Abstract
Background:
High-level evidence has established well-recognized standard treatment regimens for patients undergoing palliative chest radiotherapy (RT) for stage IV non–small cell lung cancer (NSCLC), including treating with fewer than 15 fractions of RT, and not delivering concurrent chemoradiation (CRT) because of its increased toxicity and limited efficacy in the palliative setting.
Methods:
The study included patients in the National Cancer Database from 2004 to 2012 with stage IV lung cancer who received palliative chest radiation therapy. Logistic regression was performed to determine predictors of standard vs nonstandard regimens (>15 fractions or CRT). All statistical tests were two-sided.
Results:
There were 46 803 patients in the analysis and 49% received radiotherapy for longer than 15 fractions, and 28% received greater than 25 fractions. Approximately 19% received CRT. The strongest independent predictors of long-course RT were private insurance (odds ratio [OR] = 1.40 vs uninsured, 95% confidence interval [CI] = 1.28 to 1.53) and treatment in community cancer programs (OR = 1.49, 95% CI = 1.38 to 1.58) compared with academic research programs. The strongest factors that predicted for concurrent chemoradiotherapy were private insurance (OR = 1.38 95% CI = 1.23 to 1.54) compared with uninsured patients and treatment in community cancer programs (OR = 1.44, 95% CI = 1.33 to 1.56) compared with academic programs.
Conclusions:
Approximately half of all patients with metastatic lung cancer received a higher number of radiation fractions than recommended. Patients with private insurance and treated in community cancer centers were more likely to receive longer courses of RT or CRT. This demonstrates that a substantial number of patients requiring palliative thoracic radiotherapy are overtreated and further work is necessary to ensure these patients are treated according to evidenced-based guidelines.
Metastatic non–small cell lung cancer (NSCLC) is the leading cause of cancer death in the United States (1). In patients with metastatic NSCLC, palliative thoracic radiotherapy (RT) plays an integral role in relieving symptoms such as hemoptysis, cough, shortness of breath, and chest pain (2). Historically, the optimal palliative radiation treatment duration has been an open question, with conflicting evidence suggesting that longer regimens may modestly improve overall survival but also lead to more treatment-related morbidity (3). The chosen approach must strike a delicate balance between symptom relief and local control on one hand and toxicity and patient convenience on the other, which is particularly important given the relatively short life expectancy of this population.
There have been 14 randomized controlled trials examining the optimal radiation dose-fractionation scheme in this setting (4). An initial Cochrane review of these trials first published in 2001 and updated in 2006 found no statistically significant benefit to prolonged courses of radiotherapy (5,6). Multiple evidence-based guidelines, including work from both the American College of Radiology initially published in 2009, the American Society of Radiation Oncology published in 2011, and the 2015 National Comprehensive Cancer Network guidelines, recommend that patients receive fewer than 15 fractions when thoracic radiation is given with palliative intent for lung cancer (4,7–9).
The use of concurrent chemotherapy with palliative radiation treatment has also been studied in stage IV NSCLC. The single randomized study on this question found no benefit to concurrent chemoradiation in terms of symptom palliation or overall survival, but combined treatment was associated with a statistically significant increase in toxicity (10). Based on this result and other smaller studies, national guidelines recommend that concurrent chemotherapy and radiotherapy should not be delivered in the palliative setting (4,7).
In this study, we used the National Cancer Database (NCDB) to characterize national patterns-of-care of the use of palliative radiotherapy for stage IV NSCLC. We aimed to assess the prevalence and predictors of treatment with long-course palliative radiotherapy regimens and concurrent chemoradiotherapy, aggressive treatment approaches whose intensities are not supported by high-level evidence.
Methods
Data Sources
This study examined the NCDB, which is a hospital-based cancer registry that collects data from the American College of Surgeons (ACoS)–Commission on Cancer (CoC)–accredited facilities (11). The database is sponsored by the ACoS and the American Cancer Society and includes approximately 70% of all malignant cancers diagnosed in the United States (12). The database contains information on patient demographics, primary tumor site, histology, site at diagnosis, insurance status, first course of treatment, and overall survival. The NCDB has established specific criteria to ensure the quality of the submitted data.
Analysis Population
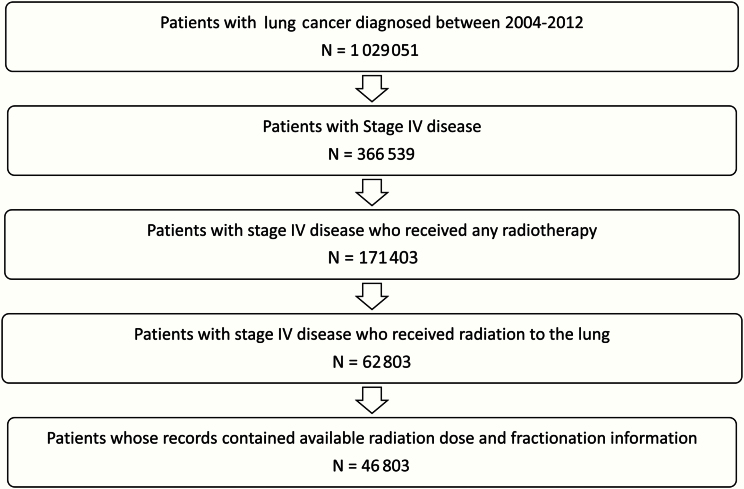
Eligible patients were diagnosed with stage IV, histologically confirmed NSCLC, who received all or part of their first course of treatment at CoC-accredited facilities; this diagnosis must have been their first cancer. Patients were included if they received palliative radiotherapy to the lung as a component of their first course of treatment, with the treatment consisting of 1–40 fractions and a total dose of 8–74 Gray (Gy). Patients who were coded as having radiotherapy to a site outside of the chest or the thoracic spine were excluded from this analysis. There were 366 539 patients diagnosed with stage IV NSCLC from 2004 to 2012, of whom 171 403 received any radiotherapy and 62 803 specifically received radiation to the lung, with the final cohort consisting of 46 803 patients whose records contained available radiation dose and fractionation information (Figure 1). The National Cancer database has a variable specifying the radiation treatment volume. All patients in this analysis were coded as having received radiation to the chest or lung, which was determined by analysis of medical records. Patients who received radiation to the thoracic spine would have been coded with a different radiation treatment volume category (ie, spine) and were excluded from this analysis. The median time from diagnosis to the start of radiation was 27 days (interquartile range = 13–51 days). The year 2004 was used as the initial time point because this was the first year that the National Cancer Database began collecting detailed information on the total radiation dose delivered and the total number of fractions delivered, while 2012 was the most recent year for which the database had information.
Figure 1.
Flowchart outlining cohort composition.
Statistical Analysis
Candidate variables were grouped into four categories: clinical (eg, histology, tumor size, age, Charlson-Deyo comorbidity index), time, socioeconomic (eg, race, insurance status), and institutional (treatment volume, facility type). Facility types are designated by the Commission on Cancer criteria and include: 1) community cancer programs, 2) comprehensive community cancer programs, and 3) academic research programs. Community cancer programs treat between 100 and 500 cancer patients a year and have a full range of services for cancer. Comprehensive community cancer programs treat at least 500 cancer patients a year and offer the same range of services. Academic research programs are affiliated with medical schools, have residency programs, and conduct ongoing cancer research. The treatment volume category was calculated by examining the average number of patients who received palliative radiotherapy treated by each facility per year and dividing it into equal sized tertiles.
Radiation treatment duration was used to dichotomize the patients into two groups: those who received more than 15 fractions (long course RT, LC-RT), and those who received 15 fractions or fewer (standard course RT, SC-RT). Fifteen fractions was chosen as the upper bound of acceptable treatment because the ASTRO and NCCN guidelines both point to the 30 Gy/10 fraction regimen as the standard approach for patients with favorable performance status (4,7). Concurrent chemoradiation was defined as patients receiving at least four weeks of radiation therapy who also received chemotherapy within three weeks of starting radiation. For this part of the analysis, 95.6% of patients had known treatment dates for inclusion.
A comparison was performed regarding clinical, socioeconomic, and institutional variables between the cohort that did and did not have radiation dose and fractionation information and revealed no differences greater than 3% between the groups.
Differences in radiation treatment duration and concurrent chemotherapy use by clinical, socioeconomic, and institutional characteristics were estimated using the chi-squared test. Multivariable stepwise logistic regressions were performed to determine independent predictors of receiving greater than 15 fractions and of receiving concurrent chemotherapy. All variables mentioned above were included as categorical covariates in the analysis. All statistical tests were two-sided, and a .05 level of significance was utilized. Data were analyzed using SPSS v21 (Armonk, NY).
Results
Patient Characteristics
A total of 46 803 patients met eligibility criteria for this study. Patient characteristics are shown in Table 1. The median age was 67 years (interquartile range [IQR] = 58–75 years). The median primary tumor size was 5cm (IQR = 3.5–7cm).
Table 1.
Patient characteristics among stage IV non–small cell lung cancer patients receiving radiation therapy, National Cancer Database 2004–2012
| Categories | All patients (n = 46 803) No. (%) |
Number of fractions (1–15) (n = 23 727) No. (%) |
Number of fractions (16–40) (n = 23 076) No. (%) |
P |
|---|---|---|---|---|
| Clinical factors | ||||
| Sex | .43 | |||
| Male | 27 454 (58.7) | 13 928 (50.7) | 13 526 (49.3) | |
| Female | 19 349 (41.3) | 9799 (50.6) | 9550 (49.4) | |
| Comorbidity Index | <.001 | |||
| 0 | 29 764 (63.6) | 14 637 (49.2) | 15 127 (50.8) | |
| 1 | 12 235 (26.1) | 6448 (52.7) | 5787 (47.3) | |
| ≥2 | 4804 (10.3) | 2642 (55) | 2162 (45) | |
| Age at diagnosis, y | <.001 | |||
| 18–59 | 13 389 (28.6) | 6518 (48.7) | 6871 (51.3) | |
| 60–69 | 14 466 (30.9) | 7147 (49.4) | 7319 (50.6) | |
| 70–79 | 12 954 (27.7) | 6665 (51.5) | 6289 (48.5) | |
| 80+ | 5994 (12.8) | 3397 (56.7) | 2597 (43.3) | |
| Histology | <.001 | |||
| Squamous | 13 845 (29.6) | 6642 (48) | 7203 (52) | |
| Nonsquamous | 32 958 (70.4) | 17 085 (51.8) | 15 873 (48.2) | |
| Size of primary tumor, cm | <.001 | |||
| 0–6.5 | 23 783 (50.8) | 11 334 (47.7) | 12 449 (52.3) | |
| 6.6–18 | 10 596 (22.6) | 5364 (50.6) | 5232 (49.4) | |
| Unknown | 12 424 (26.5) | 7029 (56.6) | 5395 (43.4) | |
| Socioeconomic factors | ||||
| Race/ethnicity | <.001 | |||
| White | 39 171 (83.7) | 19 591 (50) | 19 580 (50) | |
| Nonwhite | 7632 (16.3) | 4136 (54.2) | 3496 (45.8) | |
| Insurance status | <.001 | |||
| Not Insured | 2339 (5) | 1291 (55.2) | 1048 (44.8) | |
| Private insurance/managed care | 14 525 (31) | 6802 (46.8) | 7723 (53.2) | |
| Medicaid | 3794 (8.1) | 2088 (55) | 1706 (45) | |
| Medicare | 25 341 (54.1) | 13 063 (51.5) | 12 278 (48.5) | |
| Other government | 804 (1.7) | 483 (60.1) | 321 (39.9) | |
| Institutional factors | ||||
| Facility type | <.001 | |||
| Community cancer program | 6841 (14.6) | 3008 (44) | 3833 (56) | |
| Comprehensive community cancer program | 27 245 (58.2) | 13 476 (49.5) | 13 769 (50.5) | |
| Academic research program | 12 717 (27.2) | 7243 (57) | 5474 (43) | |
| Facility volume | <.001 | |||
| Low (0–4.33 cases/y) | 14 675 (31.4) | 6881 (46.9) | 7794 (53.1) | |
| Medium (4.34–7.88 cases/y) | 16 839 (36) | 8453 (50.2) | 8386 (49.8) | |
| High (>7.89 cases/y) | 15 289 (32.7) | 8393 (54.9) | 6896 (45.1) | |
| Year of diagnosis | <.001 | |||
| 2004–2008 | 25 066 (53.6) | 11 792 (47) | 13 274 (53) | |
| 2009–2012 | 21 737 (46.4) | 11 935 (54.9) | 9802 (45.1) | |
Treatment Duration
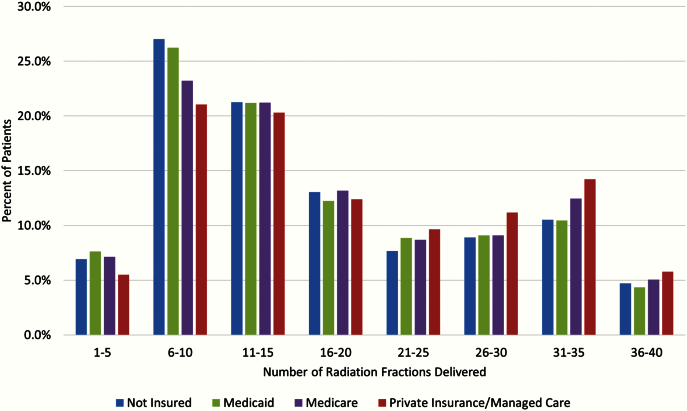
The median treatment duration was 26 days (IQR = 15–45 days). The median number of fractions delivered was 15 (IQR = 10–28). A total of 70% of all patients received a palliative radiation regimen greater than 10 fractions, 49% received more than 15 fractions, and 37% and 28% were treated with more than 20 and 25 fractions, respectively. The median total dose delivered was 39 Gy (IQR = 30–58 Gy). On univariate analysis, patients who were younger and those with a more favorable comorbidity score were more often treated with LC-RT (Table 1). In addition, white patients and individuals with private insurance were also more likely to receive LC-RT. The distribution of the number of radiation fractions stratified by insurance status is shown in Figure 2, which demonstrates a statistically significant increasing number of fractions in those with private insurance (P < .001). Patients treated in community cancer programs and at low-volume facilities were more likely to be treated with LC-RT. Patients treated with SD-RT had a median number of 16 days on treatment, while those treated with LC-RT had a median number of 44 days on treatment. Patients treated with SD-RT had a median number of 10 fractions delivered and median total dose of 30 Gy, while those treated with LC-RT had a median of 28 fractions of radiation delivered and median total dose of 57.6 Gy (P < .001).
Figure 2.
Patients grouped according to number of radiation fractions delivered and stratified by insurance status.
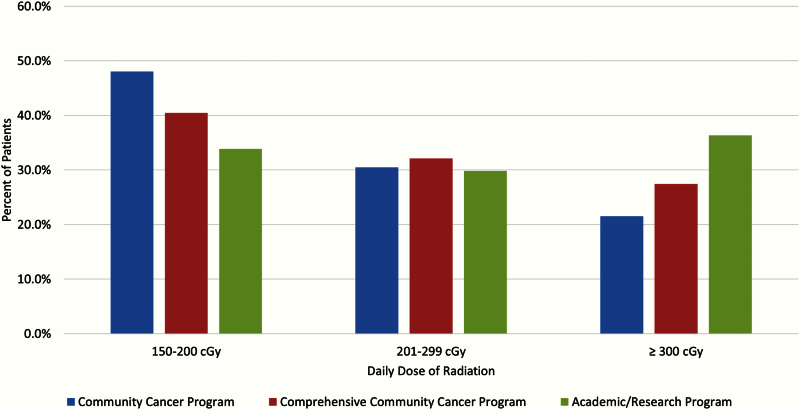
The results stratified by radiation dose paralleled those by treatment length: 41% of the cohort received 1.5–2 Gy per day, 32% received 2.01–2.99 Gy, and 27% received 3 Gy or more. When the daily dose was examined by facility type, patients treated in community cancer centers were more likely to be treated with a lower daily dose. For example, 21.5% who were treated in a Community Cancer Center received 3 Gy or more per fraction, in comparison with 36.3% treated in an academic research program (P < .0001) (Figure 3). The median number of fractions, daily dose, and total dose for community cancer centers vs academic facilities is as follows: 19 vs 15 fractions, 2.1 Gy vs 2.5 Gy, and 40 Gy vs 37.5 Gy (P < .001).
Figure 3.
Patients grouped according to daily dose of radiation delivered and stratified by facility type.
The adjusted likelihoods of treatment with LC-RT are shown in Table 2. Patients with a lower comorbidity score, younger age, squamous histology and smaller tumors were more likely to be treated with a long treatment course (more than 15 fractions). White patients were more likely to receive LC-RT (odds ratio [OR] = 1.12, 95% confidence interval [CI] = 1.06 to 1.17). In addition, patients with private insurance were statistically significantly more likely (OR = 1.40, 95% CI = 1.28 to 1.53) to be treated with LC-RT than uninsured patients, as were individuals treated in community cancer programs (OR = 1.49, 95% CI = 1.38 to 1.58 vs academic facilities) and low-volume facilities (OR = 1.24, 95% CI = 1.18 to 1.30 vs high-volume facilities). Patients treated from 2009 to 2012 were less likely to be treated with LC-RT (OR = 0.73, 95% CI = 0.70 to 0.75 vs 2004–2008).
Table 2.
Likelihood of receiving > 15 fractions of radiation therapy among clinical stage IV non–small cell lung cancer patients, National Cancer Database 2004–2012*
| Categories | OR (95% CI) | P |
|---|---|---|
| Clinical factors | ||
| Sex | .686 | |
| Male | 1.0 (Ref) | |
| Female | 1.008 (0.97 to 1.05) | |
| Comorbidity Index | <.001 | |
| 0 | 1.0 (Ref) | |
| 1 | .867 (0.83 to 0.91) | |
| ≥2 | .804 (0.76 to 0.86) | |
| Age at diagnosis, y | <.001 | |
| 18–59 | 1.0 (Ref) | |
| 60–69 | .922 (0.88 to 0.97) | |
| 70–79 | .820 (0.77 to 0.87) | |
| 80+ | .654 (0.61 to 0.7) | |
| Histology | <.001 | |
| Squamous | 1.0 (Ref) | |
| Nonsquamous | .812 (0.78 to 0.85) | |
| Size of primary tumor, cm | <.001 | |
| 0–6.5 | 1.0 (Ref) | |
| 6.6–18 | .889 (0.85 to 0.93) | |
| Unknown | .674 (0.65 to 0.71) | |
| Socioeconomic factors | ||
| Race/ethnicity | <.001 | |
| Nonwhite | 1.0 (Ref) | |
| White | 1.118 (1.06 to 1.17) | |
| Insurance status | <.001 | |
| Not insured | 1.0 (Ref) | |
| Private insurance/managed Care | 1.401 (1.28 to 1.53) | |
| Medicaid | 1.050 (0.95 to 1.17) | |
| Medicare | 1.293 (1.18 to 1.42) | |
| Other government | .904 (0.77 to 1.07) | |
| Institutional factors | ||
| Facility type | <.001 | |
| Academic research program | 1.0 (Ref) | |
| Community cancer program | 1.490 (1.38 to 1.58) | |
| Comprehensive community cancer program | 1.290 (1.18 to 1.42) | |
| Facility volume | <.001 | |
| High (>7.89 cases/y) | 1.0 (Ref) | |
| Medium (4.34–7.88 cases/y) | 1.142 (1.09 to 1.19) | |
| Low (0–4.33 cases/y) | 1.241 (1.18 to 1.30) | |
| Year of diagnosis | <.001 | |
| 2004–2008 | 1.0 (Ref) | |
| 2009–2012 | .725 (0.7 to 0.75) | |
* CI = confidence interval; OR = odds ratio.
A secondary analysis was performed using 10 fractions as the threshold to define LC-RT. The results of this multivariable analysis were nearly identical to the regression using 15 fractions: Patients with private insurance (OR = 1.40, 95% CI = 1.27 to 1.54) and those treated in community cancer programs (OR = 1.53, 95% CI = 1.42 to 1.66) were more likely to be treated with more than 10 fractions in comparison with those without insurance and treatment in academic facilities, respectively.
Concurrent Chemotherapy
A total of 19% of all patients received palliative concurrent chemoradiation. The multivariable analysis predicting treatment with concurrent chemoradiation is shown in Table 3, with predictors of this aggressive treatment echoing the prior model of LC-RT. White patients (OR = 1.19, 95% CI = 1.12 to 1.27) and patients with private insurance (OR = 1.38, 95% CI = 1.23 to 1.54 vs no insurance) were significantly more likely to be treated with concurrent chemoradiation. Individuals treated in community cancer programs were also more likely (OR = 1.44, 95% CI = 1.33 to 1.56) to receive concurrent chemoradiation than those in academic facilities. Patients treated from 2009 to 2012 were slightly less likely to be treated with concurrent chemoradiation (OR = 0.95, 95% CI = 0.90 to 0.99 vs 2004–2008).
Table 3.
Likelihood of receiving concurrent chemoradiation among clinical stage IV non–small cell lung cancer patients, National Cancer Database 2004–2012*
| Categories | OR (95% CI) | P |
|---|---|---|
| Clinical factors | ||
| Sex | <.001 | |
| Male | 1.0 (Ref) | |
| Female | .948 (0.90 to 0.99) | |
| Comorbidity Index | <.001 | |
| 0 | 1.0 (Ref) | |
| 1 | .900 (0.86 to 0.96) | |
| ≥2 | .765 (0.70 to 0.83) | |
| Age at diagnosis, y | <.001 | |
| 18–59 | 1.0 (Ref) | |
| 60–69 | .862 (0.81 to 0.92) | |
| 70–79 | .664 (0.62 to 0.71) | |
| 80+ | .316 (0.29 to 0.35) | |
| Histology | <.001 | |
| Squamous | 1.0 (Ref) | |
| Nonsquamous | .827 (0.79 to 0.87) | |
| Size of primary tumor, cm | <.001 | |
| 0–6.5 | 1.0 (Ref) | |
| 6.6–18 | 0.939 (0.89 to 0.99) | |
| Unknown | .710 (0.67 to 0.75) | |
| Socioeconomic factors | ||
| Race/ethnicity | <.001 | |
| Nonwhite | 1.0 (Ref) | |
| White | 1.193 (1.12 to 1.27) | |
| Insurance status | <.001 | |
| Not insured | 1.0 (Ref) | |
| Private insurance/managed care | 1.378 (1.23 to 1.54) | |
| Medicaid | 1.010 (0.88 to 1.15) | |
| Medicare | 1.214 (1.08 to 1.36) | |
| Other government | .716 (0.57 to 0.90) | |
| Institutional factors | ||
| Facility type | <.001 | |
| Academic research program | 1.0 (Ref) | |
| Community cancer program | 1.438 (1.33 to 1.56) | |
| Comprehensive community cancer program | 1.279 (1.21 to 1.35) | |
| Facility volume | <.001 | |
| High (>7.89 cases/y) | 1.0 (Ref) | |
| Medium (4.34–7.88 cases/y) | 1.105 (1.05 to 1.16) | |
| Low (0–4.33 cases/y) | 1.092 (1.02 to 1.16) | |
| Year of diagnosis | .02 | |
| 2004–2008 | 1.0 (Ref) | |
| 2009–2012 | 0.950 (0.90 to 0.99) | |
* CI = confidence interval; OR = odds ratio.
We performed sensitivity analyses on the definition of concurrent chemoradiation, with the RT duration ranging from a minimum of one or two weeks with chemotherapy starting within that interval, finding that 24% and 28% were defined as receiving concurrent treatment with a minimal RT duration of one and two weeks, respectively. The statistically significant parameters on multivariable analysis were identical in these secondary regressions, with only modest changes to the effect estimates.
Discussion
In this study of a large cross-section of patients with stage IV NSCLC treated with palliative radiotherapy, nearly 50% of patients received a long RT course (more than 15 fractions) that is inconsistent with the results of published phase III studies; in fact, the long-course RT arm was longer than 15 fractions in only four out of the 14 randomized dose-escalation studies of palliative radiotherapy, emphasizing the extreme nature of these treatment patterns (3). This analysis also found that 19% of patients received concurrent palliative chemoradiation, a practice that is not only unsupported by the evidence, but one that places the patient at increased risk for toxicity without an established palliative or survival advantage (10).
Multiple systematic reviews and evidence-based guidelines have endorsed shorter courses of palliative radiotherapy because they are more convenient, associated with less travel time to a radiation center, less costly, and generally associated with similar palliative relief in comparison with longer courses of radiotherapy (5,6,13). There was a large prolongation in the median number of days on treatment (16 vs 44 days) in patients treated with standard vs long-course radiation therapy, which places an unnecessary and nonbeneficial burden on patients. Treatment costs would also be much less in patients who received the median number of 10 treatments in the SD-RT cohort vs a median number of 28 fractions in the LC-RT cohort, and previous research has demonstrated the cost-effectiveness of fewer numbers of radiation treatments in the palliative setting (14). Regarding toxicity, a previous meta-analysis found physician-assessed dysphagia was increased in patients who received high-dose vs low-dose radiation (20.5% vs 14.9%, P = .01) (15). Therefore, it is reasonable to hypothesize that similar rates of increased toxicities associated with higher radiation doses and longer courses of radiation apply to this cohort of patients treated in a similar manner.
Despite the prevalence of metastatic lung cancer and the frequent need for palliative thoracic irradiation, there are relatively sparse data on the intensity of palliative chest radiotherapy in routine practice, particularly in the United States. An international practice survey suggested that United States radiation oncologists were more inclined to deliver high-dose palliation than physicians in other countries (16). A previous analysis using the Cancer Care Outcomes Research and Surveillance Consortium database found that 42% of patients received 20 or more fractions of palliative chest radiotherapy among approximately 300 individuals treated with lung RT (17). Indeed, this prevalence is similar to our finding of 37% of patients who received 20 or more fractions in a larger and more generalizable population, lending external validity to our findings.
The nonclinical predictors of LC-RT and CRT—namely, race, insurance status, and institutional characteristics—provide unique insights into these aggressive practice patterns. Previous research has shown that cancer disparities exist where nonwhite and uninsured patients are usually undertreated for their cancer diagnosis while white and insured patients receive care according to evidence-based treatment recommendations (18). This study found that in patients who underwent palliative thoracic radiotherapy a “reverse disparity” exists in that the group of patients who usually receive care consistent with evidence-based guidelines (ie, white and insured patients) were overtreated with longer courses of radiotherapy and concurrent chemoradiation. These intensive treatments are associated with higher risks of morbidity—esophagitis, in particular—without a meaningful clinical gain, and thus further investigation into this pattern is important. It is possible that insured patients may appear to have a more favorable prognosis than uninsured individuals, and thus physicians push the dose upwards. Another less savory explanation is the higher per-fraction reimbursement from private payors, and it was the financial incentive that drove the use of more treatment fractions to be prescribed (19,20).
The treating facility played a statistically significant role in influencing treatment, as patients treated at community cancer centers and low-volume institutions were more likely to treat with both longer RT courses and chemoradiotherapy. Prior studies of radiotherapy at the end-of-life have suggested institutional biases in the aggressiveness of palliative irradiation among tertiary care institutions, but there are few data on how palliative radiation treatment choices vary by institutional size and volume (21). More research is necessary to understand why these institutions are more frequently treating patients with longer radiotherapy courses and with combined modality paradigms.
A limitation of this study is that we cannot account for unknown confounding factors that may be associated with the delivered treatment, which is inherent in any retrospective population-based study. Similarly, the NCDB does not record several variables that are known to affect prognosis and potentially the physician’s choice of treatment, such as Karnofsky performance status or weight loss; however, the use of the comorbidity score did provide some adjustment for these factors. Furthermore, treatment outcomes such as relief of hemoptysis, improvement in chest pain or cough, and adverse events related to radiation, such esophagitis and pneumonitis, are not coded specifically within the database. On the other hand, this analysis benefitted from a large sample size of patients treated at several different kinds of treatment facilities, and therefore we do feel that the conclusions do accurately reflect national treatment patterns.
In conclusion, a large proportion of patients with metastatic lung cancer are treated with a long course of palliative radiotherapy and/or concurrent chemoradiation, neither of which are supported by the evidence or associated with an increased risk of treatment-related morbidity. Such aggressive treatments are also more time consuming for the patient and costly to society. Therefore, more work is necessary to educate clinicians on the proper delivery of palliative lung radiotherapy, and perhaps the release of the ASTRO clinical practice guideline on palliative lung radiotherapy in 2011 will positively inform practice. The American College of Radiology released guidelines in 2009 advocating for no more than 10 fractions of thoracic radiotherapy to be delivered in the palliative setting. The reduction in the use of LC-RT between 2004 and 2008 to 2009 and 2012 suggests that population-level progress can be made and that clinical practice guidelines can change practice patterns (8). Furthermore, early involvement with palliative care specialists can meaningfully improve outcomes and adherence to evidence-based treatment choices in the noncurative setting (22,23).
Funding
This work was supported by the University of Chicago Bucksbaum Institute for Clinical Excellence. The institute played no role in the study design, interpretation of the data, or writing of the manuscript.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65 (1):5–29. [DOI] [PubMed] [Google Scholar]
- 2. Toy E, Macbeth F, Coles B, Melville A, Eastwood A. Palliative thoracic radiotherapy for non-small-cell lung cancer: a systematic review. Am J Clin Oncol. 2003;26 (2):112–120. [DOI] [PubMed] [Google Scholar]
- 3. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26 (24):4001–4011. [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1 (2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macbeth F, Toy E, Coles B, Melville A, Eastwood A. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev. 2001;(3):CD002143. [DOI] [PubMed] [Google Scholar]
- 6. Lester JF, Macbeth FR, Toy E, Coles B. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev. 2006;(4):CD002143. [DOI] [PubMed] [Google Scholar]
- 7. Rosenzweig KE, Chang JY, Chetty IJ, et al. ACR appropriateness criteria nonsurgical treatment for non-small-cell lung cancer: poor performance status or palliative intent. J Am Coll Radiol. 2013;10 (9):654–664. [DOI] [PubMed] [Google Scholar]
- 8. Rosenzweig KE, Movsas B, Bradley J, et al. ACR appropriateness criteria on nonsurgical treatment for non-small-cell lung cancer: poor performance status or palliative intent. J Am Coll Radiol. 2009;6 (2):85–95. [DOI] [PubMed] [Google Scholar]
- 9. Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12 (12):1738–1761. [DOI] [PubMed] [Google Scholar]
- 10. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75 (5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Cancer Database. 2012 update. http://www.facs.org/cancer/ncdb/ Accessed November 19, 2014.
- 12. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15 (3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32 (26):2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol. 2009;32 (4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fairchild A, Straube W, Laurie F, Followill D. Does quality of radiation therapy predict outcomes of multicenter cooperative group trials? A literature review. Int J Radiat Oncol Biol Phys. 2013;87 (2):246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodrigues G, Macbeth F, Burmeister B, et al. International practice survey on palliative lung radiotherapy: third international consensus workshop on palliative radiotherapy and symptom control. Clin Lung Cancer. 2012;13 (3):225–235. [DOI] [PubMed] [Google Scholar]
- 17. Chen AB, Cronin A, Weeks JC, et al. Palliative radiation therapy practice in patients with metastatic non-small-cell lung cancer: a Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J Clin Oncol. 2013;31 (5):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32 (28):3118–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobson M, O’Malley AJ, Earle CC, Pakes J, Gaccione P, Newhouse JP. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood). 2006;25 (2):437–443. [DOI] [PubMed] [Google Scholar]
- 20. Lievens Y, Van den Bogaert W, Rijnders A, Kutcher G, Kesteloot K. Palliative radiotherapy practice within Western European countries: impact of the radiotherapy financing system? Radiother Oncol. 2000;56 (3):289–295. [DOI] [PubMed] [Google Scholar]
- 21. Kapadia NS, Mamet R, Zornosa C, Niland JC, D’Amico TA, Hayman JA. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer. 2012;118 (17):4339–4345. [DOI] [PubMed] [Google Scholar]
- 22. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363 (8):733–742. [DOI] [PubMed] [Google Scholar]
- 23. Rowland K, Schumann SA. PURLs. Palliative care: earlier is better. J Fam Pract. 2010;59 (12):695–698. [PMC free article] [PubMed] [Google Scholar]