccination reinforces findings from other HPV vaccine trials, which suggested that there was some VE against cervical HPV 16/18 among women who were HPV16/ or HPV 18 seropositive and cervical HPV 16/18 DNA negative at the time of vaccination (3,24,25). This study finds that these previously HPV16/18-exposed women may be protected not only at the cervix, but also at the anal and oral regions, particularly among those with lower HPV16/18 antibody titers. The lack of statistically significant cervical or multisite VE among those with higher antibody titers may be in part explained by higher titers partially protecting against cervical HPV16/18 re-infection, which may have reduced the number of cervical HPV16/18 outcomes in the control arm (26,27).
The multisite VE among previously exposed women is important to note given that this study and other randomized trials have been unable to define the location of the original HPV16/18 infection (that induced the HPV16/18 serologic response detected at enrollment). But given the multisite VE suggested in this study, there is now further evidence that HPV vaccination protects some women against acquisition of HPV16/18, including the likely site of the original HPV16/18 infection. However, further examination of the specificity and reliability of the VLP-ELISA is necessary to validate our definition of “previously infected,” as it is possible if false-positive serologic results are common that many in this group may have been truly HPV16/18 naïve (28,29).
In addition to the multisite VE among previously HPV16/18-infected women, this study also suggests some formerly infected women who were HPV16/18 vaccinated may be protected at a subset of their at-risk anatomic sites, as we observed a statistically significantly lower HPV16/18 concordance at two or more anatomic sites in HPV16/18-infected women in the HPV vaccine arm than the control arm. This difference in concordance may be explained by vaccine protection of noninfected sites among women previously exposed to HPV16/18 infection. However, it is less clear whether the vaccine provides women who are cervical HPV16/18 DNA positive at vaccination any protection at other sites given the limited sample size of this group and the lack of oral and anal sampling at enrollment.
The potential vaccine efficacy against noncervical HPV16/18 infection among previously HPV16/18-exposed women is noteworthy given that previous observational studies have suggested the prevalence of anal and oral HPV infections may be similar or even higher at older ages compared with younger individuals in their teens and early 20s (30–32). These findings differ from cervical HPV (33), which is commonly acquired closer to age of sexual debut. While the transmission and natural history of noncervical HPV infection is less understood, the initial studies suggesting a relatively higher incidence of noncervical HPV infection at older ages is noteworthy given our suggestion that HPV vaccination of older individuals may provide some protection against these infections.
While high-income countries recommend routine HPV vaccination in women in their preteen years (age 11–13 years) (34,35), the recommendations for HPV catch-up vaccination and vaccination of older women are more varied. The United States recommends HPV vaccination in women through age 26 years (34), while many other countries do not recommend vaccination for these older age groups (35). While this study and others support lower but statistically significant HPV16/18 vaccine efficacy at older ages, further study (including cost-effectiveness analyses) is necessary to best inform HPV vaccine age range and catch-up policy recommendations. A previous cervical study suggested the risk of cervical precancer is relatively low for women with HPV re-appearance (36), but the clinical relevance of noncervical HPV infections acquired at older ages (acquired sexually or through auto-inoculation) (37–39) needs better understanding.
There were limitations to this study. Specifically, this study’s lack of oral and anal sampling at enrollment precluded a completely naive population, and the outcome measures (one-time detection of cervical, anal, and oral HPV) have lower clinical significance than other commonly used outcomes in HPV vaccine trials (6- to 12-month persistent HPV16/18 infection and high-grade precancers). In addition, some analyses had relatively low power, which limits inference, particularly those related to oral HPV given its low prevalence in this population. However, this study had several strengths, including utilization of the only randomized trial that examined noncervical HPV in women and an age range of interest (age 18–25 years at vaccination) given the varying recommendations for HPV vaccination in this age group (34,35).
This is the first study to present a combined multisite woman-level HPV16/18 vaccine efficacy among women with and without HPV16/18 exposure at the time of vaccination. These results suggest that multisite HPV16/18 VE is strongest among women never exposed to HPV prior to vaccination, but VE is also evident at one or more anatomic sites among women who likely cleared their HPV16/18 infections prior to HPV vaccination.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (US Food and Drug Administration BB-IND 7920) during the four-year, randomized blinded phase of our study. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest.
Supplementary Material
The NCI and Costa Rica investigators, not the funders, are responsible for the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Registered with Clinicaltrials.gov NCT00128661.
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Loreto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Amber D’Souza, Richard Roden, and Peter Gilbert).
Investigators in the Costa Rica HPV Vaccine Trial (CVT) Group:
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica—Bernal Cortés (specimen and repository manager), Paula González (LTFU: co-principal investigator), Rolando Herrero (CVT: co-principal investigator), Silvia E. Jiménez (trial coordinator), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator).
United States National Cancer Institute, Bethesda, MD—Allan Hildesheim (co-principal investigator and NCI co-project officer), Aimée R. Kreimer (LTFU: co-principal investigator and NCI co-project officer), Douglas R. Lowy (HPV virologist), Mark Schiffman (CVT: medical monitor and NCI co-project officer), John T. Schiller (HPV virologist), Mark Sherman (CVT: QC pathologist), Sholom Wacholder (statistician).
Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD (HPV Immunology Laboratory)—Ligia A. Pinto, Troy J. Kemp.
Georgetown University, Washington, DC—Mary K. Sidawy (CVT: histopathologist).
DDL Diagnostic Laboratory, the Netherlands (HPV DNA Testing)—Wim Quint, Leen-Jan van Doorn, Linda Struijk.
University of California, San Francisco, CA—Joel M. Palefsky, Teresa M. Darragh.
University of Virginia, Charlottesville, VA—Mark H. Stoler.
References
- 1. Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila). 2009;2 (10):868–878. [DOI] [PubMed] [Google Scholar]
- 2. Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13 (1):89–99. [DOI] [PubMed] [Google Scholar]
- 3. Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1 (5):408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32 (39):5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29 (32):4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide Trends in Incidence Rates for Oral Cavity and Oropharyngeal Cancers. J Clin Oncol. 2013;31 (36):4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105 (3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365 (17):1576–1585. [DOI] [PubMed] [Google Scholar]
- 9. Fakhry C, Rosenthal B, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “Pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila). 2011;4 (9):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International Agency for Research on Cancer HPV Working Group. Primary End-points for Prophylatic HPV Vaccine Trials IARC Working Group Report. 2014;7. [PubMed] [Google Scholar]
- 11. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8 (7):e68329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12 (9):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298 (7):743–753. [DOI] [PubMed] [Google Scholar]
- 14. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26 (37):4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Closas M, Egan KM, Abruzzo J, et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10 (6):687–696. [PubMed] [Google Scholar]
- 16. Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189 (4):686–698. [DOI] [PubMed] [Google Scholar]
- 17. Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37 (8):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153 (6):1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44 (9):3292–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4 (6):425–434. [DOI] [PubMed] [Google Scholar]
- 21. Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364 (9447):1757–1765. [DOI] [PubMed] [Google Scholar]
- 22. Safaeian M, Ghosh A, Porras C, et al. Direct comparison of HPV16 serological assays used to define HPV-naive women in HPV vaccine trials. Cancer Epidemiol Biomarkers Prev. 2012;21 (9):1547–1554. [DOI] [PubMed] [Google Scholar]
- 23. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44 (4):1049–1060. [PubMed] [Google Scholar]
- 24. Szarewski A, Poppe WA, Skinner SR, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131 (1):106–116. [DOI] [PubMed] [Google Scholar]
- 25. Castellsague X, Munoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105 (1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Safaeian M, Porras C, Schiffman M, et al. Epidemiological Study of Anti-HPV16/18 Seropositivity and Subsequent Risk of HPV16 and -18 Infections. J Natl Cancer Inst. 2010;102 (21):1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356 (19):1915–1927. [DOI] [PubMed] [Google Scholar]
- 28. Wang SS, Schiffman M, Shields TS, et al. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br J Cancer. 2003;89 (7):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferguson M, Heath A, Johnes S, Pagliusi S, Dillner J, Collaborative Study Participants. Results of the first WHO international collaborative study on the standardization of the detection of antibodies to human papillomaviruses. Int J Cancer. 2006;118 (6):1508–1514. [DOI] [PubMed] [Google Scholar]
- 30. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307 (7):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2550–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreimer AR, Campbell CMP, Lin H, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382 (9895):877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22 (4):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 35. Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction--the first five years. Vaccine. 2012;30 Suppl 5:F139-48. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez AC, Schiffman M, Herrero R, et al. Low risk of type-specific carcinogenic HPV re-appearance with subsequent cervical intraepithelial neoplasia grade 2/3. Int J Cancer. 2012;131 (8):1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beachler DC, Sugar EA, Margolick JB, et al. Risk factors for oral HPV infection acquisition and clearance among HIV-infected and HIV-uninfected adults. Am J Epidemiol. 2015;181 (1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castro FA, Quint W, Gonzalez P, et al. Prevalence of and risk factors for anal human papillomavirus infection among young healthy women in Costa Rica. J Infect Dis. 2012;206 (7):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edelstein ZR, Schwartz SM, Hawes S, et al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis. 2012;39 (11):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.