Summary
Objective
To characterize local field potentials, high frequency oscillations, and single unit firing patterns in microelectrode recordings of human limbic onset seizures.
Methods
Wide bandwidth local field potential recordings were acquired from microelectrodes implanted in mesial temporal structures during spontaneous seizures from six patients with mesial temporal lobe epilepsy.
Results
In the seizure onset zone, distinct epileptiform discharges were evident in the local field potential prior to the time of seizure onset in the intracranial EEG. In all three seizures with hypersynchronous (HYP) seizure onset, fast ripples with incrementally increasing power accompanied epileptiform discharges during the transition to the ictal state (p < 0.01). In a single low voltage fast (LVF) onset seizure a triad of evolving HYP LFP discharges, increased single unit activity, and fast ripples of incrementally increasing power were identified ~20 s prior to seizure onset (p < 0.01). In addition, incrementally increasing fast ripples occurred after seizure onset just prior to the transition to LVF activity (p < 0.01). HYP onset was associated with an increase in fast ripple and ripple rate (p < 0.05) and commonly each HYP discharge had a superimposed ripple followed by a fast ripple. Putative excitatory and inhibitory single units could be distinguished during limbic seizure onset, and heterogeneous shifts in firing rate were observed during LVF activity.
Significance
Epileptiform activity is detected by microelectrodes before it is detected by depth macroelectrodes, and the one clinically identified LVF ictal onset was a HYP onset at the local level. Patterns of incrementally increasing fast ripple power are consistent with observations in rats with experimental hippocampal epilepsy, suggesting that limbic seizures arise when small clusters of synchronously bursting neurons increase in size, coalesce, and reach a critical mass for propagation.
Keywords: Hypersynchronous, Low voltage fast, High frequency oscillation, Ripple, Fast ripple, Limbic seizure
Understanding the fundamental neuronal mechanisms that govern the transition from interictal to ictal states in focal seizures is critical to improving pharmacological and surgical treatments for epilepsy, as well as in seizure prediction. Antiseizure medicines may function by preventing this state change, and defining the precise location is essential to determining the site and extent of epilepsy surgery.
Mesial temporal lobe epilepsy (MTLE) is the most common form of medically refractory epilepsy1 and several animal models have been developed to understand its pathogenesis.2 In MTLE, two common depth electrode recorded ictal EEG onset patterns are hypersynchronous (HYP) with ictal discharges <2 Hz and low voltage fast (LVF) consisting of low amplitude oscillations >12 Hz.3–6 Often the HYP onset transitions to LVF activity later in the seizure when there is propagation, but rarely does HYP activity appear at sites of seizure spread.
Electrophysiological features occurring at the local field potential (LFP) and cellular levels have been described for these two ictal onset types in human tissue and animal models of MTLE, and involve changes in synaptic plasticity,7 the occurrence of high-frequency oscillations (HFOs),8 and cell type specific changes in single unit spike firing.9 HFOs are brief (10–200 ms) and consist chiefly of ripples (80–200 Hz) or fast ripples (200–600 Hz). In the kainic acid model of MTLE, fast ripples of incrementally increasing power were observed prior to HYP seizure onset,8 whereas in the bicuculline model of MTLE, LVF onset seizures were associated with a sustained firing of inhibitory interneurons without firing of excitatory neurons in the entorhinal cortex.9
Analysis of ictal microelectrode recordings during the HYP and LVF onset seizures in patients undergoing intracranial EEG (iEEG) monitoring can begin to address the clinical relevance of these findings. In the present study, we quantified microelectrode-recorded LFPs, HFOs, and single unit spike firing in the seizure onset zone before and during depth electrode-recorded spontaneous seizures in six presurgical patients with pharmacoresistant MTLE.
Methods
Subjects
Subjects for this retrospective study were patients with suspected mesial temporal lobe epilepsy who were candidates for resective surgery, but required diagnostic intracranial EEG (iEEG) studies because results from non-invasive tests were not conclusive. Each patient was bilaterally implanted with clinical depth electrodes (Ad-Tech Medical, Inc.); each electrode was specially adapted with microelectrodes that extended beyond the distal tip10,11 and localized to anatomical temporal lobe regions as described previously.12 Under the approval of the Internal Review Board of the UCLA Office for Protection of Research Subjects, patients gave their informed consent to participate in these research studies prior to depth electrode implantation.
Recordings
Clinical depth iEEG (0.1–70 Hz; 200 samp/s; reference scalp Fz) was recorded using a Stellate (XLTEK, San Diego, CA, U.S.A) or a Nihon-Kohden 128-channel NK 1200 long-term monitoring system (Nihon-Kohden America, Foothill Ranch, CA, U.S.A.). Simultaneously, wide bandwidth (0.001–6 kHz) high-frequency EEG (30 ksamp/s; gain × 10,000) was recorded from microelectrodes using a Cheetah recording system (Neuralynx, Bozeman, MT, U.S.A) or a NeuroPort recording system (Blackrock, Salt Lake City, UT, U.S.A).
Data analysis
Clinical depth electrode-recorded ictal iEEG onset patterns were classified as HYP, LVF, rhythmic spiking, or polyspike using clinical depth electrode recordings.3–6 Seizure onset was defined as the time at which the clinical macroelectrode EEG qualitatively evolved in frequency and amplitude that could be clearly distinguished from the background activity. In LVF onset seizures, time of onset was identified by the dominance of low voltage frequencies >12 Hz in the EEG that were clearly different from the background, whereas in the other ictal EEG onset types, time of onset was immediately prior to initial EEG discharge.
HFOs were visually reviewed from bandpass filtered (80–600 Hz; zero-phase, 500th order FIR) microelectrode-recorded EEG. Microelectrodes exhibiting distinct HFOs > 15 μV in amplitude were selected for subsequent analysis. Ripple- and fast ripple-frequency HFOs contained peak spectral power between 80 and 150 Hz, and 200 and 600 Hz respectively.12 Spike separation into putative single units was carried out using wavelet clustering and temporal autocorrelations.13 Clusters were distinct and well separated using this methodology during both extended preictal intervals and seizure onset (Fig. S4). Microelectrode recording that contained high-frequency noise or spikes rates <0.01 Hz were excluded from the analysis. Excitatory and inhibitory neurons were distinguished using previously established criteria.14 Morlet wavelet decomposition on a variable number of scales and frequency ranges were used for the analysis of ictal onset and HFOs in the LFP.15
A custom algorithm for detecting individual HFOs was written in MATLAB that used a Hilbert transform to derive the amplitude envelope corresponding to ripple and fast ripple power in the bandpass filtered EEG, which was normalized using a segment of the LFP that included the preictal baseline. HFOs were detected when the bandpass filtered amplitude exceeded 2 SD for a minimum duration of 8 ms prior to falling below 1.5 SD of the mean.16 HFO power in the ripple and fast ripple band were calculated using a power spectral density function. A power law fit using the equation log(y) = log(A) + n log(x) implemented in Igor Pro (Lake Oswego, OR, U.S.A.) was used to demonstrate the incremental growth of fast ripples at seizure onset, where y is the relative fast ripple power, x is the order of each event in the fast ripple sequence, and A and n are constants determined by the program. The performance of the detector was compared with results from visual review. In a small fraction of microelectrodes, spectral power calculated for consecutive longer duration HFOs that occurred within 150 ms of each other were combined because visual analysis suggested these were not individual events. The HFO detector was applied to discrete interictal and ictal epochs selected on visual review. The interictal epochs were recorded during wakefulness while the subject rested with eyes closed. No seizures were recorded 12 h before or after the interictal sample.
Statistical analysis of single unit activity was performed using a MATLAB toolset provided by Dr. Fabrizio Gabbiani. Comparisons of single unit interspike interval values and rates of HFO occurrence between preictal and seizure onset epochs were performed with the unpaired t-test and α = 0.01.
Results
Description of recordings
We analyzed recordings from 410 clinical depth electrode contacts and 152 microelectrodes during seven seizures in six patients (Table 1). The distal depth electrode contacts and adjacent microelectrodes were positioned in the amygdala, hippocampus, entorhinal cortex, fusiform gyrus, and cingulate cortex, which corresponded to the site of the seizure onset zone (SOZ) or secondary spread determined by the clinical EEG. Microelectrodes had varying yields of units and HFOs (Table S1).
Table 1.
Patient and seizure information for the study population
| Patient# | Age (sex) | sMRI | Onset Type | SOZ | Phase III | Outcome | Pathology |
|---|---|---|---|---|---|---|---|
| 1 | 41M | TBI (bilateral hippocampal atrophy) | HYP | Bi- AH | None | Not applicable (independent bilateral onsets) | Not applicable |
| 2 | 34F | Nonlesional | HYP | RAH | R. ATL | Class I @ >60 months observation | Normal |
| 3 | 51F | Nonlesional | HYP | REC | R. ATL | Class I @ 15 months observation | Chaslin's gliosis |
| 4 | 49M | Left MTS | Rhythmic spiking | LEC | L. ATL | Class I @ 60 months observation | Normal |
| 5 | 41F | Nonlesional | LVF | Bi-EC, AH | None | Not Applicable (independent bilateral onsets) observation | Not applicable |
| 6 (two seizures) | 57M | Left PCA stroke involving hippocampus | Polyspike and wave | LEC | L. ATL | Class I @ 8 months observation | Chaslin's gliosis |
sMRI, structural MRI; Phase III, resective surgery; TBI, traumatic brain injury; MTS, mesial temporal lobe sclerosis; AH, anterior hippocampus; EC, entorhinal cortex; ATL, anterior temporal lobectomy; PCA, posterior cerebral artery.
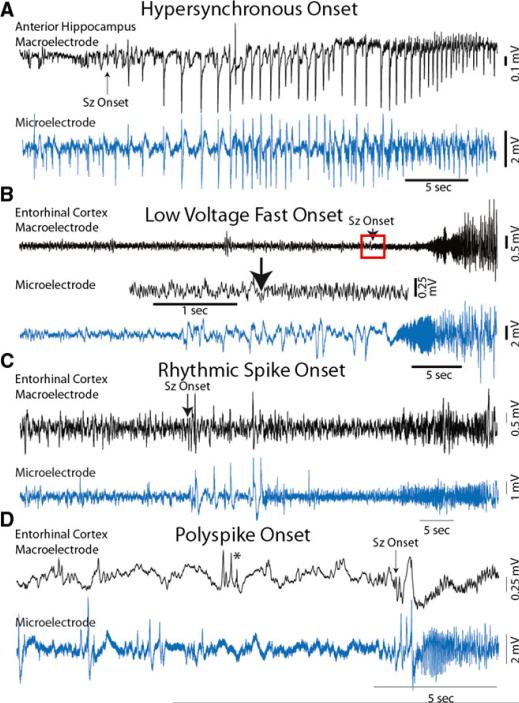
Ictal iEEG onset patterns were classified on the basis of manual review of clinical depth electrode recordings as HYP (n = 3) (Fig. 1A), LVF (n = 1) (Fig. 1B), and two others: a rhythmic spiking onset (n = 1) that consisted of discharges with a frequency (<1 Hz) that was distinct from HYP with respect to waveform morphology (Fig. 1C), and a polyspike onset (n = 2 in the same patient) that began with a brief burst of spikes immediately followed by larger amplitude slow wave (Fig. 1D). The polyspike and rhythmic spike were also seen in the absence of an accompanying seizure during the interictal epoch. With the exception of the rhythmic spiking onset pattern, ictal EEG onset patterns were preceded by epileptiform discharges that were clearly evident in the microelectrode recordings that had very little or no electrographic correlate in the clinical EEG (Fig. 1A, B,D). All ictal iEEG onset patterns included or were followed by an epoch of LVF activity, and all seizures occurred during wakefulness with the exception of the two poly-spike and wave seizures.
Figure 1.
Different ictal EEG onsets recorded by clinical electrodes (black) and microelectrodes (blue) positioned in mesial temporal limbic structures. (A) The hypersynchronous (HYP) onset pattern. (B) The low voltage fast (LVF) onset pattern with discharges evident preceding seizure onset exclusively in the microelectrode. Red box indicates expanded time epoch of macroelectrode recording shown directly below. (C) The rhythmic spiking pattern. (D) A polyspike and wave onset pattern. Asterisk indicates burst of spikes evident on the macroelectrode but not microelectrode.
For two of three HYP onset seizures the clinically defined SOZ was in the hippocampus, for the LVF onset seizure the SOZ was hippocampus and entorhinal cortex, and for the remaining seizures the SOZ was entorhinal cortex. Four patients had resective surgery that resulted in seizure freedom, while two patients with bilateral independent seizure onsets (one non-lesional, the other bilateral hippocampal atrophy) did not have resective surgery (Table 1).
Fast ripples of incrementally increasing power before HYP seizure onset
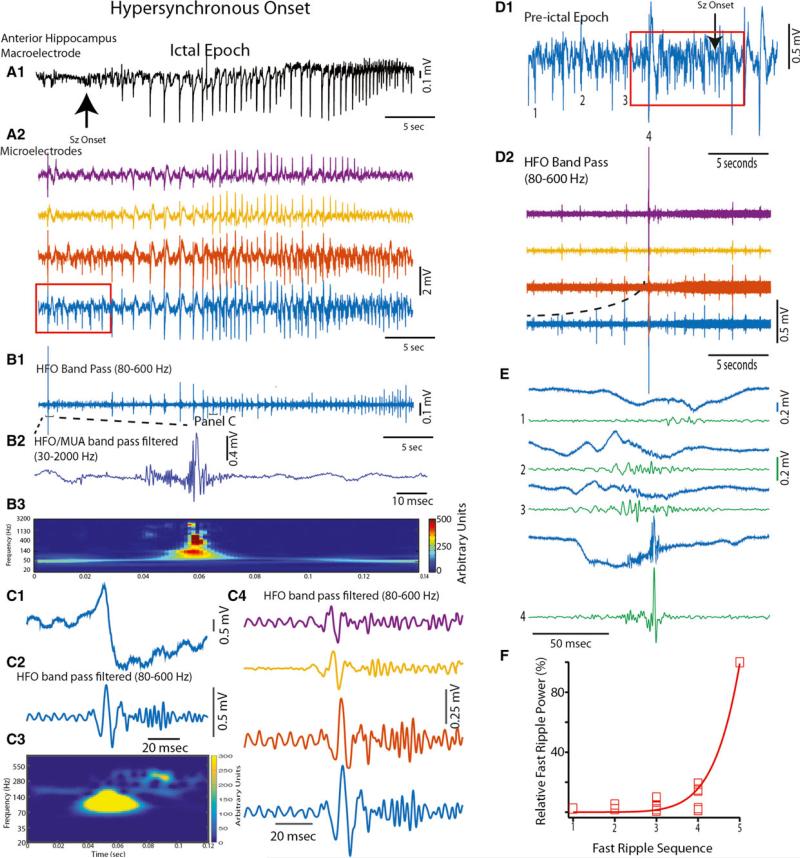
As indicated in the preceding section, microelectrode recorded discharges preceded the appearance of the depth electrode-recorded HYP onset seizure (Fig. 2A). These LFP discharges were morphologically distinct from the HYP discharges that occurred at the time of seizure onset, but after several seconds the discharges were similar to, and nearly synchronous with, the HYP discharges recorded on the adjacent depth electrode (Fig. 2A). In the example outlined by the red box in Fig. 2A2, and also Fig. 2D,E where the recording time base was extended, these LFPs were accompanied by fast ripples of incrementally increasing amplitude recorded by multiple microelectrodes located in the SOZ (see also Fig. S1A). Although each sequential discharge usually contained a corresponding fast ripple of greater amplitude than its predecessor, this was not always the case and some LFPs were not associated with fast ripples (Fig. 2D2. Fig. S1A2). However, even when the fast ripples occurred irregularly they were associated with an incremental increase in power (Fig. 2F, Fig. S1A4, d.f. = 13,9,26, χ2 = 0.052, 0.014, 1.118, p < 0.01). In two of the HYP onset seizures, the largest fast ripple in the sequence preceded seizure onset by 1.98 ± 0.01 (Fig. 2D) and 9.71 ± 2.47 s respectively. Between the largest fast ripples and the depth EEG onset were other discharges of variable morphology, as well as smaller amplitude fast ripples in some of the microelectrodes (Fig. 2D2). In the other HYP seizure, the largest amplitude fast ripple occurred 0.5 ± 0.10 s after onset (Fig. S1A), but the first fast ripple in the sequence preceded seizure onset. The duration of the incremental fast ripple increase for the three HYP seizures was 5.76 ± 1.02, 13.95 ± 0.9, and 3.31 ± 1.24 s. The largest amplitude fast ripple that immediately preceded a HYP onset seizure was >1 mV (Fig. 2B2), was associated with gamma oscillations before and after the fast ripple oscillation, and contained spectral power up to 800 Hz (Fig. 2B3).
Figure 2.
Hypersynchronous (HYP) seizure onset in this human hippocampus was preceded by fast ripples of incrementally increasing power that persisted during seizure onset. (A1) HYP onset seizure recorded simultaneously from the most distal macroelectrode (black, arrow indicates seizure onset), and by four microelectrodes as shown in A2. The red box, referenced in panel D1 in which the preictal epoch is extended, corresponds to the preictal epoch during which discharges are clearly evident in the microelectrode but not the clinical recording. (B1) HFO band pass filtering (80–600 Hz) revealed that an HFO accompanies each HYP ictal discharge. (B2) HFO accompanying the largest preictal EEG spike consisted of gamma oscillations prior to and following a fast ripple as shown in B3. (C) A typical HYP ictal discharge, referenced from panel B1, with an accompanying ripple and fast ripple shown in the band-pass filtered LFP and spectrogram in C2 and C3, respectively. C4 demonstrates that the HFOs were evident in several microelectrodes. (D1) During the preictal epoch extended from panel A2 as indicated by the red box, HFO band pass filtering (D2) revealed a progressive increase in HFO amplitude (shown here as recorded in four microwires) prior to HYP onset illustrated by the dotted curve in D2. (E) Expanded unfiltered LFP (blue) and HFO bandpass filtered LFP traces (green) at the four time points (1,2,3,4) denoted in D1. (F) The increase in the scaled power of sequential fast ripples recorded from five microelectrodes prior to HYP onset exhibited exponential growth (d.f. = 26, χ2 = 0.0522, p < 0.01).
Fast ripples of incrementally increasing power before LVF seizure onset
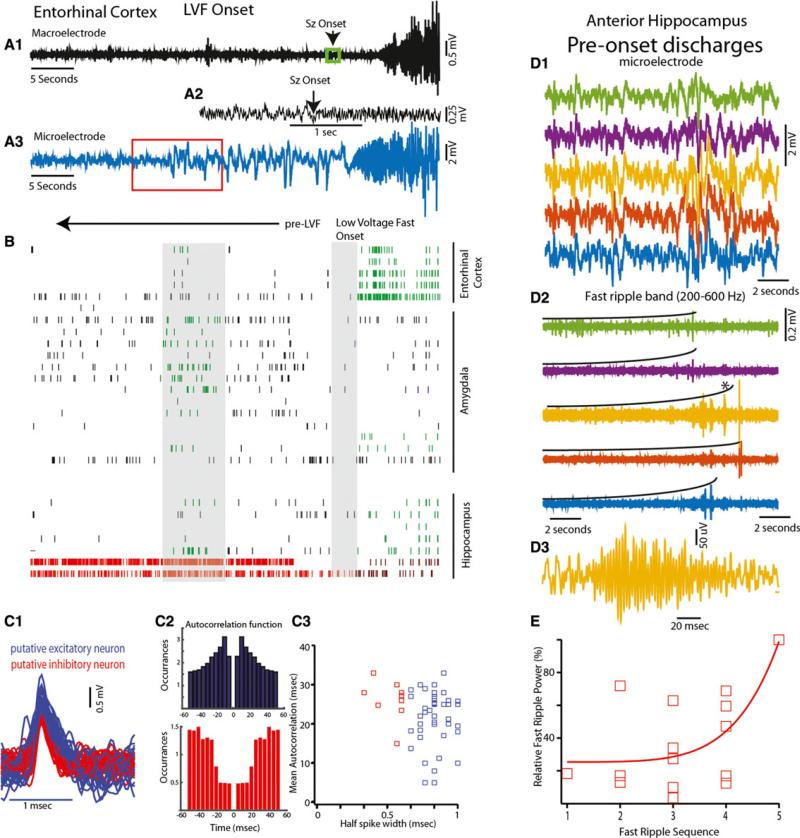
Analysis of the microelectrode recording before the appearance of the depth electrode-recorded LVF onset seizure again showed an abrupt increase in LFP discharges that did not correspond with the depth EEG (Fig. 3A). These microelectrode discharges appeared ~20 s prior to the depth clinical onset and then transitioned to LVF activity that did correspond to the depth LVF onset pattern (Fig. 3A). Importantly, the onset of microelectrode discharges was associated with fast ripples that incrementally increased in power (Fig. 3D,E, d.f. = 14, χ2 = 0.091, p < 0.01). The largest amplitude event in the series of fast ripples preceded depth LVF onset by 12.3 ± 1.45 s. Following the largest fast ripple in this sequence were mixed frequency LFPs associated with smaller amplitude fast ripples. The mean duration of the sequence of incrementally increasing fast ripples was 7.18 ± 1.26 s, and in some of the microelectrodes the initial fast ripple in the sequence occurred at the time of onset of the LFP discharges (Fig. 3D1,2).
Figure 3.
A LVF onset seizure recorded from the entorhinal cortex and hippocampal SOZs was preceded by preictal discharges in the LFP that were accompanied by incrementally increasing fast ripples and increased unit activity. Following these discharges bidirectional shifts in excitatory and inhibitory single unit firing activity were evident during LVF onset (A) Distal most clinical electrode (A1, black) and microelectrode (A3, blue) recording of a LVF onset seizure from the entorhinal cortex demonstrating that prior to LVF onset, shown at an extended time scale in A2, a series of large amplitude discharges was evident exclusively in the microelectrode recording (red box corresponds to panel D1). (B) Raster plot of putative excitatory (black) and inhibitory (red) neuronal firing in limbic structures relative to LVF onset. First shaded bar in B defines the epoch of increased neuronal firing during the fast ripples of incrementally increasing power, the second shaded bar decreased neuronal firing during LVF activity. Green and purple ticks correspond to epochs of statistically significant (p < 0.01) increases and decreases in excitatory unit firing rate, dark red tick marks reflect decreased inhibitory cell firing. (C1) Individual spike waveforms, and autocorrelation histograms (C2) of a putative excitatory (blue) and inhibitory (red) neuron. (C3) Scatter plot of the spike half width and mean autocorrelation time for all the sorted units. (D1) LFP of the preictal epoch as shown in panel A, red box) recorded by five microelectrodes in the hippocampus. (D2) Fast ripples incrementally increased during this epoch as illustrated by the black curves, shown in panel D3 is the HFO indicated by as asterisk in D2 at an expanded time scale. (E) The increase in the scaled power of sequential fast ripples recorded from four microelectrodes prior to LVF onset exhibited exponential growth (d.f. = 14, χ2 = 0.091, p < 0.01).
Fast ripples of incrementally increasing power during a rhythmic spiking onset to LVF activity but not during the polyspike onsets
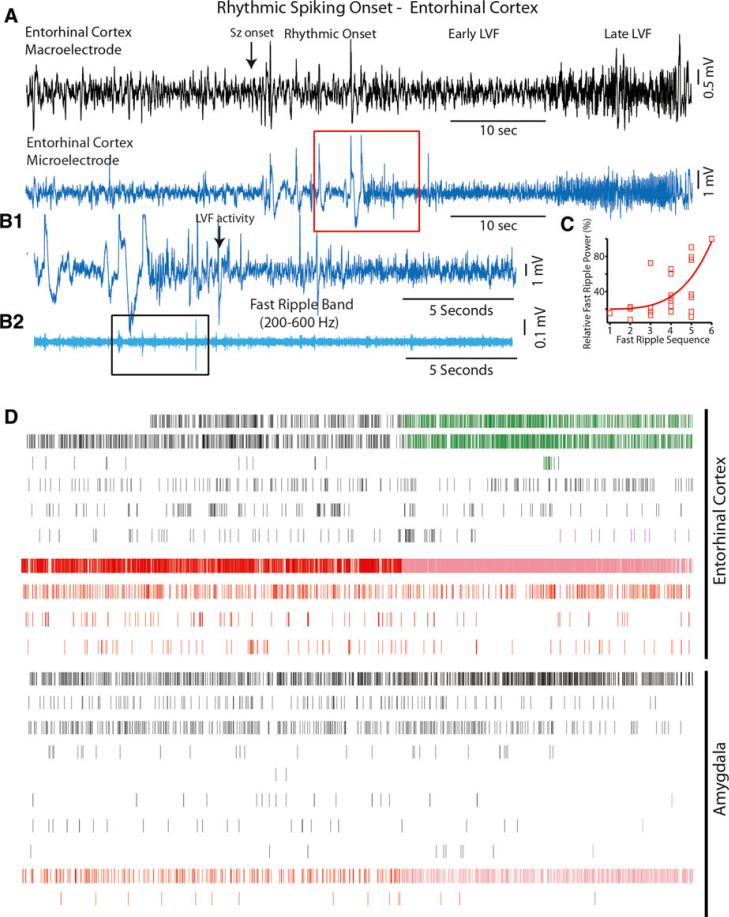
Unlike the HYP and LVF onset seizures illustrated in Figs. 2 and 3 microelectrodes recorded only a few irregular LFPs that were not associated with fast ripples in the period immediately preceding the depth electrode rhythmic spiking onset (Fig. 4A). However, fast ripples were detected after the rhythmic spiking onset during the transition from rhythmic spiking to LVF activity (Fig. 4B,C, d.f. = 23, χ2 = 1.14, p < 0.01). Fast ripples preceded the transition to LVF activity by 9.8 ± 3.54 s and during the intervening period between the largest fast ripple and LVF activity, there were irregular epileptiform discharges and a paucity of fast ripples. In contrast, the microelectrode recordings did not contain incrementally increasing fast ripples either before or during the depth polyspike onset seizures (Fig. S2).
Figure 4.
A rhythmic spiking onset seizure is associated with fast ripples of incrementally increasing power during the transition to LVF activity and increases in excitatory and inhibitory single unit firing rates during LVF activity. (A) A rhythmic spiking onset seizure recorded by the most distal macroelectrode contact in the entorhinal cortex (top, black), and corresponding recording from the adjacent microelectrode (bottom, blue). (B1) Expansion of the transition from rhythmic spiking to LVF activity (red box A), (B2) HFO band pass filtering (cyan) reveals incrementally increasing fast ripples as shown in the black box. (C) The increase in the scaled power of sequential fast ripples recorded from seven microelectrodes in the entorhinal cortex prior to LVF activity exhibited exponential growth (d.f. = 23, χ2 = 1.14, p < 0.01). (D) Raster plot of putative excitatory (black) and inhibitory (red) cell spike firing in the entorhinal cortex and amygdala. Light red tick marks indicate epochs of increased inhibitory cell spike firing.
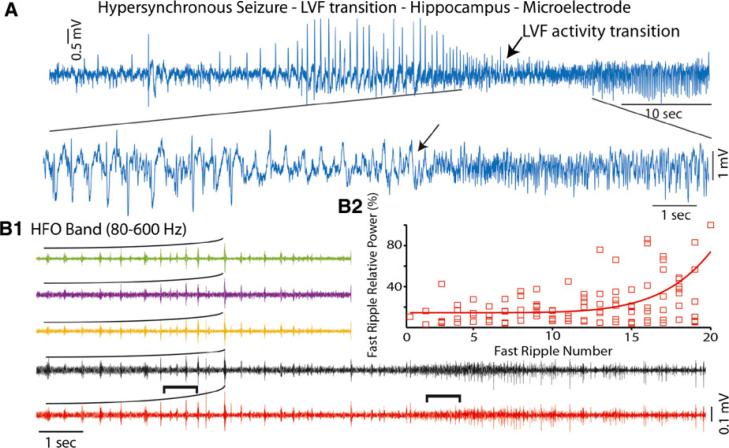
Fast ripples of incrementally increasing power during a HYP to LVF transition
The incrementally increasing fast ripple motif in the band pass filtered LFP was also recorded during a transitional period from a HYP onset pattern to LVF activity (Fig. 5A, B) in one of the three HYP onset seizures. The incrementally increasing fast ripples (Fig. 5B2, d.f. = 89, χ2 = 4.45, p < 0.01) occurred approximately 50 s after seizure onset and accompanied the terminal sharply contoured HYP discharges (Fig. 5A). Following these discharges were several bluntly contoured discharges that lasted approximately 4.5 s before the transition to LVF activity (Fig. 5A,B). These bluntly contoured discharges were associated mostly with low amplitude ripples, although some fast ripples were evident. The incrementally increasing fast ripples lasted 6.81 ± 0.51 s and consisted of more discrete events than the number of fast ripples detected near seizure onset.
Figure 5.
The transition from HYP onset to LVF activity was associated with an incremental increase in fast ripple power at the termination of the HYP epoch followed by ripple-frequency HFOs during LVF activity. (A) The HYP onset seizure recorded by a microelectrode in the left hippocampus exhibits a transition to LVF activity as shown by the arrow. The HYP to LVF transition is shown at an expanded time scale in the bottom trace. (B1) HFO band pass filtered recordings from five of the microelectrodes demonstrating an incremental increase in HFO amplitude, illustrated by the black curves, during the end of the HYP epoch. (B2) The increase in the scaled power of sequential fast ripples recorded from seven microelectrodes prior to LVF activity exhibited exponential growth (d.f. = 89, χ2 = 4.45, p < 0.01). Epilepsia ILAE
Rates of HFO increase during both HYP and LVF ictal epochs
To further investigate HFO activity during preictal and ictal epochs we calculated the ripple and fast ripple rates. Overall, in the SOZ during HYP onset seizures, the occurrence of both ripples and fast ripples increased (n = 18, p < 0.05). In each of the HYP onset seizures, ripples and fast ripples occurred in a recurrent pattern where most ictal HYP discharges were accompanied by a ripple followed by a fast ripple. During LVF activity there was also an increase in the ripple (n = 53, p < 0.001) and fast ripple rates (p < 0.001) although these changes were not consistent within each individual LVF recording.
Interictal HFOs did not demonstrate incrementally increasing power
In three of the patients who exhibited incrementally increasing fast ripples at seizure onset we analyzed 10–15 min epochs of interictal LFPs recorded from microelectrodes in the SOZ at least 12 h prior to seizure onset to determine whether this same motif occurred independently of seizure onset. This analysis found that the rates of ripples and fast ripples during these interictal epochs were comparable to the rates during preictal epochs (p = 0.22 ripple, p = 0.07 fast ripple, n = 20). Quantification of fast ripple events across multiple microelectrodes, combined with visual review of the temporal pattern of events, found no evidence for incrementally increasing fast ripples during the interictal epoch (Fig. S3).
Changes in single unit spike firing during seizures with fast ripples of incrementally increasing power
In the LVF onset seizure, an increase in single unit firing rates corresponded closely with the occurrence of fast ripples of incrementally increasing power (Fig. 3A,B). Overall, nine of 13 putative excitatory neurons in the amygdala, two of five excitatory neurons in the hippocampus, and one of five excitatory neurons in the entorhinal cortex increased their firing rate during this epoch (paired t-test, p < 0.01). However, in two other seizures that exhibited fast ripples of incrementally increasing power, there was no change in unit firing rates (Fig. 4A,D, Fig. S1).
Statistically significant shifts in single unit firing rate were also detected after seizure onset during LVF activity. In two seizures, single unit firing rates changed twice during LVF activity (Fig. S1B,C, Fig. 3A,B). In one HYP onset seizure, a decrease in putative excitatory neuron firing in the entorhinal cortex SOZ was observed during the transition to LVF activity (paired t-test, n = 5, p < 0.01), which was then followed by a robust rebound of increased neuronal firing compared to baseline (Fig. S1B,C,D, n = 4, p < 0.01). Single unit ensembles were not recorded during the other HYP seizures (Table S1).
Similar to the changes in spike firing associated with one HYP onset seizure described in the preceding paragraph, during the LVF onset seizure, excitatory neuronal firing in the entorhinal cortex and amygdala initially decreased during LVF activity (Fig. 3B, Table S2, p < 0.01), and then increased primarily in the entorhinal cortex and hippocampus later during LVF activity (Fig. 3B, Table S2, p < 0.01). In addition, two putative inhibitory neurons in the hippocampus exhibited a decrease in firing during the period of LVF activity (Fig. 3B, Table S2, p < 0.01).
The opposite trend was observed in the rhythmic spiking onset seizure. Both excitatory and inhibitory neurons, which were in entorhinal cortex inside the SOZ and amygdala outside the SOZ, increased their firing rate during the transition to LVF activity (Fig. 4D, Table S3, p < 0.01).
Changes in single unit spike firing during seizures without fast ripples of incrementally increasing power
In the two polyspike onset seizures that were not associated with fast ripples, single units in the entorhinal cortex SOZ also increased their spike firing during the transition to LVF activity (Fig. S2, p < 0.01).
Discussion
Depth electrode EEG
The ictal onset patterns described here are similar to those described by us and others in previous studies of human MTLE3–6 and animal models of this disorder,8,17,18 while the transition to LVF activity after seizure onset is consistent with a depth EEG pattern associated with seizure spread.
Microelectrode LFP
In most of the seizures recorded, microelectrodes that extended up to 5 mm from the tip of each depth electrode recorded brief epileptiform discharges before the apparent clinical depth EEG seizure onset, which indicates the microelectrode(s) were recording from a different volume of tissue than the adjacent clinical contact or that the volume of tissue was too small to be detected by the macroelectrode.19
In some cases, like the polyspike onset seizures, the LFP epileptiform discharges occurred as infrequent brief bursts, whereas before the HYP onset seizures, LFP discharges appeared continuously for 10 min or more before clinical EEG onset. In these latter seizures, our recordings were not of sufficient duration to unequivocally determine the onset of this LFP activity, or explicitly distinguish whether this activity reflects baseline LFPs or possibly preictal epilepti-form discharges as described in previous human epileptogenic tissue slices.7
By contrast, we observed a clear evolution in LFP amplitude and frequency before the LVF onset seizure, and the waveform morphology of these discharges resembled HYP discharges typical of HYP onset seizures. In addition, the LFP discharges were preceded by fast ripples that incrementally increased in power and an increase neuronal spike firing, as described for HYP seizures below. Collectively, these data suggest the microelectrode actually recorded a HYP onset seizure that was misclassified as a LVF onset seizure in the clinical EEG, and could be similar to microseizures described in another microelectrode studies.19
Microelectrode-recorded HFOs
The PIN (pathologically interconnected neurons) cluster hypothesis postulates that pathological HFOs reflect summated action potentials from small groups of neurons that are aberrantly linked structurally and/or functionally. Changes in local inhibitory processes cause individual clusters to increase in size, coalesce, and synchronize activity. Once a critical volume of tissue is recruited, propagation occurs and a seizure ensues.20 Experimental evidence for this mechanism of seizure onset was observed in intrahippocampal kainic acid–treated rats with spontaneous seizures that exhibited fast ripples of incrementally increasing amplitude just prior to HYP seizure onset.8
In the current study, we detected fast ripples of incrementally increasing power across multiple microelectrodes before each of the HYP onset seizures, including the one that appeared to be LVF on clinical recordings, but not the seizures beginning with rhythmic spiking or polyspike onset pattern. While microelectrode recordings improve the granularity of analysis, accuracy can be reduced due to limited spatial sampling, which could explain the absence of incrementally increasing fast ripples in these latter ictal recordings. This could also contribute to the temporal jitter between the occurrence of fast ripples and ictal onset, which in some cases could reach 9.7 s after the terminal fast ripple with the largest amplitude, but in others it was nearly simultaneous or 0.5 s after seizure onset in the clinical electrodes. These results could reflect activity from fast ripple-generating sites positioned on the periphery of a network that feedback onto more central or “hub” sites which triggered the seizure.21 If this is correct, then the LFP pattern after the terminal fast ripple and before the apparent clinical seizure onset could correspond to a period of seizure propagation. Higher density microelectrode array recordings like those used in neocortex would be needed to evaluate this alternative possibility.22,23
In two different seizures, fast ripples of incrementally increasing power occurred after seizure onset and before the transition of LVF activity. In each of these seizures, the terminal fast ripple with the largest amplitude occurred several seconds before appearance of LVF activity. It is plausible that these results reflect the continued extension of PIN clusters and further recruitment of surrounding areas of tissue. The significance of the bluntly contoured epileptiform discharges before LVF activity is not clear, but might be associated with changes in local neuronal spike firing that facilitates seizure propagation after the HYP epoch EEG discharges.
Additional evidence for involvement of HFOs in seizure spread derives from significant changes in rates of fast ripples and ripples. In the HYP onset seizures, rates of fast ripple occurrence increased during the HYP ictal discharges, which contained a ripple that was followed by a fast ripple. The appearance of a ripple-fast ripple complex during HYP ictal discharges might correspond to a transient increase in inhibitory activity followed by a rebound in principal cell firing. We observed inconsistent increases in ripple and fast ripple rates during LVF activity. Additional microelectrode recordings will clarify whether ripples generated by synaptic inhibition24 are involved in network synchronization and seizure spread during LVF. An increase in ripples and interneuron spike firing was reported to be associated with LVF onset seizures,25 whereas an increase in the fast ripple rate was associated with HYP seizures in the pilocarpine model18 and patients with MTLE.5
Evidence that the patterns described here were unique to ictal events include our observation that fast ripples of incrementally increasing power were not recorded during the interictal state, and ripple and fast ripple rates during the interictal epochs did not approach those seen during HYP or LVF activity.
Microelectrode single unit activity
Results from the single unit analysis showed that in most of the seizures recorded, there was little change in spike firing rates during the transition to ictus. These data are consistent with the PIN cluster hypothesis, as well as previous human single neurons studies,26,27 that predict relatively few synchronously firing neurons are involved in seizure genesis.28,29 Moreover, in those seizures with incrementally increasing fast ripples preceding ictal onset, the occurrence of population spikes might have obscured single unit spikes and our ability to detect changes in firing rate.30 However, our analysis found more prominent changes in spike firing occurred late in the seizure, typically after the episode of incrementally increasing fast ripples. It should be considered, because of sampling error, that we were less likely to see changes in unit firing at onset, but only after many neurons were recruited into the seizure and propagation occurred could we observe significant changes in unit activity.
With respect to the question as to whether LVF activity reflects an epoch of increased inhibitory tone,9 a reduction in firing rate among some putative principal cells was seen during LVF in two seizures (Fig. 3B; Fig. S1C). We only had a very limited sample of putative interneurons and some increased firing during LVF, but others showed no change, or decreased their rate of firing (Figs. 3B, 4B). Overall, these data are consistent with a role of inhibition from some, but not all, types of interneurons that might serve to synchronize principal cell spike firing during limbic seizures.
Conclusions
In a single LVF onset seizure, a triad of evolving HYP LFP discharges, increased single unit activity, and fast ripples of incrementally increasing power was present ~20 s prior to clinical depth EEG seizure onset suggesting that LVF onset seizures can be preceded by a HYP onset pattern isolated to a microdomain. All the HYP onset seizures also exhibited fast ripples of incrementally increasing power prior to seizure onset, while the incrementally increasing fast ripple motif was also identified after seizure onset and before the transition to LVF activity, but was not found during interictal episodes. These findings support the PIN cluster hypothesis of limbic seizure genesis and suggest that PIN clusters may also be involved in ictal recruitment and propagation. The LVF activity that followed fast ripples of incrementally increasing power was characterized by changes in excitatory and inhibitory unit firing rate. Unraveling the meaning of these shifts in single unit activity and its relevance to the network that support human limbic seizure propagation will require a significantly greater number of ictal recordings from diverse neuron populations.
Supplementary Material
Key Points.
HYP onset seizures exhibited fast ripples of incrementally increasing power prior to seizure onset.
HYP onset seizures were associated with an increase in the rate of ictal fast ripple and ripples, and individual HYP EEG spikes contained a ripple followed by a fast ripple.
LVF activity was associated with changes in excitatory and inhibitory single unit firing rate.
LVF onset seizures recorded from the human hippocampus and entorhinal cortex can be preceded by a HYP onset pattern isolated to a microdomain.
Acknowledgments
Dr. Shennan Aibel Weiss is supported by an Epilepsy Foundation Award Research and Training Fellowships for Clinicians, Dr. Fried is supported by NINDS grant NS033221, Dr. Engel is supported by NS002808, NS033310, and NS080181, Dr. Staba is supported by NS071048, and Dr. Bragin is supported by NS065877. Dr. Shennan Aibel Weiss would like to thank Stephanie Moy for assistance with preparing the manuscript.
Biography
 Dr. Shennan Aibel Weiss is a clinical epilepsy fellow at the University of California Los Angeles Seizure Disorder Center.
Dr. Shennan Aibel Weiss is a clinical epilepsy fellow at the University of California Los Angeles Seizure Disorder Center.
Footnotes
Disclosure
All authors report no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 2.White SH. Animal models of epileptogenesis. Neurology. 2002;59:S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 3.Velasco AL, Wilson CL, Babb TL, et al. Functional and Anatomic Correlates of two Frequently Observed Temporal Lobe Seizure-Onset Patterns. Neural Plast. 2000;7:49–63. doi: 10.1155/NP.2000.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogren JA, Bragin A, Wilson CL, et al. Three-dimensional hippocampal atrophy maps distinguish two common temporal lobe seizure-onset patterns. Epilepsia. 2009;50:1361–1370. doi: 10.1111/j.1528-1167.2008.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain. 2014;137:183–196. doi: 10.1093/brain/awt299. [DOI] [PubMed] [Google Scholar]
- 6.Memarian N, Madsen SK, Macey PM, et al. van Luijtelaar G, editor. Ictal Depth EEG and MRI Structural Evidence for Two Different Epileptogenic Networks in Mesial Temporal Lobe Epilepsy. PLoS ONE. 2015;10:e0123588–16. doi: 10.1371/journal.pone.0123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huberfeld G, Menendez de la Prida L, Pallud J, et al. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- 8.Bragin A, Azizyan A, Almajano J, et al. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 9.Gnatkovsky V, Librizzi L, Librizzi L, et al. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- 10.Fried I, Wilson CL, Maidment NT, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J Neurosurg. 1999;91:697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- 11.Staba RJ, Ekstrom AD, Suthana NA, et al. Gray matter loss correlates with mesial temporal lobe neuronal hyperexcitability inside the human seizure-onset zone. Epilepsia. 2012;53:25–34. doi: 10.1111/j.1528-1167.2011.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staba RJ, Wilson CL, Bragin A, et al. Quantitative analysis of high-frequency oscillations (80-500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 13.Quian Quiroga R, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- 14.Le van Quyen M, Bragin A, Staba R, et al. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;24:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakatos P, O'Connell MN, Barczak A, et al. The leading sense: supra-modal control of neurophysiological context by attention. Neuron. 2009;64:419–430. doi: 10.1016/j.neuron.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragin A, Claeys P, Vonck K, et al. Analysis of initial slow waves (ISWs) at the seizure onset in patients with drug resistant temporal lobe epilepsy. Epilepsia. 2007;48:1883–1894. doi: 10.1111/j.1528-1167.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 18.Lévesque M, Salami P, Gotman J, et al. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragin A, Wilson C, Engel J., Jr Chronic Epileptogenesis Requires Development of a Network of Pathologically Interconnected Neuron Clusters: a Hypothesis. Epilepsia. 2000;41:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 21.Jefferys JGR, de la Prida LM, Wendling F. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schevon CA, Weiss SA, McKhann G, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;11:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss SA, Banks GP, McKhann GM, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. 2013;136:3796–3808. doi: 10.1093/brain/awt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel J, Jr, Bragin A, Staba R, et al. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 25.Shiri Z, Manseau F, Lévesque M, et al. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol. 2015;77:541–546. doi: 10.1002/ana.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower MR, Bower MR, Stead M, et al. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia. 2012;53:807–816. doi: 10.1111/j.1528-1167.2012.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truccolo W, Ahmed OJ, Harrison MT, et al. Neuronal ensemble synchrony during human focal seizures. J Neurosci. 2014;34:9927–9944. doi: 10.1523/JNEUROSCI.4567-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bragin A, Engel J, Jr, Wilson C, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 29.Bragin A, Mody I, Wilson CL, et al. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bragin A, Benassi SK, Kheiri F, et al. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.