Abstract
Ampicillin-ceftriaxone combination therapy has become a predominant treatment for serious Enterococcus faecalis infections, such as endocarditis. Unfortunately, ceftriaxone use is associated with future vancomycin-resistant enterococcus colonization. We evaluated E. faecalis in an in vitro pharmacodynamic model against simulated human concentration-time profiles of ampicillin plus ceftaroline, cefepime, ceftriaxone, or gentamicin. Ampicillin-cefepime and ampicillin-ceftaroline demonstrated activities similar to those of ampicillin-ceftriaxone against E. faecalis.
TEXT
Enterococcus faecalis is one of the most common causes of infective endocarditis in hospitalized and/or immunocompromised patients. The combination regimen of ampicillin plus ceftriaxone has averted high-level aminoglycoside resistance (HLAR) and improved the safety profile in E. faecalis endocarditis treatment over the traditional regimen of ampicillin plus gentamicin (1–4). Accordingly, ampicillin and ceftriaxone were recently added as an option to treat both HLAR and non-HLAR E. faecalis endocarditis, according to national guidelines (5). While this regimen has increased safety for patients with serious E. faecalis infections, it may create long-term collateral damage, as ceftriaxone carries an increased risk of vancomycin-resistant Enterococcus (VRE) gastrointestinal colonization (6, 7). This increase in VRE colonization is likely due to ceftriaxone's high biliary excretion and is associated with increased risk for VRE bacteremia (6–9). Cefepime and ceftaroline are cephalosporins with different spectra of activity, distinct structures, and less biliary excretion; therefore, they should carry less risk of VRE colonization (6, 10). We therefore evaluated the activities of these dual β-lactam combinations that have less potential for VRE colonization in a high-inoculum in vitro pharmacodynamic (IVPD) infection model (6, 10, 11).
(A portion of the results were presented as a poster at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], 8 September 2014, Washington, DC.)
We evaluated two previously described strains of E. faecalis: one ampicillin-susceptible and gentamicin-susceptible strain (OG1X), and a β-lactamase-producing although still ampicillin-susceptible and HLAR gentamicin-resistant strain (HH22) (12, 13). Mueller-Hinton broth (MHB; Becton Dickinson, Sparks, MD, USA), adjusted to 25 mg/liter calcium and 12.5 mg/liter magnesium, was used for in vitro pharmacodynamic models (14). Colony counts were determined using tryptic soy agar (TSA; Difco, Becton Dickinson). Ampicillin (App Pharmaceuticals, Schaumberg, IL), ceftriaxone (Hospira, Lake Forest, IL), cefepime (Sagent Pharmaceuticals, Schaumberg, IL), ceftaroline research powder (Forest Laboratories, New York, NY), and gentamicin (Sigma-Aldrich, St. Louis, MO) were evaluated.
A previously described IVPD model was used to evaluate several antibiotic regimens against enterococci at a high inoculum (∼108 CFU/ml) over 24 h (15, 16). A 250-ml working-volume glass model with inflow and outflow ports controlled by peristaltic pumps was used to achieve desired antibiotic concentrations and half-lives. The regimens simulated free serum concentrations of human doses, including 2 g of ampicillin every 4 h (maximum concentration in serum [Cmax], 150 μg/ml; protein binding, 20%; half-life, 1 h), 2 g of ceftriaxone every 12 h (Cmax, 257 μg/ml; protein binding, 90%; half-life, 6 h), 2 g of cefepime every 12 h (Cmax, 163.9 μg/ml; protein binding, 20%; half-life, 2 h), 600 mg of ceftaroline every 12 h (Cmax, 21.3 μg/ml; protein binding, 20%; half-life, 2.66 h), and 6 mg/kg of body weight of gentamicin every 24 h (Cmax, 24 μg/ml; protein binding, 0%; half-life, 2 h) (17–22). For combination regimens, the rate was set for the drug with the shorter half-life; the drug with the longer half-life was supplemented. The following regimens were tested: ampicillin alone, ceftriaxone alone, cefepime alone, ampicillin plus ceftriaxone, ampicillin plus cefepime, ampicillin plus ceftaroline, ampicillin plus gentamicin, and no antibiotic (growth control).
All model experiments were performed at least in duplicate to ensure reproducibility. Samples were removed from each model at 0, 4, 8, and 24 h. Once removed, samples were serially diluted, plated on TSA, and incubated at 37°C for 24 h before colony count enumeration. The limit of detection for this method is 2.0 log10 CFU/ml. Antimicrobial carryover was minimized by serial dilution (1:10 to 1:10,000) of plated samples in conjunction with vacuum filtration, if needed, as previously described (23).
The MICs of the antimicrobial agents were determined by Etest methodology (Table 1). All samples were incubated at 37°C in ambient air for 24 h. Etests were also used to assess changes in MIC at 24 h to detect resistance.
TABLE 1.
MICs by Etest against two strains of E. faecalis
| Drug | MIC (mg/liter) (CLSI susceptibility)a |
|
|---|---|---|
| OG1X, aminoglycoside susceptible | HH22, high-level aminoglycoside resistant | |
| Ampicillin | 0.19 (S) | 2 (S) |
| Ceftriaxone | >256* | >256* |
| Cefepime | 3* | 16* |
| Ceftaroline | 0.047* | 0.5* |
| Gentamicin | 12 (S) | >500 (R) |
S, susceptible per CLSI guidelines; R, resistant per CLSI guidelines. *, no CLSI breakpoints for cephalosporins against enterococci.
Changes in log10 CFU/ml were plotted to demonstrate reduction by each regimen over 24 h. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/ml reduction and bacteriostatic activity as a <3-log10 CFU/ml change in colony count from the initial inoculum.
Changes in bacterial growth (log10 CFU/ml) at 24 h were compared by analysis of variance with Tukey's post hoc test. A P value of <0.05 was considered significant. All statistical analyses were performed using SPSS statistical software (SPSS 22, Inc., Chicago, IL).
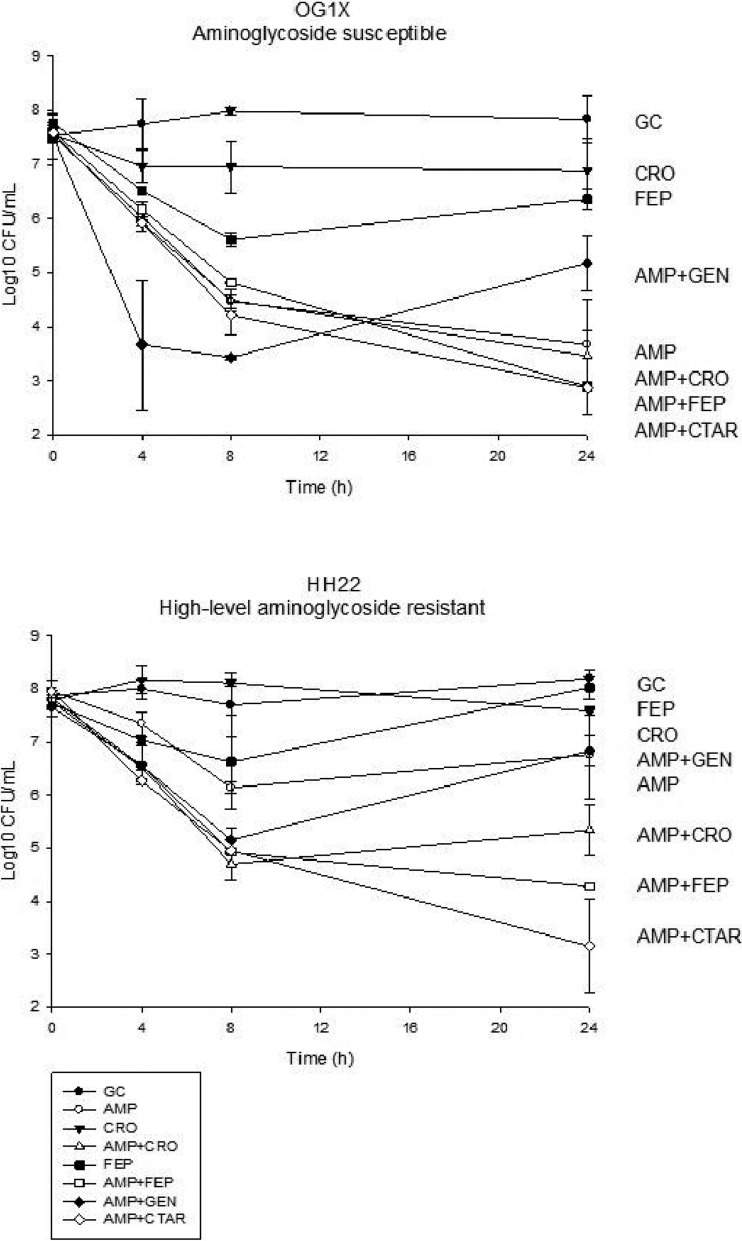
Against both isolates, ampicillin-cefepime and ampicillin-ceftaroline demonstrated greater activity than that of ampicillin-gentamicin at 24 h (mean difference in log10 CFU/ml, 2.29 to 3.69; P ≤ 0.02 for all) (Fig. 1). The activity of ampicillin-ceftriaxone was not significantly different than that of ampicillin-ceftaroline or ampicillin-cefepime. Ampicillin-gentamicin was no more active than ampicillin alone against either strain, likely due to high-level aminoglycoside resistance of HH22 and once-daily gentamicin dosing.
FIG 1.
Activities (mean log10 CFU/ml ± standard deviation) of ampicillin (AMP), ceftriaxone (CRO), cefepime (FEP), ceftaroline (CTAR), gentamicin (GEN), and growth control (GC) regimens against two E. faecalis isolates over 24 h in an in vitro pharmacodynamic model.
Against the gentamicin-susceptible E. faecalis OG1X, ampicillin alone and all ampicillin-cephalosporin combinations demonstrated bactericidal activity. Due to the considerable activity of ampicillin alone, ampicillin-cephalosporin combinations were not significantly more active. Ampicillin-gentamicin demonstrated regrowth at 24 h.
We used a high-dose once-daily gentamicin regimen in this study, similar to that recommended by recent European guidelines for enterococcal endocarditis (24). Previous studies have found no difference in humans or rabbits with gentamicin intervals of once, twice, or thrice daily (25, 26). Of importance, penicillins can accelerate the degradation of aminoglycosides in vitro (41). It remains possible, however, that activity against the gentamicin-susceptible isolate could have been increased by using gentamicin every 8 or 12 h. Additionally, an increase in other antibiotics, such as ceftaroline every 8 h, might increase activity against enterococci, particularly those with higher ceftaroline MICs.
Against our high-level aminoglycoside-resistant (HLAR), β-lactamase-producing ampicillin-susceptible isolate (HH22), ampicillin alone demonstrated bacteriostatic activity, but combinations with cefepime or ceftaroline demonstrated bactericidal activity at 24 h. These ampicillin-cefepime and ampicillin-ceftaroline combinations were more active than ampicillin alone (mean difference in log10 CFU/ml, 2.49; 95% confidence interval [95% CI], 0.46 to 4.52; P = 0.01, and 3.62; 95% CI, 1.59 to 5.65; P = 0.001). Ampicillin-ceftriaxone did not significantly increase activity over that of ampicillin alone but was also not significantly different from the other cephalosporin combinations. Against this HLAR isolate, there was regrowth at 24 h with all monotherapy regimens and ampicillin-gentamicin. Despite regrowth, no increases in MIC were seen with any combination or ampicillin monotherapy. There was no regrowth with ampicillin-cefepime or ampicillin-ceftaroline.
Enterococcal β-lactamase production has a negligible effect on ampicillin MIC at standard testing inocula but raises the MIC when tested at high inocula (27). The significant differences we observed in the activities of ampicillin alone against the two strains in this study are consistent with these prior studies of HH22. Our results are encouraging in that the reduced efficacy of ampicillin against the β-lactamase-producing strain was overcome by the addition of cefepime or ceftaroline. Our study is limited by the use of two strains and the 24-h duration. Additional research will be required before the efficacy of cefepime and ceftaroline against E. faecalis endocarditis in the clinical setting can be determined.
Overall, ampicillin-cephalosporin combinations demonstrated the greatest activity against both strains. Activity with dual-β-lactam therapy may be isolate dependent and likely depends on the isolate's susceptibility to ampicillin alone. While the rate of susceptibility to ampicillin remains at >95% in E. faecalis, up to 60% of bloodstream isolates in the United States in 2010 were gentamicin resistant, necessitating other synergistic treatment options (28–30). The synergy of ampicillin-cephalosporin combinations is thought to be due to complementary penicillin binding protein (PBP) saturation (31). Cephalosporins bind to PBP 2 and 3 at low concentrations, providing total saturation. Ampicillin binds to PBP 4 and 5, inhibiting cell wall synthesis (31). This same mechanism has been shown with amoxicillin-cefotaxime and amoxicillin-ceftriaxone (31, 32). The synergistic effect demonstrated in our study between ampicillin-cefepime and ampicillin-ceftaroline is predictable and increases the possibilities of additional treatment options for enterococcal endocarditis.
Previous studies have associated ceftriaxone use with both VRE colonization and bacteremia (7, 8). The high biliary excretion of ceftriaxone selects for the survival of VRE; this increased colonization does not occur with other cephalosporins that do not undergo significant biliary excretion (6, 7, 9, 11). The high levels of ceftriaxone in the gastrointestinal tract (up to 67% biliary excretion) inhibit colonic microbiota, but due to their intrinsic resistance to cephalosporins (along with ampicillin and vancomycin resistance), VRE growth is left unchecked (7, 33). The complex interactions between colonic flora, innate immunity, antimicrobial spectrum, and gastrointestinal antimicrobial concentration likely all contribute to VRE colonization, but these relationships have not been clearly determined (7, 33). Antienterococcal and antianaerobic activities are important for VRE colonization, but VRE expansion does not always correlate with the numbers of anaerobes present (10, 34). Both gut anaerobes and Gram-negative bacteria interact with VRE growth and the immune regulation of VRE (34, 35). In hospitalized patients, rates of VRE acquisition can be as high as 41% (30). Bacteremia with HLAR enterococci, which has demonstrated increased mortality over that with non-HLAR bacteremia, was also associated with previous third-generation cephalosporin use, likely ceftriaxone (36).
Cefepime carries less risk of promoting VRE colonization in animals and has not been associated with VRE in humans (6, 10). This may be due to the low biliary excretion (∼95% renal excretion) and lack of antianaerobic activity (10, 19). The narrower spectrum of activity of ceftaroline, coupled with its renal excretion and high activity in this study, make ampicillin-ceftaroline a promising combination for E. faecalis endocarditis. Despite intrinsic resistance, ceftaroline monotherapy has demonstrated in vitro and in vivo activity against E. faecalis (37). The ampicillin-ceftaroline combination has previously demonstrated synergy against E. faecalis in an in vitro time-kill study (38). Further study of VRE colonization with ceftaroline is needed, as the antianaerobic activity is ∼4- to 8-fold greater than that of ceftriaxone, but biliary excretion is lower (∼6% excreted in feces) (39, 40).
In our study, ampicillin-cephalosporin combinations demonstrated the most activity against both strains of E. faecalis over 24 h. Ampicillin-cefepime and ampicillin-ceftaroline significantly increased activity over that of ampicillin alone for one strain. Dual-β-lactam regimens should be investigated further, not only for activity, but also with regard to colonization and infection with vancomycin-resistant enterococci.
ACKNOWLEDGMENTS
We thank Kayla Babcock and Thomas Rylah for laboratory assistance. Ceftaroline research powder was provided by Forest Laboratories, Inc.
The research reported in this publication was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant 2P20GM103430.
This material is the result of work supported with resources at the Providence Veterans Affairs Medical Center.
The content of this paper does not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Megan K. Luther declares research funding from Pfizer and Cubist. Louis B. Rice declares no conflicts of interest. Kerry L. LaPlante declares research funding, an advisory position, and/or consultancy with Merck (Cubist), Allergan (Forest), Cempra, Melinta, The Medicines Company, Bard/Davol, Marvao Medical, and Pfizer.
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Hidalgo N, Almirante B, Gavaldà J, Gurgui M, Peña C, de Alarcón A, Ruiz J, Vilacosta I, Montejo M, Vallejo N, López-Medrano F, Plata A, López J, Hidalgo-Tenorio C, Gálvez J, Sáez C, Lomas JM, Falcone M, de la Torre J, Martínez-Lacasa X, Pahissa A. 2013. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcus faecalis infective endocarditis. Clin Infect Dis 56:1261–1268. doi: 10.1093/cid/cit052. [DOI] [PubMed] [Google Scholar]
- 3.Gavaldà J, Len O, Miró JM, Muñoz P, Montejo M, Alarcon A, de la Torre-Cisneros J, Peña C, Martinez-Lacasa X, Sarria C, Bou G, Aguado JM, Navas E, Romeu J, Marco F, Torres C, Tornos P, Planes A, Falco V, Almirante B, Pahissa A. 2007. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med 146:574–579. doi: 10.7326/0003-4819-146-8-200704170-00008. [DOI] [PubMed] [Google Scholar]
- 4.Pericas JM, Cervera C, Del Rio A, Moreno A, Garcia de la Maria C, Almela M, Falces C, Ninot S, Castaneda X, Armero Y, Soy D, Gatell JM, Marco F, Mestres CA, Miro JM, Hospital Clinic Endocarditis Study Group. 2014. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 20:O1075–O1083. [DOI] [PubMed] [Google Scholar]
- 5.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 6.Rice LB, Hutton-Thomas R, Lakticova V, Helfand MS, Donskey CJ. 2004. Beta-lactam antibiotics and gastrointestinal colonization with vancomycin-resistant enterococci. J Infect Dis 189:1113–1118. doi: 10.1086/382086. [DOI] [PubMed] [Google Scholar]
- 7.Rice LB, Lakticova V, Helfand MS, Hutton-Thomas R. 2004. In vitro antienterococcal activity explains associations between exposures to antimicrobial agents and risk of colonization by multiresistant enterococci. J Infect Dis 190:2162–2166. doi: 10.1086/425580. [DOI] [PubMed] [Google Scholar]
- 8.McKinnell JA, Kunz DF, Chamot E, Patel M, Shirley RM, Moser SA, Baddley JW, Pappas PG, Miller LG. 2012. Association between vancomycin-resistant enterococci bacteremia and ceftriaxone usage. Infect Control Hosp Epidemiol 33:718–724. doi: 10.1086/666331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. 2000. Effect of parenteral antibiotic administration on the establishment of colonization with vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis 181:1830–1833. doi: 10.1086/315428. [DOI] [PubMed] [Google Scholar]
- 10.Stiefel U, Pultz NJ, Helfand MS, Donskey CJ. 2004. Increased susceptibility to vancomycin-resistant Enterococcus intestinal colonization persists after completion of anti-anaerobic antibiotic treatment in mice. Infect Control Hosp Epidemiol 25:373–379. doi: 10.1086/502408. [DOI] [PubMed] [Google Scholar]
- 11.Laktićová V, Hutton-Thomas R, Meyer M, Gurkan E, Rice LB. 2006. Antibiotic-induced enterococcal expansion in the mouse intestine occurs throughout the small bowel and correlates poorly with suppression of competing flora. Antimicrob Agents Chemother 50:3117–3123. doi: 10.1128/AAC.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ike Y, Craig RA, White BA, Yagi Y, Clewell DB. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A 80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mederski-Samoraj BD, Murray BE. 1983. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis 147:751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.LaPlante KL, Sakoulas G. 2009. Evaluating aztreonam and ceftazidime pharmacodynamics with Escherichia coli in combination with daptomycin, linezolid, or vancomycin in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 53:4549–4555. doi: 10.1128/AAC.00180-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15(Suppl A):125–130. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett JG, Auwaerter PG, Pham PA. 2010. Johns Hopkins POC-IT Center ABX guide: diagnosis & treatment of infectious diseases. Jones and Bartlett Publishers, Burlington, MA. [Google Scholar]
- 18.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20:634–641. doi: 10.1128/AAC.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apotex Corp. 2007. Cefepime for injections, USP for intravenous or intramuscular use. Apotex Corp, Weston, FL: https://www.apotex.com/us/en/products/downloads/pil/cefe_sinj_ins.pdf. [Google Scholar]
- 20.Drusano GL. 2010. Pharmacodynamics of ceftaroline fosamil for complicated skin and skin structure infection: rationale for improved anti-methicillin-resistant Staphylococcus aureus activity. J Antimicrob Chemother 65(Suppl 4):iv33–iv39. [DOI] [PubMed] [Google Scholar]
- 21.McNamara DR, Nafziger AN, Menhinick AM, Bertino JS Jr. 2001. A dose-ranging study of gentamicin pharmacokinetics: implications for extended interval aminoglycoside therapy. J Clin Pharmacol 41:374–377. doi: 10.1177/00912700122010221. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert DN, Moellering RC Jr, Eliopoulos GM. 2012. The Sanford guide to antimicrobial therapy, 42nd ed Antimicrobial Therapy, Inc, Sperryville, VA. [Google Scholar]
- 23.Steed M, Vidaillac C, Rybak MJ. 2011. Evaluation of ceftaroline activity versus daptomycin (DAP) against DAP-nonsusceptible methicillin-resistant Staphylococcus aureus strains in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:3522–3526. doi: 10.1128/AAC.00347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Erol C, Nihoyannopoulos P, Aboyans V, Agewall S, Athanassopoulos G, Aytekin S, Benzer W, Bueno H, Broekhuizen L, Carerj S, Cosyns B, De Backer J, De Bonis M, Dimopoulos K, Donal E, Drexel H, Flachskampf FA, Hall R, Halvorsen S, Hoen B, Kirchhof P, Lainscak M, Leite-Moreira AF, Lip GY, Mestres CA, Piepoli MF, Punjabi PP, Rapezzi C, Rosenhek R, Siebens K, et al. 2015. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 25.Dahl A, Rasmussen RV, Bundgaard H, Hassager C, Bruun LE, Lauridsen TK, Moser C, Sogaard P, Arpi M, Bruun NE. 2013. Enterococcus faecalis infective endocarditis: a pilot study of the relationship between duration of gentamicin treatment and outcome. Circulation 127:1810–1817. doi: 10.1161/CIRCULATIONAHA.112.001170. [DOI] [PubMed] [Google Scholar]
- 26.Gavaldà J, Cardona PJ, Almirante B, Capdevila JA, Laguarda M, Pou L, Crespo E, Pigrau C, Pahissa A. 1996. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob Agents Chemother 40:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray BE, Church DA, Wanger A, Zscheck K, Levison ME, Ingerman MJ, Abrutyn E, Mederski-Samoraj B. 1986. Comparison of two beta-lactamase-producing strains of Streptococcus faecalis. Antimicrob Agents Chemother 30:861–864. doi: 10.1128/AAC.30.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Families. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 29.Patel R, Allen SL, Manahan JM, Wright AJ, Krom RA, Wiesner RH, Persing DH, Cockerill FR, Thompson RL. 2001. Natural history of vancomycin-resistant enterococcal colonization in liver and kidney transplant recipients. Liver Transpl 7:27–31. doi: 10.1053/jlts.2001.20784. [DOI] [PubMed] [Google Scholar]
- 30.Sidler JA, Battegay M, Tschudin-Sutter S, Widmer AF, Weisser M. 2014. Enterococci, Clostridium difficile and ESBL-producing bacteria: epidemiology, clinical impact and prevention in ICU patients. Swiss Med Wkly 144:w14009. [DOI] [PubMed] [Google Scholar]
- 31.Mainardi JL, Gutmann L, Acar JF, Goldstein FW. 1995. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 39:1984–1987. doi: 10.1128/AAC.39.9.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desbiolles N, Piroth L, Lequeu C, Neuwirth C, Portier H, Chavanet P. 2001. Fractional maximal effect method for in vitro synergy between amoxicillin and ceftriaxone and between vancomycin and ceftriaxone against Enterococcus faecalis and penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 45:3328–3333. doi: 10.1128/AAC.45.12.3328-3333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvidsson A, Alvan G, Angelin B, Borga O, Nord CE. 1982. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother 10:207–215. doi: 10.1093/jac/10.3.207. [DOI] [PubMed] [Google Scholar]
- 34.Pultz NJ, Stiefel U, Subramanyan S, Helfand MS, Donskey CJ. 2005. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J Infect Dis 191:949–956. doi: 10.1086/428090. [DOI] [PubMed] [Google Scholar]
- 35.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. 2008. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang HC, Lee S, Song KH, Jeon JH, Park WB, Park SW, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW. 2010. Clinical features, risk factors and outcomes of bacteremia due to enterococci with high-level gentamicin resistance: comparison with bacteremia due to enterococci without high-level gentamicin resistance. J Korean Med Sci 25:3–8. doi: 10.3346/jkms.2010.25.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacqueline C, Caillon J, Le Mabecque V, Miegeville AF, Ge Y, Biek D, Batard E, Potel G. 2009. In vivo activity of a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, ceftaroline, against vancomycin-susceptible and -resistant Enterococcus faecalis strains in a rabbit endocarditis model: a comparative study with linezolid and vancomycin. Antimicrob Agents Chemother 53:5300–5302. doi: 10.1128/AAC.00984-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werth BJ, Abbott AN. 2015. The combination of ampicillin plus ceftaroline is synergistic against Enterococcus faecalis. J Antimicrob Chemother 70:2414–2417. doi: 10.1093/jac/dkv125. [DOI] [PubMed] [Google Scholar]
- 39.Citron DM, Tyrrell KL, Merriam CV, Goldstein EJ. 2010. In vitro activity of ceftaroline against 623 diverse strains of anaerobic bacteria. Antimicrob Agents Chemother 54:1627–1632. doi: 10.1128/AAC.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rymarz A, Brodowska-Kania D, Gomolka M, Jozefczak-Bergier E, Dzierzanowska M, Niemczyk S. 2014. Vancomycin dosing in patients undergoing maintenance hemodialysis. Int Urol Nephrol 46:1681–1682. doi: 10.1007/s11255-014-0707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trissel LA. 2005. Trissel's stability of compounded formulations. American Pharmacists Association, Washington, DC. [Google Scholar]