Abstract
The aim of this study was to determine aztreonam (ATM) membrane permeability using Calu-3 cells and its plasma and pulmonary epithelial lining fluid (ELF) pharmacokinetics in rats after intratracheal nebulization and intravenous administration (15 mg · kg−1). ATM exhibits low Calu-3 permeability (0.07 ± 0.02 × 10−6 cm · s−1), and a high area under the ELF/unbound plasma concentration time curve between 0 and infinity (AUCELF/AUCu,plasma) ratio of 1,069 was observed after nebulization in rats. These results confirm that ATM is a low-permeability molecule and a good candidate for nebulization.
TEXT
Antibiotic inhalation is frequently combined with systemic administration to treat lung infections (1). The rational for inhaled antibiotics is to obtain high concentrations at the target site (i.e., lungs) and low systemic concentrations and therefore limited side effects (2). Three antibiotics are currently available for pulmonary administration: tobramycin (TOB), colistin (CST) administered as colistin methansulfonate (CMS), and aztreonam (ATM) (2). We have previously shown in healthy rats that with TOB and CST, much higher concentrations were obtained within the lungs after nebulization (NEB) than after intravenous (i.v.) administration (3, 4). The aim of this study was to confirm these results with ATM, using the same standardized protocol.
ATM for parenteral administration was used for all experiments (1 g Azactam; Sanofi Aventis). The membrane apparent permeability (Papp) was measured by in vitro transepithelial transport experiments across Calu-3 monolayers (5, 6). Briefly, cells were cultured on Transwell inserts (Corning) for 15 days, and on the day of the experiment, they were incubated in the presence of 250 μg · ml−1 ATM in the donor compartment. After 60 min of incubation, the ATM concentration was measured in the acceptor compartment. The Papp measurements were obtained from two different experiments and in triplicate (n = 6). The animal experiments were approved by the local ethics committee (COMETHEA) and registered by the French Ministry of Higher Education and Research (no. 01733.01). Briefly ATM was administered under anesthesia at a dose close to 15 mg · kg−1, either by an i.v. bolus in the tail vein (1 ml) or by intratracheal nebulization (NEB) (100 μl, MicroSprayer model 1A-1B; Penn-Century, Wyndmoor, PA, USA) (7) in two groups of rats (male Sprague-Dawley rats; n = 27, 307 ± 15 g for the i.v. group and n = 26, 302 ± 15 g for the NEB group; Janvier Laboratories, Le Genest-Saint-Isle, France). Bronchoalveolar lavage (BAL) fluid and blood samples were collected at 0.25, 0.5, 1, 2.5, and 4 h after administration (4 to 7 rats per sampling time) in both groups. Assays were conducted by liquid chromatography-tandem mass spectroscopy (LC-MS/MS). The system included an Agilent high-performance liquid chromatography (HPLC) system module (HP1100; Agilent Technologies, Les Ulis, France) coupled with an API 3000 mass spectrometer (Sciex, Les Ulis, France). An XBridge C18 column (5.0 μm, 150 by 2.1 mm inside diameter [ID]; Waters, Saint-Quentin en Yvelines, France) was used, and a mobile phase composed of 2 mM ammonium acetate and acetonitrile (75:25 [vol/vol]) was delivered isocratically at 0.2 ml · min−1. The mass spectrometer was operated in the positive mode. Ions were analyzed by multiple reaction monitoring (MRM). The transitions were m/z 436.1/313 for ATM and 442.1/313 for its deuterated internal standard. The same standard curves were performed for BAL fluid and plasma samples (5, 3.5, 2, 0.5, 0.2, 0.05, 0.02, and 0 μg · ml−1). Four levels of concentrations (0.02, 0.05, 0.5, and 5 μg · ml−1) were tested for intraday variability with precision and accuracy of <10% (n = 18 per medium). The between-day variability was studied at 0.05, 0.5, and 3.5 μg · ml−1 with a precision and a bias of <10% (n = 6). The urea concentrations in plasma and BAL fluid samples were measured as previously described (7). The epithelial lining fluid (ELF) antibiotic concentrations were calculated from the measured BAL fluid concentrations after correction by the dilution of urea in BAL fluid compared with that in plasma (7). S-ADAPT software (8) was used to simultaneously analyze the concentrations versus time concentrations of ATM in plasma and ELF. The final structural pharmacokinetic (PK) model was similar to the one used previously for TOB (3) except that the ATM plasma concentrations versus times were described by two compartments. Only the unbound plasma concentration of ATM was assumed to distribute into lung compartments, and the unbound fraction in plasma was fixed at 48.5% (9). The area under the unbound plasma (AUCu,plasma) and ELF concentration time curve between 0 and infinity (AUCELF) and the elimination half-lives for plasma (t1/2,plasma) and ELF (t1/2,ELF) were derived from the model (Berkeley Madonna, version 8.3.18; University of California). Statistical comparisons were conducted with Prism 5 (GraphPad, La Jolla, CA, USA).
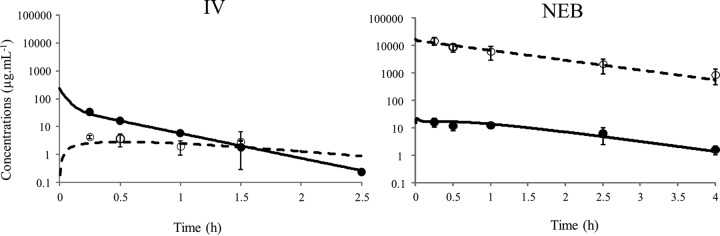
The ATM Papp in Calu-3 monolayers was estimated to be 0.07 ± 0.02 × 10−6 cm · s−1, which is relatively low but close to the values found for CST and TOB (Table 1) (3, 4) and lower than those estimated under similar experimental conditions for ciprofloxacin (CIP) and moxifloxacin (MOX) (10). The ATM total plasma maximum concentrations derived from the model were significantly lower after NEB than after i.v. administration (16.2 ± 5.5 μg · ml−1 versus 34.3 ± 6.6 μg · ml−1 at t = 0.5 h) (P < 0.05; Mann-Whitney test), and the t1/2,plasma was almost 3 times higher after NEB than after the i.v. bolus (t1/2,plasma,NEB = 0.98 h versus t1/2,plasma,i.v. = 0.34 h), suggesting that ATM absorption from the lung is the elimination rate-limiting step (Fig. 1). The ratio of the plasma AUCs after NEB and i.v. administrations was 1.09, indicating that with the Penn-Century system the dose administered was fully delivered and then absorbed systemically. The ATM ELF concentrations were much higher after NEB than after the i.v. bolus at the same dose (Fig. 1), with an AUCELF/AUCu,plasma ratio that was approximately 2,673 times higher (1,069 versus 0.39) (Table 1). The AUCELF/AUCu,plasma ratio being lower than unity after the i.v. bolus (Table 1) suggests that ATM could be the substrate of a transporter, limiting its penetration within ELF after systemic administration, although to our knowledge, no such transporters have been described, but it could also be that ATM slowly distributes into the ELF. This low AUCELF/AUCu,plasma ratio after i.v. administration also differentiates ATM from TOB and CST (Table 1). However, this ratio is noticeably close to 1,000 for ATM after NEB, consistent with the value obtained with CST (1,214) (4) and several fold higher than that obtained with TOB (230) (3), for reasons that still need to be explained (Table 1). Yet apart from these differences, ATM, CST, and TOB essentially present similarities. They are hydrophilic (low log P) with low permeability (low Papp), leading to mostly similar biopharmaceutical characteristics after nebulization (Table 1). Therefore, together with the two previous studies conducted under similar experimental conditions (3, 4), this new investigation provides a biopharmaceutical rational for using ATM, CST, and TOB as aerosols for the treatment of pulmonary infections, consistent with the current practice. Complementary experiments are now being conducted with other compounds presenting different characteristics in terms of solubility and permeability that will constitute the basis of a biopharmaceutical classification of nebulized antimicrobial agents.
TABLE 1.
Molecular weight, lipophilicity (log P), in vitro permeability across Calu-3 cells (Papp), and lung distribution in rats after NEB and i.v. administrations (AUCELF/AUCu,plasma) for ATM, COL, and TOB
FIG 1.
Predicted concentration-time profiles of ATM (15 mg · kg−1) in plasma (solid line) and in ELF (dashed line) samples from simultaneous PK modeling after i.v. and NEB administrations. Closed and open symbols represent, respectively, the means ± standard deviations (SD) of experimental concentrations in plasma (total) and in ELF.
ACKNOWLEDGMENT
We thank Hélène Fayard for her technical assistance in this study.
REFERENCES
- 1.Flume PA, VanDevanter DR. 2015. Clinical applications of pulmonary delivery of antibiotics. Adv Drug Deliv Rev 85:1–6. doi: 10.1016/j.addr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Trigkidis KK, Vardakas KZ. 2015. Inhaled antibiotics beyond aminoglycosides, polymyxins and aztreonam: a systematic review. Int J Antimicrob Agents 45:221–233. doi: 10.1016/j.ijantimicag.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Marchand S, Gregoire N, Brillault J, Lamarche I, Gobin P, Couet W. 2015. Biopharmaceutical characterization of nebulized antimicrobial agents in rats. 3. Tobramycin. Antimicrob Agents Chemother 59:6646–6647. doi: 10.1128/AAC.01647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gontijo AV, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats. 2. Colistin. Antimicrob Agents Chemother 58:3950–3956. doi: 10.1128/AAC.02819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother 53:1457–1462. doi: 10.1128/AAC.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brillault J, De Castro WV, Couet W. 2010. Relative contributions of active mediated transport and passive diffusion of fluoroquinolones with various lipophilicities in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother 54:543–545. doi: 10.1128/AAC.00733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother 54:3702–3707. doi: 10.1128/AAC.00411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. 2011. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J 13:201–211. doi: 10.1208/s12248-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW. 2007. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using Monte Carlo simulation. Antimicrob Agents Chemother 51:3049–3055. doi: 10.1128/AAC.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gontijo AV, Brillault J, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats. 1. Ciprofloxacin, moxifloxacin and grepafloxacin. Antimicrob Agents Chemother 58:3942−3949. doi: 10.1128/AAC.02818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]