ABSTRACT
Intestinal dual oxidase (DUOX) activation is the first line of host defense against enteric infection in Drosophila. DUOX enzymatic activity is mainly controlled by phospholipase C-β (PLCβ)-dependent calcium mobilization, whereas DUOX gene expression is mainly controlled by the MEKK1-p38 mitogen-activated protein kinase pathway. Furthermore, bacterial-derived uracil molecules act as ligands for DUOX activation. However, our current understanding of uracil-induced signal transduction pathways remain incomplete. We have recently found that uracil stimulates Hedgehog signaling, which in turn upregulates cadherin99C (Cad99C) expression in enterocytes. Cad99C molecules, along with PLCβ and protein kinase C, induce the formation of signaling endosomes that facilitate intracellular calcium mobilization for DUOX activity. These observations illustrate the complexity of signaling cascades in uracil-induced signaling pathways. Here, we further demonstrated the role of lipid raft formation and calmodulin-dependent protein kinase-II on endosome formation and calcium mobilization, respectively. Moreover, we will provide a brief discussion on two different models for uracil recognition and uracil-induced DUOX activation in Drosophila enterocytes.
Keywords: Calmodulin-dependent kinase II, Drosophila, dual oxidase, gut immunity, hedgehog signaling, lipid raft, reactive oxygen species, Uracil
Introduction
As a protective mechanism, animals have evolved to sense different types of life-threatening microorganisms.1,2 However, not all microorganisms are pathogenic; in fact, many animal-associated microorganisms are commensal and/or symbiotic.3-5 Therefore, one of the best strategies for animals is to distinguish pathogens from commensals or symbionts in order to specifically eliminate pathogens.6 However, the details of this strategy are poorly understood. The best known strategy currently is the so-called ‘pattern recognition’ mechanism wherein eukaryotic cells sense common cell wall components (i.e. patterns) that exist in most bacterial cells, regardless of whether they are pathogenic, commensal, or symbiotic.7 For example, in the Drosophila gut epithelium, the host cells are able to recognize bacteria by sensing peptidoglycan, a component of the bacterial cell wall, for the initiation of the immune deficiency (a Drosophila homolog of the mammalian p105-like NF-βB pathway) pathway activation.2 Although a pattern recognition mechanism revolutionizes our understanding of how animals can initiate the defense system in response to pathogen invasion, it does not provide a sufficient explanation of how animals protect symbionts while eliminating pathogens.
A bacterial-derived uracil molecule acts as a ‘pathogen-specific’ signature that enables the host to distinguish uracil-positive microorganisms (e.g., pathogens or pathobionts) from uracil-negative microorganisms (e.g.,, commensals or symbionts) in the gut mucosal epithelium.8 Although it remains to be elucidated why pathogens, but not symbionts, secrete uracil, it has been clearly shown that the host cells recognize uracil to induce the enzymatic activation of dual oxidase (DUOX), a member of the nicotinamide adenine dinucleotide phosphate oxidase family,9 involved in microbicidal reactive oxygen species (ROS) generation. This pathogen-specific host defense system is intriguing because this system makes it possible to explain how gut epithelial immunity tolerates the presence of uracil-negative symbionts.8,10 Therefore, gut immunity may react in response to the quality of contacting bacteria (i.e., by uracil-secreting ability) rather than to the quantity of contacting bacteria. Further molecular dissection of the uracil-induced host signaling pathway will be of great value to increase the understanding of the molecular mechanism governing bacterial species-specific gut immunity.
Toward this goal, we recently analyzed the whole transcriptome profiles of Drosophila midgut cells following uracil stimulation.11 Functional enrichment analyses further revealed that the Hedgehog (Hh) signaling pathway, initially involved in early embryogenesis, is required for the full activation of DUOX. Uracil-induced Hh signaling is responsible for the expression of cadherin 99C (Cad99C), a member of the cadherin family, in the apical region of the enterocyte.11 Upon uracil stimulation, uracil is recognized by a yet unknown uracil receptor, and Cad99C molecules, along with phospholipase C-β (PLCβ) and protein kinase C (PKC), induce a signaling endosome that in turn facilitates intracellular calcium mobilization for DUOX enzymatic activity. All of these studies illustrate the complexity of uracil-induced innate immune signaling pathways in the Drosophila gut epithelium.
In this Extra View, we further define the role of lipid raft formation and calmodulin-dependent kinase-II (CaMKII) in uracil-induced DUOX activation. Moreover, we will discuss 2 different models for uracil recognition and uracil-induced DUOX activation in Drosophila enterocytes.
Involvement of enterocyte membrane lipid rafts in DUOX activation
How is Cad99C involved in DUOX activation? Uracil stimulation rapidly induces membrane dynamics including endosome formation.11 The roles of caveolae and lipid rafts in ligand-dependent endocytosis are well-established.12,13 As the signal-dependent clustering of many membrane proteins is associated with lipid rafts, which are specific microdomains of the plasma membrane,12,13 we examined membrane lipid rafts before and after uracil stimulation.
When we examined flotillin-1 (Flo-1), a marker of lipid rafts, we found more intense Flo-1 staining following uracil treatment, indicating that uracil induces the clustering of lipid rafts (Fig. 1A). Interestingly, Cad99C co-localized with Flo-1 upon uracil stimulation (Fig. 1B). Furthermore, we found that the oral ingestion of methyl-β-cyclodextrin, an inhibitor of lipid raft formation that acts by removing cholesterol from the membrane, was able to block uracil-induced Cad99C clustering as well as uracil-induced ROS generation (Fig. 1C-D). These findings indicate that Cad99C molecules are lipid raft-associated molecules and that uracil rapidly induces Cad99C clustering in a lipid raft-dependent manner, an event that is required for uracil-induced DUOX activation.
Figure 1.

Cad99C clustering in membrane lipid rafts of the enterocytes is required for DUOX activation.In the case of uracil stimulation, flies were subjected to uracil ingestion (20 nM) for 90 min. (A) Uracil stimulation induces clustering of the lipid raft. Flo-1 was stained as a lipid raft marker. (B) Co-localization of Cad99C and Flo-1 upon uracil stimulation. (C-D) Uracil stimulation induces clustering of Cad99C and DUOX-dependent ROS generation in a lipid raft-dependent manner. Cad99C localization (C) and percentage of ROS-positive intestines (D) were shown in the absence or presence of methyl-β-cyclodextrin (MβCD) (1 mM for 30 min before uracil ingestion). Data were analyzed using an ANOVA followed by Tamhane's T2 post hoc test; the values are expressed as the mean ± SEM (**P < 0.005) of at least 3 independent experiments.
Identification of the role of CaMKII in DOUX activation
To fill the knowledge gap in the uracil-induced intracellular signal transduction pathway, we focused on the protein kinases that play pivotal roles in ligand-induced intracellular signal transduction. Among the uracil-induced transcriptome, 35 kinases were found to be inducible (Fig. 2A). To see whether these kinases are involved in DUOX-dependent host defense, we examined the survival rates of knockdown (KD) animals for different uracil-induced kinases following pathogen infection because animals having impaired uracil-induced DUOX-activating ability (e.g., flies having impaired Hh signaling or impaired calcium signaling) show high rates of host lethality following enteric infection.11 Among 35 uracil-induced kinases, we used KD animals for 16 kinases that were subjected to enteric infection (Fig. 2A). Our mini-screening revealed that only one KD animal having reduced the expression of CaMKII was susceptible to gut infection (Fig. 2B), suggesting that CaMKII, along with previously identified PKCs, is the protein kinase involved in DUOX activation.11 By using quantitative PCR analyses, we could also confirm that uracil stimulation was able to induce the expression of CaMKII in the midgut (Fig. 2C).
Figure 2.

CaMKII is a uracil-induced kinase required for host survival against enteric infection. (A) Identification of the protein kinase genes upregulated by uracil treatment. The details of mRNA-sequencing data were described previously.11 Color bar, gradient of log2-fold-changes at 2 or 16 h following uracil ingestion. Sixteen KD animals indicated by bold letters were subjected to gut infection experiments. (B) CaMKII is required for host survival against gut infection. The host survival rate following gut infection with E. carotovora is shown. A log rank analysis on the Kaplan-Meier data showed a significant difference in survival between control flies and CaMKII-KD flies. (C) CaMKII is induced following uracil ingestion. The expression levels of CaMKII were analyzed in anterior midguts obtained from adult flies following uracil ingestion (20 nM for 16 h). Target gene expression in the untreated control flies was taken arbitrarily as 1, and the results were shown as the relative levels of expression. Comparisons of 2 samples were made by Student's t-test; the values were expressed as the mean ± SEM (*P < 0.05) of at least 3 independent experiments.
To determine whether the high infection-induced lethality observed in CaMKII-KD flies was due to impaired DUOX-dependent ROS generation, we measured uracil-induced ROS generation in these animals. We found that CaMKII-KD animals failed to generate ROS following uracil ingestion (Fig. 3A), indicating that CaMKII is required for DUOX activation. Epistatic analyses further revealed that constitutive ROS generation found in flies carrying constitutive Hh signaling could be abolished in the presence of CaMKII-KD (Fig. 3B). These results indicate that CaMKII acts as a downstream component of Hh signaling.
Figure 3.
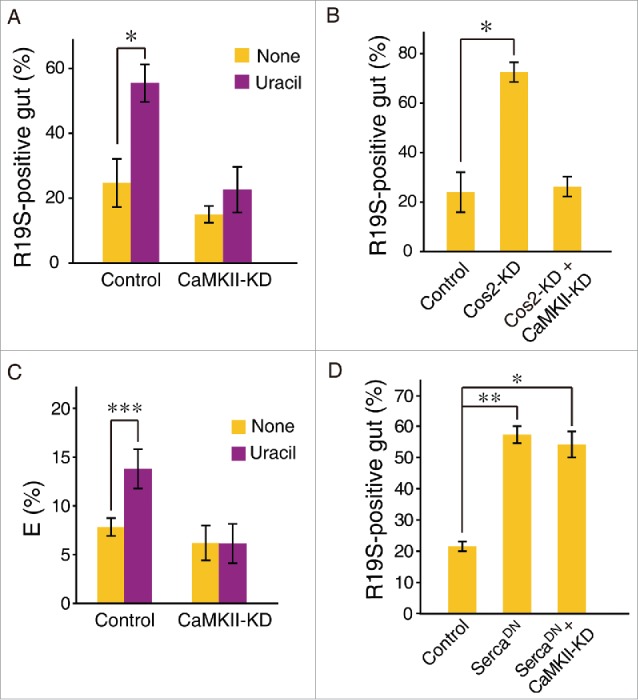
CaMKII acts as a downstream component of Hh-Cad99C, which required for DUOX activity. (A) Uracil-induced DUOX activation was abolished in CaMKII-KD flies. Uracil (20 nM) was administered orally to 3-day-old adult flies for 90 min, and DUOX-dependent ROS production in the midgut was visualized by HOCl−specific R19S dye (orange). Representative confocal microscopic images and the percentage of ROS-positive intestines is shown. (B) Epistatic analyses. Constitutive ROS generation found in flies carrying constitutive Hh signaling (Cos2-KD) can be abolished in the presence of CaMKII-KD. (C) CaMKII is required for uracil-induced Ca2+ mobilization. FRET analysis for the measurement of Ca2+ using flies expressing cameleon calcium sensor was performed. FRET efficiency (E %) was expressed as the mean ± SD from at least 30 flies. (D) Forced calcium increase is sufficient to induce DUOX activation in a CaMKII-KD animals. Flies over-expressing dominant-negative form of sarco/endoplasmic reticulum Ca2+-ATPase (SercaDN) were used. Data in A-D were analyzed using an ANOVA followed by Tukey post hoc test (C) or by Tamhane's T2 post hoc test (A,B and D); values represent mean ± SEM (*P < 0.05, **P < 0.005, ***P < 0.001) of at least three independent experiments.
How does CaMKII regulate DUOX activity? CaMKII is known to be involved in calcium amplification, i.e., it is initially activated by calcium ions released from the endoplasmic reticulum (ER), then activated-CaMKII in turn phosphorylates Ryanodine receptors (RyR, a calcium channel of ER), resulting in increased intracellular calcium ion concentration by maintaining prolonged openings of RyR.14,15 To test this possibility, we first examined uracil-induced calcium in the enterocytes of CaMKII-KD flies. We were unable to detect uracil-induced calcium in CaMKII-KD animals (Fig. 3C), supporting the possibility of calcium amplification by CaMKII. Furthermore, we found that forced cytoplasmic calcium increase by reducing calcium transport from the cytosol to the ER (e.g.,, by overexpressing dominant-negative form of sarco/endoplasmic reticulum Ca2+-ATPase) is sufficient to induce DUOX activation (Fig. 3D). Interestingly, constitutive ROS generation observed in these flies is not affected in the absence of CaMKII activity, suggesting that CaMKII activation is an upstream event that is required to reach maximum level of intracellular calcium concentration necessary for full DUOX activation (Fig. 3D). This result supports the notion in that CaMKII may be involved in calcium amplification. All of these results demonstrate that CaMKII is an essential signaling component for uracil-induced DUOX activation.
Two uracil receptors model versus one uracil receptor model
Uracil-induced Hh signaling operates in a cell-autonomous manner to maintain proper Cad99C expression for DUOX-activating signaling.11 Given that strong Hh/Cad99C signaling activation alone (in the absence of bacteria or uracil) is insufficient to induce DUOX activation and that the presence of bacterial-derived uracil is necessary to induce DUOX activation in these flies, it has been speculated that two separate receptors are involved in uracil recognition16: one that induces Hh signaling and another that acts in PLCβ-dependent calcium mobilization in concert with Cad99C (i.e. GPCR 1 and GPCR 2 in the Fig. 4). In this model, uracil recognition occurs twice via two different receptors: before and after Hh-Cad99C signaling. Although this model enables us to explain the sequential events (i.e., Hh induction, Cad99C induction, and DUOX activation), we propose an alternative activation model in this extra view.
Figure 4.

Two possible models for uracil recognition and uracil-induced DUOX activation. Uracil is recognized either by two different receptors or by a single receptor. See text for additional details.
The alternative model is a one uracil receptor model with Hh signaling as positive feedback for signaling amplification (Fig. 4). In this system, uracil-induced signaling activation consists of two sequential phases: the initial (I) phase and the amplified (A) phase (Fig. 4). In the I phase, uracil is first recognized by a putative uracil receptor that in turn induces signaling endosomes with basally expressed Cad99C molecules. This initial endosome formation may be responsible for a basal level of calcium mobilization as well as a basal level of DUOX-dependent ROS. As intracellular calcium mobilization occurs within a few seconds following uracil stimulation, we believe that all of these events in the I phase occur very rapidly. Considering that the level of calcium and ROS during the I phase is very low and the cellular responses are very rapid, it may be difficult to detect the cellular responses of the I phase using conventional methods. In the I phase, uracil can induce Hh gene expression via a presently unknown signaling pathway, possibly a MEKK1-dependent pathway. The Hh ligand then initiates Hh signaling pathway activation, leading to Ci-dependent Cad99C expression in the apical region of the enterocytes. As the overexpression of Cad99C is sufficient to induce DUOX-dependent ROS generation under conventional gut conditions (where we could find a basal level of uracil that is normally insufficient to induce DUOX-dependent ROS generation),11 this Hh-induced Cad99C expression is an essential event for signal amplification. In the A phase, a high Cad99C level amplifies all of the downstream signaling events, showing enhanced Cad99C clustering and endosome formation as well as higher calcium mobilization for maximum DUOX activation. In our experiment, we believe that we routinely detected these delayed (i.e., approximately 1.5 h following uracil ingestion) and amplified signaling events that occurred in the A phase via our detection methods. As putative uracil receptors are presently unknown, it is unclear whether enterocytes use one or 2 receptors. Future identification of the uracil receptor(s) would clarify the uracil-induced signaling pathways.
Outlook
The discoveries of the DUOX-activating effect of uracil nucleobase via Hh-Cad99C signaling activation raise several interesting questions for future research directions. What are the uracil receptors? What is the molecular mechanism by which uracil induces Hh expression? Are signaling components (or pathways) other than the PKC/PLCβ/Cad99C complex required for uracil-induced endosome formation? What is the mechanism by which the Cad99C-containing endosomes degrade via the endo-lysosomal pathway? Does protocadherin 15, a mammalian homolog of Drosophila Cad99C, have similar roles in DUOX activation in mammalian epithelial cells? Further investigation in this direction will provide a more precise mechanism by which the host animals recognize and control different bacterial species via DUOX regulation in the case of Drosophila and, hopefully, in more complex vertebrate organisms including humans.
Methods
Fly strains
The fly lines used in this study were: w,1118 UAS-Cad99C,17 UAS-cameleon2.1,18 UAS-DUOX-KD,9 Myo1A-GAL4 (NP1-GAL4),19 NP3084-GAL4 (Drosophila Genetic Resource at the National Institute of Genetics, Shizuoka, Japan), All kinase KD flies as well as UAS-SercaDN flies were obtained from Vienna Drosophila Resource Center (Vienna, Austria).
In vivo ROS measurement
HOCl−specific rhodamine-based R19S dye was used to measure DUOX-dependent in vivo ROS generation.20
Immunostaining
Adult flies of different genotypes were orally administered 5% sucrose solution containing uracil (20 nM) for 1.5 hr. The midgut samples were dissected in PBS and then prepared for immunostaining exactly as described previously.11 The samples were incubated with anti-Cad99C antibody (1:500 dilution) or anti-Flo-1 antibody (1:500 dilution; Abcam) for 16 hr at 4°C. The samples were then washed 5 times for 5 min with 0.1% Triton X-100 in PBS, and Alexa Fluor® 568 goat anti-rabbit IgG or Alexa Fluor® 488 goat anti-rabbit or anti-mouse IgG (Invitrogen) was used as the secondary antibody. Following three washes with PBS for 5 min each, the samples were mounted in mounting buffer (Vectorshield, Vector Laboratories Inc.., Burlingame, CA, USA) and analyzed by confocal microscopy LSM 700 (Carl Zeiss, Oberkochen, Germany). Nuclear staining was performed with DAPI.
Real-time qPCR analysis
Fluorescence real-time PCR was performed to quantify gene expression, using the double-stranded DNA dye, SYBR Green (Perkin Elmer, Waltham, MA, USA). Primer pairs for CaMKII (sense, 5′-CGA CCA GAA ATT TTT CGA GC-3′; antisense, 5′- TGT ATC CAT CAA AGT CGC CA-3′) the control, Rp49 (sense, 5′-AGA TCG TGA AGA AGC GCA CCA AG-3′; antisense, 5′-CAC CAG GAA CTT CTT GAA TCC GG-3′) were used to detect target gene transcripts.
FRET analysis for Ca2+measurement
The dissected guts of flies expressing cameleon calcium sensor were fixed and plated onto coverslips for FRET analysis using a LSM700 Confocal Microscope (Carl Zeiss, Germany). FRET analysis by the acceptor bleaching method was exactly performed as described previously.21
Gut infection and Survival experiment
Adult male flies (5–6-day-old) were used for gut infection experiment using E. carotovora exactly as described previously.11
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Creative Research Initiative programs (NRF-2015R1A3A2033475) of the National Research Foundation of the Ministry of Science, ICT, and Future Planning of Korea. K.-A. Lee was supported by Basic Science Research Program (NRF-2013R1A1A2013219).
References
- 1.Poltorak A, He XL, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al.. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998; 282:2085-8; PMID:9851930; http://dx.doi.org/ 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol 2007; 25:697-743; http://dx.doi.org/ 10.1146/annurev.immunol.25.022106.141615 [DOI] [PubMed] [Google Scholar]
- 3.Lee WJ, Brey PT. How Microbiomes Influence Metazoan Development: Insights from History and Drosophila Modeling of Gut-Microbe Interactions. Annu Rev Cell Dev Bi 2013; 29:571-92; PMID:23808845; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122333 [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837-48; http://dx.doi.org/ 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 5.McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al.. Animals in a bacterial world, a new imperative for the life sciences. P Natl Acad Sci USA 2013; 110:3229-36; http://dx.doi.org/ 10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Lee WJ. Role of DUOX in gut inflammation: lessons from Drosophila model of gut-microbiota interactions. Front Cell Infect Mi 2014; 3:116; PMID:24455491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janeway CA, Medzhitov R. Innate immune recognition. Annual Rev Immunol 2002; 20:197-216; http://dx.doi.org/ 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 8.Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, Kim MJ, Kwon Y, Ryu JH, Lee WJ. Bacterial-Derived Uracil as a Modulator of Mucosal Immunity and Gut-Microbe Homeostasis in Drosophila. Cell 2013; 153:797-811; http://dx.doi.org/ 10.1016/j.cell.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 9.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science 2005; 310:847-50; http://dx.doi.org/ 10.1126/science.1117311 [DOI] [PubMed] [Google Scholar]
- 10.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nature Immunol 2009; 10:949-U19; http://dx.doi.org/ 10.1038/ni.1765 [DOI] [PubMed] [Google Scholar]
- 11.Lee KA, Kim B, Bhin J, Kim DH, You H, Kim EK, Kim SH, Ryu JH, Hwang D, Lee WJ. Bacterial Uracil Modulates Drosophila DUOX-Dependent Gut Immunity via Hedgehog-Induced Signaling Endosomes. Cell Host Microbe 2015; 17:191-204; http://dx.doi.org/ 10.1016/j.chom.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009; 45:625-33; PMID:19324409; http://dx.doi.org/ 10.1016/j.ceca.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Bio 2000; 1:31-9; http://dx.doi.org/ 10.1038/35036052 [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, Brown JH. The delta(C) isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circulation Res 2003; 92:912-9; http://dx.doi.org/ 10.1161/01.RES.0000069686.31472.C5 [DOI] [PubMed] [Google Scholar]
- 15.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Brown JH, Bers DM, et al.. Increased sarcoplasmic reticulum calcium leak but unaltered Contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circulation Res 2006; 98:235-44; http://dx.doi.org/ 10.1161/01.RES.0000200739.90811.9f [DOI] [PubMed] [Google Scholar]
- 16.Bier E, Nizet V. Hedgehog: Linking Uracil to Innate Defense. Cell Host Microbe 2015; 17:146-8; http://dx.doi.org/ 10.1016/j.chom.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Schlichting K, Wilsch-Brauninger M, Demontis F, Dahmann C. Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells (vol 119, pg 1184, 2006). J Cell Sci 2006; 119:1197; http://dx.doi.org/ 10.1242/jcs.02831 [DOI] [PubMed] [Google Scholar]
- 18.Fiala A, Spall T. In vivo calcium imaging of brain activity in Drosophila by transgenic cameleon expression. Sci STKE 2003; 2003:PL6. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 2009; 136:483-93; PMID:19141677; http://dx.doi.org/ 10.1242/dev.026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Lee KA, Ha EM, Lee KM, Seo YY, Choi HK, Kim HN, Kim MJ, Cho CS, Lee SY, et al.. A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem Commun (Camb) 2011; 47:4373-5; PMID:21399827; http://dx.doi.org/ 10.1039/c1cc10589b [DOI] [PubMed] [Google Scholar]
- 21.Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the G α q-Phospholipase C β-Ca2+ Pathway in Drosophila Gut Immunity. Dev Cell 2009; 16:386-97; http://dx.doi.org/ 10.1016/j.devcel.2008.12.015 [DOI] [PubMed] [Google Scholar]


