Abstract
Existing therapies for leishmaniases present significant limitations, such as toxic side effects, and are rendered inefficient by parasite resistance. It is of utmost importance to develop novel drugs targeting Leishmania that take these two limitations into consideration. We thus chose a target-based approach using an exoprotein kinase, Leishmania casein kinase 1.2 (LmCK1.2) that was recently shown to be essential for intracellular parasite survival and infectivity. We developed a four-step pipeline to identify novel selective antileishmanial compounds. In step 1, we screened 5,018 compounds from kinase-biased libraries with Leishmania and mammalian CK1 in order to identify hit compounds and assess their specificity. For step 2, we selected 88 compounds among those with the lowest 50% inhibitory concentration to test their biological activity on host-free parasites using a resazurin reduction assay and on intramacrophagic amastigotes using a high content phenotypic assay. Only 75 compounds showed antileishmanial activity and were retained for step 3 to evaluate their toxicity against mouse macrophages and human cell lines. The four compounds that displayed a selectivity index above 10 were then assessed for their affinity to LmCK1.2 using a target deconvolution strategy in step 4. Finally, we retained two compounds, PP2 and compound 42, for which LmCK1.2 seems to be the primary target. Using this four-step pipeline, we identify from several thousand molecules, two lead compounds with a selective antileishmanial activity.
INTRODUCTION
The protozoan parasite Leishmania is the causative agent of leishmaniasis, a potentially fatal disease with worldwide distribution. Depending on the species, three clinical forms of the disease can be distinguished, cutaneous leishmaniasis (CL; e.g., Leishmania major), mucocutaneous leishmaniasis (MCL; e.g., Leishmania braziliensis), and fatal visceral leishmaniasis (VL; e.g., Leishmania donovani) (1). Several treatment options are available, which either show important side effects or are unaffordable. In all regions where these infections are endemic, the first line of treatment is pentavalent antimonials, despite their important side effects and the appearance of parasite resistance. Although their target has not been identified, one member, sodium stibogluconate was shown to inhibit the energy metabolism and macromolecule biosynthesis (2, 3). The second line of treatment is amphotericin B, a very potent but highly toxic antifungal drug. This compound creates pores targeting ergosterol only present in the plasma membrane of parasites but not in that of mammalian cells. Its less toxic lipid formulation is extremely expensive and thus incompatible with treatment in developing countries (3, 4). Among the other drugs that have been recently developed, miltefosine was a major breakthrough in leishmaniasis therapy as this anticancer drug is the first oral treatment against VL. Unfortunately, its teratogenicity excludes the treatment of pregnant women and its slow turnover could promote the emergence of clinical parasite resistance (4). Miltefosine plays a role in the perturbation of the lipid metabolism and the induction of apoptosis-like cell death and has immunostimulatory effects; however, its mode of action has not been precisely identified (5). The activity of miltefosine is due to its accumulation inside the parasite (6), which is prevented in resistant lines generated in vitro by the overexpression of members of the ABC (ATP-binding cassette) transporter family and/or mutation of the flippase LdMT (6, 7). Three more drugs complete the list of available treatments for leishmaniasis: (i) pentamidine, which has been used for VL, CL, and MCL treatment, induces the inhibition of polyamine biosynthesis and a decrease of the mitochondrial inner membrane potential; (ii) the aminoglycosidic antibiotic paromomycin, which is restricted to areas where such infections are endemic, cures both VL and CL efficiently by targeting mitochondria; and (iii) sitamaquine, an 8-aminoquinoline, which intercalates within biological membranes to accumulate in Leishmania cytosolic acidic compartments (4).
Despite the various drugs available, none of these treatments are ideal because of two main aspects: (i) their side effects, due mainly to off-target effects that cannot be eliminated by drug optimization as the target responsible for the antileishmanial effect is unknown, and (ii) the emergence of parasite resistance, due to the plasticity of the parasite. Therefore, there is an urgent need to discover new molecules and to develop new drug discovery pipelines that take these two aspects into consideration. First, the use of known validated targets for drug screening represents a major advantage as the compound could be optimized to fit exclusively the target, avoiding off-target effects mainly responsible for side effects. Protein kinases are among the best candidates as drug targets for leishmaniasis because: (i) kinase inhibitors are one of the most important group of U.S. Food and Drug Administration-approved drugs for the treatment of diseases such as cancer or Alzheimer's disease; (ii) they are considered valid targets for diseases caused by unicellular parasites, such as malaria; and (iii) kinases regulate many key processes, such as cell cycle or signal transduction, and thus the inhibition of their activity decreases cell viability (8, 9–14). Second, targeting proteins secreted by the parasite could delay the emergence of drug resistance. Indeed, it has been recently shown that parasitic proteins could be exported, via exosomes, into the host cell to modify its biology or its innate immune response (15, 16). To perform their function in the host, these proteins need to interact with host proteins, and thus any mutations could abrogate their functions in the host cell, which could be detrimental for the intracellular parasite survival.
Among the proteins identified in the recent proteomic analysis of Leishmania exosomes, 13 could qualify as good drug targets as defined above because they are excreted kinases. Most of these kinases are involved in purine or glucose metabolism, and only one is involved in signal transduction, casein kinase 1 (CK1). A member of the highly conserved Ser/Thr protein kinase family (17), CK1 contains six isoforms in Leishmania (15, 18–20). LmjF35.1010 (LmCK1.2), the major isoform, has been validated pharmacologically as a drug target based on the findings that the inhibition of CK1 activity by the specific inhibitor D4476 strongly compromises axenic amastigote viability and decreases the percentage of infected macrophages (21). We hypothesize that the capacity of LmCK1.2 to recognize and phosphorylate host proteins could allow the parasite to regulate essential host cell processes (22) and therefore to survive. This hypothesis is based on our previous findings showing that (i) the protein sequence of LmCK1.2 kinase domain is 100% identical in all sequenced Leishmania species (except the lizard-isolated Leishmania tarentolae and unclassified Leishmania sp. strain MAR LEM2494), suggesting that there is a selection pressure to maintain the integrity of the protein sequence, and (ii) LmCK1.2 is the most closely related kinase to its human orthologs in Leishmania. These two elements suggest that LmCK1.2 cannot be mutated without compromising the survival of the intracellular parasite, which would render the emergence of drug-resistant parasites expressing mutated LmCK1.2 unlikely (21).
We present here a four-step pipeline that allows the discovery of novel lead compounds. First, we generated an active recombinant LmCK1.2 and purified mammalian CK1 from porcine brain (SsCK1 [23]). We developed an enzymatic assay to screen 4,030 compounds from kinase-biased and focused libraries, as well as 988 analogs with both Leishmania and mammalian kinases, in order to identify hit compounds and assess their specificity. We selected 88 compounds with a 50% inhibitory concentration (IC50) below 10 μM. Second, we tested the antileishmanial effect of these compounds on cultured parasites using a rezasurin-based assay, as well as on intracellular parasites using a high-content phenotypic screen. We retained 75 compounds with an antileishmanial activity. Third, after evaluation of the toxicity of the selected antileishmanial compounds against mouse macrophages and human cell lines, only four compounds had a selectivity index (SI) greater than 10. Fourth, the affinity for LmCK1.2 for these compounds was tested using a target deconvolution approach. Two compounds for which LmCK1.2 seems to be the primary target were eventually selected. The identification of these two lead compounds validates our pipeline, which will be used to screen diversified libraries to identify more lead compounds.
MATERIALS AND METHODS
L. donovani culture and axenic amastigote differentiation.
L. donovani 1S2D (MHOM/SD/62/1S-CL2D), clone LdB, was obtained from Steve Beverley, Washington University School of Medicine, St. Louis, MO, and cultured as described previously (24–26).
Parasite growth inhibition assay.
L. donovani promastigotes and axenic amastigotes (2 × 106 cells/ml) in their respective media were distributed in 96-well plates (125 μl/well). An equal volume of medium containing inhibitor at the indicated concentrations (in 1% dimethyl sulfoxide [DMSO]) was added. After 24 h of incubation in the dark at 26°C (promastigotes) or 37°C (amastigotes), 25 μl of resazurin solution at 0.001% was added, and the plates were incubated for an additional 24 h in the dark at appropriate temperatures. The plates were read (excitation, 544 nm; emission, 590 nm) using a fluorescent microplate reader (Safas Xenius XML) (27).
Human cell line MTT assay.
HFF1, SH-SY5Y, and U-2 OS cells were cultured in Dulbecco modified Eagle medium (DMEM); hTERT RPE-1 cells were cultured in DMEM/F-12 medium. All media were supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 50 IU of penicillin and streptomycin. Cell viability was assayed using a Promega CellTiter96 AQueous nonradioactive cell proliferation assay according to the manufacturer's instructions.
Macrophage infection and assessment of intracellular parasite survival.
A high-content, biologically relevant, cell-based assay was used to determine the antileishmanial activity of D4476 as previously described (21, 28). Briefly, the assay combines (i) the use of primary bone marrow-derived mouse macrophages as natural host cells and DsRed2-expressing L. amazonensis (MPRO/BR/1972/M1841) amastigotes, the clinically relevant parasite stage of Leishmania, with (ii) the detection of fluorescent markers as reporter molecules. A total of 10,000 macrophages were counted per well depending on the number of replicates per tested compound.
Ethics statement.
All animals were housed in our A3 animal facilities in compliance with the guidelines of the A3 animal facilities at the Institut Pasteur, which is a member of Committee 1 of the Comité d'Ethique pour l'Expérimentation Animale (CEEA), Ile de France, France. Animal housing conditions and the protocols used in the work described here were approved by the Direction des Transports et de la Protection du Public, Sous-Direction de la Protection Sanitaire et de l'Environnement, Police Sanitaire des Animaux, B75-15-27 and B75-15-28, in accordance with the Ethics Charter of animal experimentation that includes appropriate procedures to minimize pain and animal suffering. G.S. and E.P. are authorized to perform experiments on vertebrate animals (licenses B75-1159 and 75-1265, respectively) issued by the Direction Départementale de la Protection des Populations de Paris and were responsible for all experiments conducted personally or under supervision as governed by the laws and regulations relating to the protection of animals.
Automated microtiter plate CK-S kinase assay.
A mixture of native CK1 isoforms (essentially CK1δ and CK1ε) was extracted from porcine brain (SsCK1) and purified by affinity chromatography on immobilized axin (23). LmCK1.2 was produced and purified as previously described (21). Both SsCK1 and recombinant LmCK1.2 were assayed, with 27 μM CK-specific peptide substrate CK-S (RRKHAAIGpSAYSITA) synthesized by Proteogenix (Oberhausbergen, France) in buffer C (pH 7; 60 mM β-glycerophosphate, 30 mM p-nitrophenyl phosphate, 25 mM morpholinepropanesulfonic acid, 5 mM EGTA, 15 mM MgCl2, 1 mM dithiothreitol [DTT], 0.1 mM sodium vanadate), with 15 μM [γ-33P]ATP in a final volume of 30 μl. After 30 min of incubation at 30°C, 30-μl aliquots were filtered onto Whatman P81 phosphocellulose paper. The filters were washed with a solution of 1% phosphoric acid and then counted in the presence of 20 μl of scintillation fluid per well. Blank values were subtracted and activities calculated as the pmol of phosphate incorporated during 30 min of incubation. The activities were expressed as the percentages of maximal activity, i.e., in the absence of inhibitors. Controls were performed with appropriate dilutions of DMSO.
ATP depletion and competition.
The axenic amastigote total protein extract (7 mg) was dialyzed overnight at 4°C in dialysis solution (1× phosphate-buffered saline, 1 mM EDTA, 1 mM DTT) using a Slide-A-Lyzer 10kD dialysis cassette (Pierce) to eliminate free ATP. Then, 1 mg of dialyzed extract per condition was mixed with the binding solution (1× binding solution, 1 mM DTT, 1× protease inhibitor) of the ATP affinity test kit (Jena Bioscience) and 500 μM inhibitor. The samples were incubated 30 min at 4°C and added to a mixture containing 12.5 μl of each ATP agarose (ATP affinity test kit from Jena Bioscience). ATP binding proteins from the assay and competition samples were enriched according to the manufacturer's instructions. Finally, eluted samples were concentrated using Amicon Ultra-10K centrifugal filters (Millipore) to a final volume of approximately 100 μl.
Next, 12.5 μl of the flowthrough and 30 μl of the eluate were separated on Novex NuPAGE 4 to 12% Bis-Tris gel (Life Technologies) from both the assay and the competition. The gel was stained with SYPRO Ruby (Life Technologies) according to the manufacturer's instructions and revealed using a Typhoon scanner. Alternatively, the proteins separated by SDS-PAGE were transferred onto a polyvinylidene difluoride membrane and probed with SY3535 antibody (21). Signals were revealed by SuperSignal-ECL (Pierce).
Compound libraries.
We screened 5,018 compounds from the Roscoff library including 588 purine derivatives (29) and 400 indirubin derivatives (30, 31, 32).
Homology modeling and structural alignment.
The amino acid sequence of L. major CK1.2 was retrieved from the NCBI database (accession number XP_003722496) in FASTA format. The homology modeling of the sequence was performed by Swiss-Model program (33), and the protein with PDB code 3SV0 was selected as a template. The PyMOL program (34) was used for the structural alignment of Schizosaccharomyces pombe CK1 in complex with the specific CK1 inhibitor, IC261 (PDB code 1EH4) and Homo sapiens CK1δ (PDB code 4KB8) to the generated L. major CK1.2 homology model. The figures were also prepared using PyMOL (34).
RESULTS
We present below a comprehensive drug discovery pipeline encompassing four steps.
Primary screening comparing SsCK1 and LmCK1.2 to identify specific LmCK1.2 inhibitors.
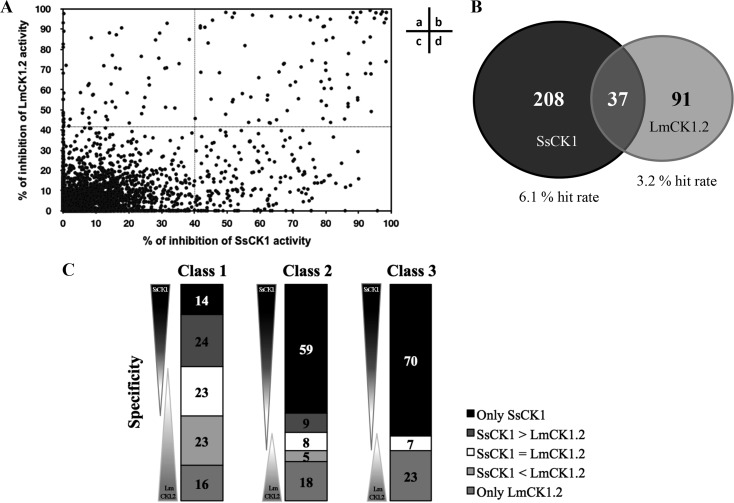
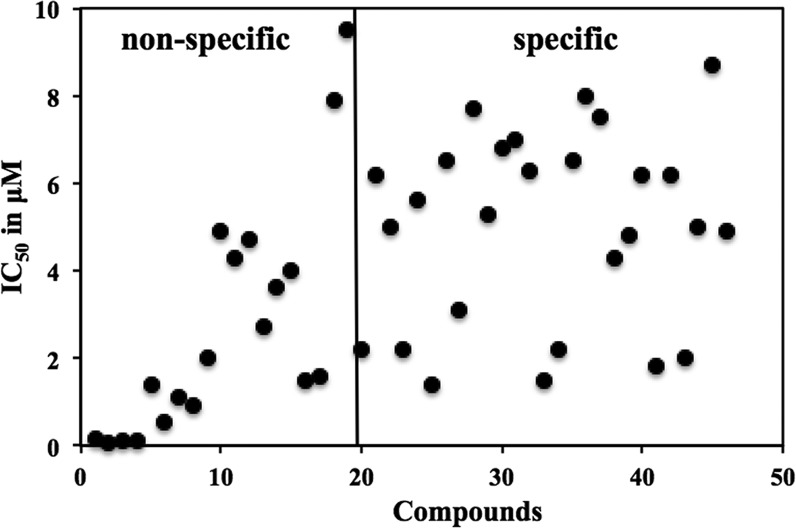
Primary screening comparing SsCK1 and LmCK1.2. We purified recombinant LmCK1.2-V5-His6 from Escherichia coli and a mixture of Sus scrofa CK1δ and CK1ε (SsCK1) from porcine brain (21, 23). The conditions used previously for the manual kinase assay were adapted to an automated 96-well plate format (21, 23). We used CK-S as the substrate for both kinases and 15 μM [γ-33P]ATP for the assay (21, 23). We screened 4,030 compounds at 10 μM from a kinase-biased library that has been previously tested on mammalian kinases, such as cyclin-dependent kinases (29). For each compound, the percent inhibition of LmCK1.2 versus that of SsCK1 is presented in Fig. 1A. We classified as hit compounds those that decreased the kinase activity by >40% (Fig. 1Aa and b). We obtained twice as many hit compounds for the mammalian CK1 than for LmCK1.2 (Fig. 1B). Indeed, we identified 245 hit compounds against SsCK1 (6.1% hit rate) and 128 against LmCK1.2 (3.2% hit rate), with only 37 compounds with a similar potency against both kinases. This finding, which is surprising considering the high similarity between the protein sequences of LmCK1.2 and the mammalian CK1s (ca. 70% [21]), suggests that the ATP binding pocket of the two kinases is sufficiently divergent to accommodate different inhibitors. We next classified the compounds according to their potency and specificity (class 1, compounds that inhibit the kinase activity between 80 and 100%; class 2, compounds that inhibit the kinase activity between 60 and 80%; and class 3, compounds that inhibit the kinase activity between 40 and 60%; Fig. 1C). We obtained a similar number and distribution of hit compounds active on SsCK1 (30 compounds) and on LmCK1.2 (31 compounds) in class 1. In contrast, we observed an increased number of compounds inhibiting specifically SsCK1 in classes 2 and 3 (Fig. 1C). This could suggest that the ATP binding pocket of SsCK1 could be more permissive than that of LmCK1.2. We selected 45 compounds either belonging to class 1 that inhibited LmCK1.2 activity by >90% or belonging to class 2 and were specific to LmCK1.2 to determine their IC50. As shown in Fig. 2, all the compounds with an IC50 below 1.3 μM (most potent) were nonspecific, whereas all the specific compounds had an IC50 above 1.3 μM.
FIG 1.
Differential target-based screen of 4,030 compounds from various libraries. (A) Representation of the percentage of inhibition toward LmCK1.2 activity versus the percentage of inhibition toward SsCK1 activity. The compounds in sectors a and b are potent toward LmCK1.2 since they show more than 40% inhibition, whereas the compounds in sectors b and d are potent toward SsCK1. (B) A total of 336 hit compounds were identified in the screen, of which 245 inhibit SsCK1 (6.1% hit rate) and 128 inhibit LmCK1.2 (3.2% hit rate). Only 37 compounds showed equal potency against both CK1s. (C) Compounds were classified according to their specificity: compounds only potent against SsCK1 (only SsCK1), more potent against SsCK1 than LmCK1.2 (SsCK1 > LmaCK1.2), equally potent on both kinases (SsCK1 = LmCK1.2), more potent against LmCK1.2 than SsCK1 (SsCK1 < LmaCK1.2), and only potent on LmCK1.2 (only LmCK1.2). Compounds were also classified according to their percent inhibition: class 1 corresponds to compounds that inhibit the kinase activity between 80 and 100%, class 2 corresponds to compounds that inhibit the kinase activity between 60 and 80%, and class 3 corresponds to compounds that inhibit the kinase activity between 40 and 60%. A total of 23 compounds were more potent toward LmCK1.2 than SsCK1, and 68 compounds were specific to LmCK1.2 (the numbers in the histograms indicate the percentage of compounds in each category).
FIG 2.
Determination of the IC50 of the 45 compounds belonging to class 1 that have a percent inhibition above 90%. Each point represents the IC50 of a particular compound toward LmCK1.2. Nonspecific compounds have a potency below 10 μM toward both kinases, whereas specific compounds have a potency below 10 μM only toward LmCK1.2.
Among the 45 compounds that were potent against LmCK1.2, we identified several inhibitors described to have antileishmanial activity, including known CK1 inhibitors, such as anthraquinone (35–37), or compounds for which we revealed CK1 as a new target, such as gossypol, purpurogallin, and some flavonoids (38–42, 43). These compounds, identified from several libraries, were found at least twice with similar IC50s, indicating that our assay is reproducible (data not shown). Altogether, these data demonstrate the efficiency of using CK1 as a target to identify compounds with antileishmanial activities and confirm LmCK1.2 as a valid drug target. However, we did not retain these compounds for subsequent characterization since they have been already extensively studied.
Secondary screening of purine and indirubin libraries.
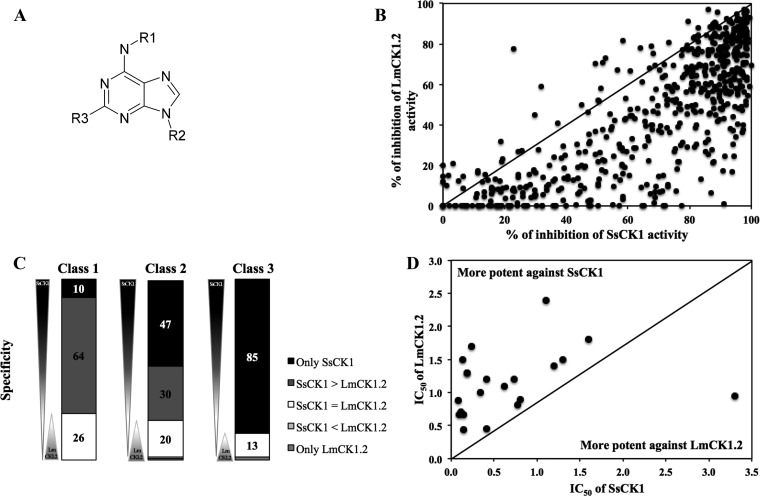
Of the 45 compounds for which we determined the IC50, we selected two compounds with low IC50s but only moderate specificity toward LmCK1.2, purvalanol B, and indirubin-3′-monoxime, and we tested analog libraries to perform structure-activity relationship (SAR) analysis in order to identify more specific compounds. We chose purvalanol B because we showed previously that Leishmania CK1.2 binds to purvalanol B better than its mammalian counterpart, suggesting that the sensitivity to purvalanol B could be higher for LmCK1.2 than for SsCK1 (44). Confirming this finding, the IC50 of purvalanol B toward LmCK1.2 (2 ± 0.3 μM) is slightly lower than that toward SsCK1 (2.9 ± 1.2 μM). We tested the potency of 588 purine analogs (Fig. 3A) at 10 μM against LmCK1.2 and SsCK1 to identify compounds with better potency and/or specificity. As shown in Fig. 3B and C, most compounds were more potent against mammalian CK1 than against LmCK1.2 (below the black line in Fig. 3B and dark gray in Fig. 3C). Next, we determined the IC50s of the 21 most potent purine derivatives against LmCK1.2 (Fig. 3D). For all of the compounds except one the IC50 was systematically higher against LmCK1.2 (0.44 to 2.4 μM) than against mammalian CK1 (0.081 to 1.6 μM). Only compound 26 had a lower IC50 against LmCK1.2 (1 ± 0.4 μM) than against SsCK1 (3.7 ± 1.2 μM), which could be due to the presence of a long carbon chain, a unique feature compared to the other derivatives. Thus, although the purines were very potent toward LmCK1.2, they present a higher affinity for SsCK1.
FIG 3.
Screening of the purine derivative library. (A) Structure of the purine backbone. R1, R2, and R3 represent different substitutions of the purines. (B) We performed a target-based screening of 588 derivatives. Each point represents the percent inhibition toward LmCK1.2 activity versus the percent inhibition toward SsCK1 activity of each compound. The compounds in the top left are more potent toward LmCK1.2, whereas the compounds in the bottom right are more potent toward SsCK1. (C) Compounds were classified according to their specificity: only potent on SsCK1, more potent on SsCK1 than LmCK1.2 (SsCK1 > LmCK1.2), equally on both kinases (SsCK1 = LmCK1.2), more potent on LmCK1.2 than SsCK1 (SsCK1 < LmCK1.2), and only potent on LmCK1.2. Compounds were also classified according to their % of inhibition: class 1 corresponds to compounds that inhibit kinases between 80 and 100%, class 2 corresponds to compounds that inhibit kinases between 60 and 80%, and class 3 corresponds to compounds that inhibit kinases between 40 and 60%. Only 4% of the compounds are more potent toward LmCK1.2 than SsCK1 or specific to LmCK1.2. (D) We determined the IC50 of the 21 compounds belonging to class 1 that have a percent inhibition above 90%. Each point represents the IC50 of a particular compound toward LmCK1.2 versus SsCK1. The IC50 values are lower toward SsCK1 than LmCK1.2.
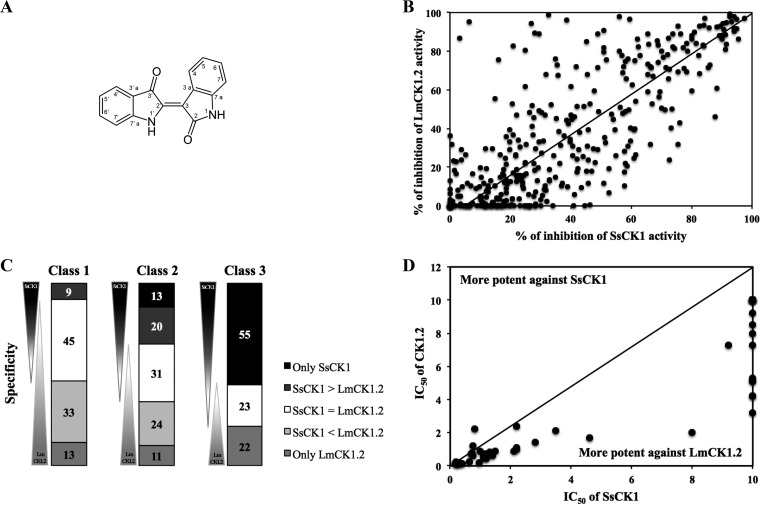
The second family of compounds we screened are the indirubins (Fig. 4A). We showed that the IC50 of indirubin-3′-monoxime is lower against LmCK1.2 (0.13 ± 0.03; see Table S1 in the supplemental material) than against mammalian CK1 (0.39 ± 0.08 μM; see Table S1 in the supplemental material). To identify better compounds with higher selectivity, we tested 400 indirubin derivatives (Fig. 4B). In contrast to the purine analogs, the indirubins were globally more potent against LmCK1.2 than against SsCK1 (Fig. 4B). For instance, in class 1 a total of 46 compounds were more specific toward LmCK1.2 than SsCK1, whereas only 9 were more specific toward SsCK1 than LmCK1.2 (Fig. 4C). Indeed, the most active compounds were the most selective toward LmCK1.2, whereas compounds with lower potency (class 3) were more specific toward SsCK1 (Fig. 4C). We selected the 55 most potent compounds to measure their IC50s. The IC50s were between 0.08 and 10 μM, and for almost all the compounds the IC50 against LmCK1.2 was systematically lower than that against mammalian CK1 (Fig. 4D), suggesting that the indirubin compounds have more affinity toward LmCK1.2 than toward SsCK1.
FIG 4.
Screening of the indirubin derivative library. (A) Structure of the indirubin backbone. (B) Target-based screening of 400 derivatives. Each point represents the percent inhibition toward LmCK1.2 activity versus the percent inhibition toward SsCK1 activity for each compound. The compounds in the top left are more potent toward LmCK1.2, whereas the compounds in the bottom right are more potent toward SsCK1. (C) Compounds were classified according to their specificity: only potent on SsCK1, more potent on SsCK1 than LmCK1.2 (SsCK1 > LmaCK1.2), equally on both kinases (SsCK1 = LmCK1.2), more potent on LmCK1.2 than SsCK1 (SsCK1 < LmaCK1.2), and only potent on LmCK1.2. Compounds were also classified according to their percent inhibition: class 1 corresponds to compounds that inhibit the kinases between 80 and 100%, class 2 corresponds to compounds that inhibit the kinases between 60 and 80%, and class 3 corresponds to compounds that inhibit the kinases between 40 and 60%. Fifty-seven percent of the compounds are more potent toward LmCK1.2 than SsCK1, and 46% are specific to LmCK1.2. (D) IC50s of the 55 compounds that are specific to LmCK1.2 or that belong to class 1 with a percent inhibition above 90%. Each point represents the IC50 of a particular compound toward LmCK1.2 versus SsCK1. The IC50 are lower against LmCK1.2 than SsCK1.
The differences in specificity observed with the purine and indirubin compound families confirm that important differences exist between the ATP binding pocket of both kinases (21). It also suggests that due to the strong affinity of the purine for the mammalian CK1, it is likely that these compounds will be toxic for the host cell.
Among all the compounds identified in the primary and secondary screenings, we eliminated all the compounds for which the chemical optimization was unfeasible and selected 12 compounds from the main library, 21 compounds from the purine library, and 55 compounds from the indirubin library to assess their antileishmanial activity.
(Step 2) Evaluation of the antileishmanial activity of selected compounds.
We evaluated the antileishmanial activity of the 88 compounds selected in step 1 on cultured L. donovani promastigotes and axenic amastigotes by measuring the percentage of metabolically active parasites in liquid culture using a resazurin-based assay (27) (see Table S1 in the supplemental material). As a positive control, we treated the parasites with a 1 μM concentration of the antileishmanial reference drug, AMB, and obtained growth inhibitions of 90.4% ± 1.5% and 79.9% ± 1.3% for promastigotes and amastigotes, respectively, with excellent reproducibility, as reported by the small standard deviation (SD) values.
We then tested the compounds against intracellular L. amazonensis using a visual high-content phenotypic assay (21, 28). We measured three parameters: (i) the percentage of cells remaining after treatment compared to the vehicle control (DMSO) to evaluate cell detachment (total macrophages), (ii) the percentage of healthy cells compared to the total number of cells remaining after treatment to evaluate cell mortality (viability index [VI]), and (iii) the percentage of parasitophorous vacuoles per healthy cell to evaluate the parasite burden (PB). We used AMB at 0.5 μM and cycloheximide at 150 μM as antileishmanial and cytotoxic control compounds, respectively. Figure S1 in the supplemental material presents the data for all of the controls performed during the screening campaign. As expected, AMB reduced the parasite burden without affecting the number of macrophages or their viability, whereas cycloheximide did not significantly affect the number of macrophages but decreased dramatically the viability, since it is extremely toxic to macrophages. The data were reproducible, as judged by the small SD values (see Fig. S1 in the supplemental material).
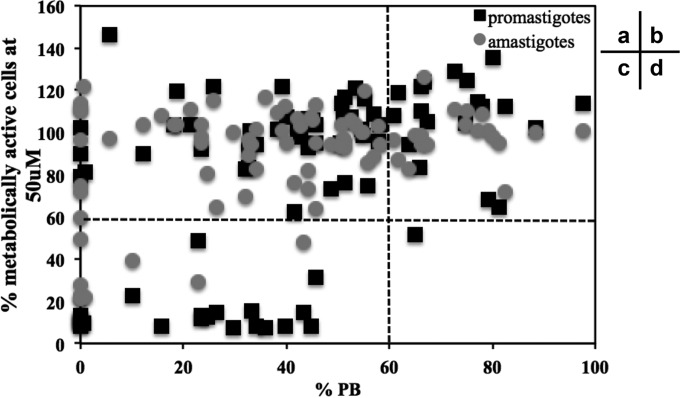
For each of the 88 compounds, we plotted the percentages of metabolically active promastigotes or amastigotes at 10 μM versus the percentages of parasite burden at 10 μM (Fig. 5). We considered efficient any compounds that decreased the percentages of metabolically active parasites or the percentages of parasite burden by 40%. We eliminated compounds that had no effect on the percentage of intracellular parasites; remarkably, these compounds were also mainly inefficient on cultured parasites (Fig. 5b and d). Among the 65 compounds that were potent toward the intracellular parasites, we identified two categories: compounds equally efficient on cultured and intracellular parasites (Fig. 5c) and compounds that were only efficient on intracellular parasites, which represent the majority of the compounds (43 of 65 for promastigotes and 57 of 65 for amastigotes, Fig. 5a). This finding suggests that the exclusion of compounds based on their lack of efficacy on cultured parasites could lead to the elimination of compounds that are very efficient on intracellular parasites, including inhibitors that indirectly kill parasites by targeting host cell proteins.
FIG 5.
Comparison of the antileishmanial activity of compounds on cultured and intracellular parasites. We performed a screening of 88 compounds from the main, the purine and the indirubin libraries on cultures promastigotes, axenic amastigotes, and intracellular parasites. Each point represents the percentage of metabolically active promastigotes or amastigotes at 10 μM versus the percentage of parasite burden at 10 μM for each compound. Black squares correspond to the percentage of metabolically active promastigotes at 10 μM versus the percentage of parasite burden at 10 μM, and gray dots correspond to the percentage of metabolically active amastigotes at 10 μM versus the percentage of parasite burden at 10 μM. Sectors: a, compounds that are potent against intracellular parasites but not against cultured parasites; b, compounds that are not potent against intracellular and cultured parasites; c, compounds that are potent against intracellular and cultured parasites; and d, compounds that are not potent against intracellular parasites but potent against cultured parasites.
Main library.
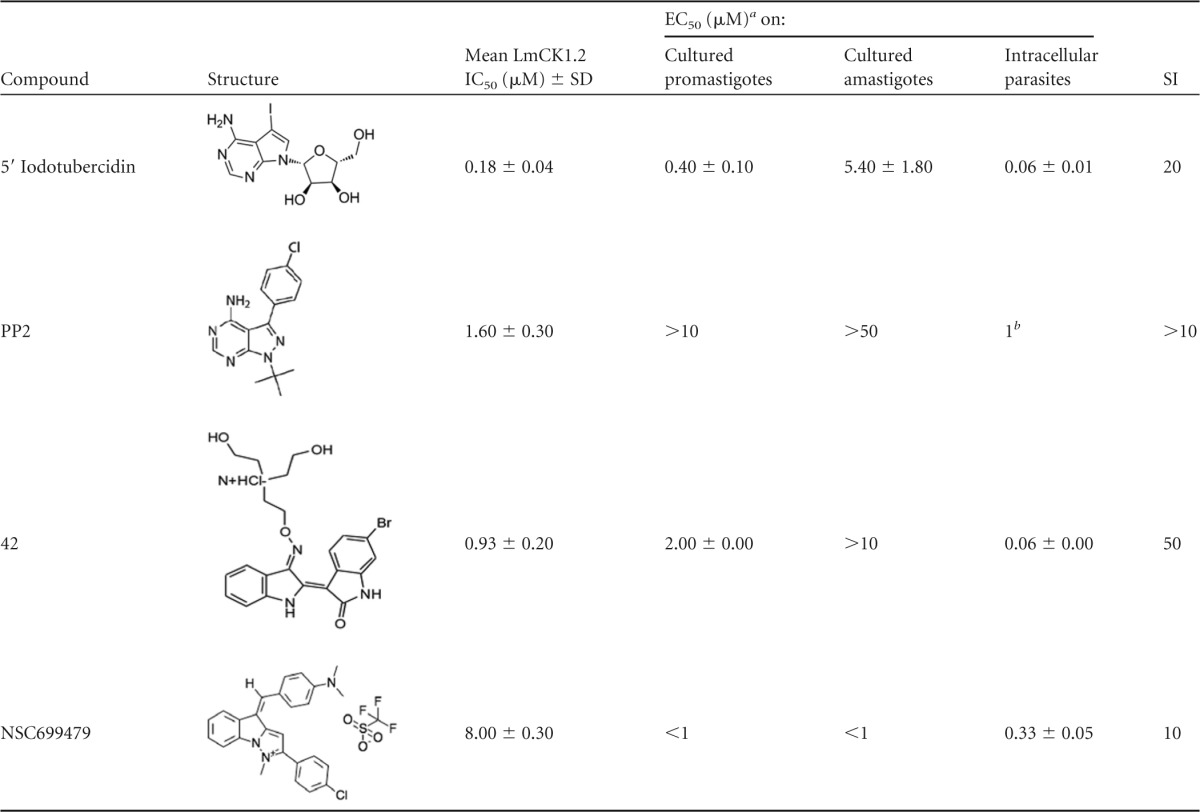
We selected 7 of 12 compounds from the main library, including (i) rottlerin, NSC146771, gefitinib, and sunitinib (all potent against intracellular parasites at 10 μM) and (ii) 5′ITu, PP2 [1-tert-butyl-3-(4-chlorophenyl)-1 h-pyrazolo[3,4-d]pyrimidin-4-amine (45)] and NSC699479, which were efficient against intracellular parasites at 10 and 1 μM, as well as against cultured parasites (see Table S1 in the supplemental material). 5′ITu, which is described as a general kinase inhibitor (46), is potent against promastigotes and axenic amastigotes, with EC50s of 0.4 ± 0.1 and 5.4 ± 1.8 μM, respectively (see Table S1 in the supplemental material). It has also a strong effect at 10 and 1 μM on intracellular parasites with only 10% ± 1% and 13 ± 4.5% remaining PB, respectively, an observation similar to that for 0.5 μM AMB. PP2 is potent against intracellular amastigotes at 1 μM (54% ± 3% PB; see Table S1 in the supplemental material). NSC699479 {4-[(E)-[2-(4-chlorophenyl)-1-methylpyrazolo[1,5-a]indol-1-ium-4-ylidene]methyl]-N,N-dimethylaniline-trifluoromethane sulfonate} is known for anticancer activity and has been shown to target a wide range of proteins, including the DNA polymerase iota (47). It is extremely potent against promastigotes and axenic amastigotes, with an EC50 below 1 μM, as well as intracellular parasites at 10 μM (3.6% ± 1.9% PB) and 1 μM (11% ± 1.4% PB; see Table S1 in the supplemental material), an activity that is comparable to that of AMB.
Purine library.
We selected 13 of 21 purine compounds that were able to kill efficiently intracellular parasites at either 1 or 10 μM (compounds 21 to 30, see Table S1 in the supplemental material). Consistent with their high potency against recombinant LmCK1.2, most compounds were active against intracellular parasites. Surprisingly, the purine derivatives were not very potent against promastigotes and axenic amastigotes. With the exception of compounds 22 and 30, which present EC50s of 0.72 ± 0.03 μM and 6.2 ± 0.8 μM, respectively, against promastigotes, most of the compounds were weakly active against promastigotes and inactive against axenic amastigotes at 50 μM (see Table S1 in the supplemental material). This lack of potency against cultured parasites cannot be explained by cell permeability since these compounds efficiently decrease the parasite burden of infected macrophages.
Indirubin library.
A total of 55 indirubins were tested against promastigotes and axenic amastigotes. Twenty-one compounds showed an EC50 below 10 μM against promastigotes (range, 0.4 to 2 μM), whereas only 6 showed an EC50 below 10 μM against amastigotes (range, 3.5 to 8 μM). These compounds were all members of a subfamily of indirubins, containing a diethanolamine substitution at position 3′, suggesting that the presence of this substitution could be important for their antileishmanial activity against cultured parasites. It is remarkable that the EC50s against promastigotes were systematically lower than those against axenic amastigotes (21). We next tested all the indirubin derivatives against intracellular parasites. In contrast to purine derivatives, all indirubin compounds were efficient against intracellular parasites at 10 μM, with 9 also being efficient at 1 μM (see Table S1 in the supplemental material). The most efficient indirubin was compound 42, with a remaining 22% ± 5% PB, corresponding to a decrease of 78% compared to the DMSO-treated controls. Altogether, these data confirm what we observed with recombinant LmCK1.2 (Fig. 4C and D) that the indirubin compound family, which has a stronger affinity for LmCK1.2, also shows a greater antileishmanial activity.
(Step 3) Evaluation of the toxicity of the compounds: cytotoxicity against mouse bone marrow-derived macrophages.
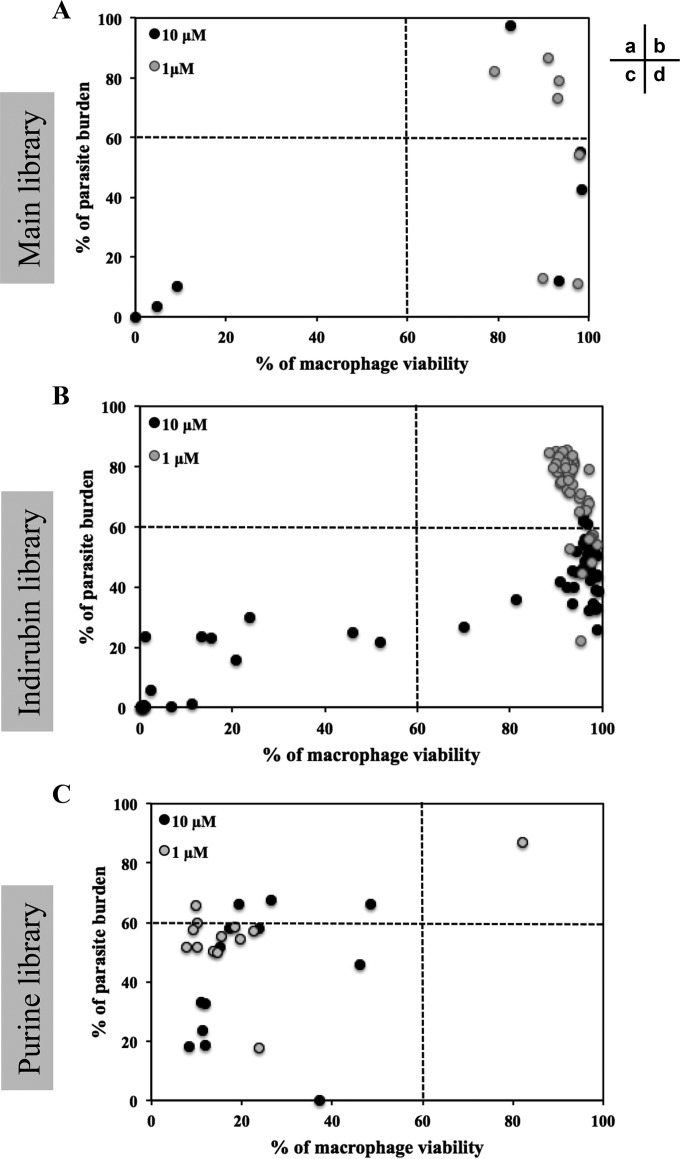
We first assessed toxicity toward mouse bone marrow-derived macrophages of 75 compounds that displayed antileishmanial activity against intracellular parasites (see Table S1, column VI%, in the supplemental material). We plotted the percentage of parasite burden versus the percentage of viable macrophages for each of the three libraries (Fig. 6). As shown in Fig. 6A, three compounds of the seven selected from the main library were toxic toward macrophages at 10 μM (Fig. 6Ac, black dots), but none were toxic at 1 μM (Fig. 6Ab and d, gray dots). However, decreasing their concentration from 10 to 1 μM to prevent cytotoxicity led, in some cases (such as with sunitinib), to a decrease in potency against intracellular parasites. Nevertheless, we identified compounds that were not toxic and were able to efficiently decrease PB (Fig. 6Ad). We obtained a similar result with the indirubin derivatives (Fig. 6Bb and d) since we identified compounds with antileishmanial activity and no toxicity against macrophages: 35 compounds at 10 μM and 9 compounds at 1 μM (Fig. 6Bd). These results are in contrast to the results obtained for the purine library, since most of the 21 purine derivatives that we tested led to cell death, preventing proper analysis of their effects on intracellular parasites. Indeed, we did not identify any compound that decreased the percentage of the parasite burden without cytotoxicity (Fig. 6Cd). To investigate whether these derivatives could be efficient toward intracellular parasites at lower concentrations, we tested compounds 16, 22, and 30 at 0.01 and 0.1 μM (data not shown). These compounds were no longer toxic to host cells at 0.1, 0.1, and 0.01 μM, respectively, and were no longer active against intracellular parasites. It seems that the efficient concentration to kill intracellular parasites could be similar to the cytotoxic concentration. This finding could be explained by the higher affinity of this compound family for the mammalian CK1 compared to Leishmania CK1.2 (Fig. 3B and C).
FIG 6.
Parasite burden versus macrophage viability. Using a visual high-content phenotypic assay, we calculated the percentage of viable macrophages and the percentage of infected cells. We analyzed the antileishmanial effect of the selected compounds from the main library (A), the indirubin library (B), and the purine library (C) versus their toxicity against macrophages. Sectors: a, compounds that are not potent against intracellular parasites but cytotoxic; b, compounds that are not potent against intracellular parasites and not cytotoxic; c, compounds that are potent against intracellular parasites but cytotoxic; and d, compounds that are potent against intracellular parasites and not cytotoxic.
Based on these results, we eliminated the sunitinib from the main library, all the remaining compounds from the purine library, and 11 compounds from the indirubin library because of their toxicity against macrophages. We thus retained six compounds from the main library and seven indirubins from those with antileishmanial activity at 1 μM and without cytotoxicity against BMDM.
Cytotoxicity against human cell lines.
To establish the selectivity index (SI; this unit corresponds to the ratio between the EC50 against intracellular parasites and the EC50 against mammalian cells), we determined the EC50s against intracellular parasites, macrophages, and human cell lines (RPE-1, SHSY-5Y, HFF-1, and U2OS) for the remaining 13 compounds (see Table S1 in the supplemental material). The SIs ranged from 0.15 to 50, which is consistent with small molecules being able to discriminate between Leishmania CK1.2 and mammalian CK1, as we identified compounds that show leishmanicidal activity without cytotoxicity (see Table S1 in the supplemental material). We eliminated all the compounds with an SI below 10, since they were likely to lead to side effects, retaining only five compounds: 5′ITu, PP2, and NSC699479 from the main library and compounds 38 and 42 from the indirubin library. The EC50 of 5′ITu against intracellular parasites was in the nanomolar range (0.06 ± 0.01 μM), whereas that against mouse macrophages was in the micromolar range (3.5 μM ± 0 μM), which represents a 60-fold difference between the cytotoxic and antileishmanial concentrations (see Table S1 in the supplemental material). The toxicity of this compound toward the human cell lines seems to be cell-dependent; indeed, the EC50 against RPE-1 and U2OS was 1.2 μM, whereas it was >25 μM against SHSY-5Y and HFF-1 (see Table S1 in the supplemental material). Taking in account both cell lines and macrophages the minimum SI is thus 20, indicating that the leishmanicidal concentration is 20-fold lower than the toxic concentration. NSC699479 has also an EC50 against intracellular parasites in the nanomolar range (0.33 ± 0.05 μM) but an SI of only 10 due to the low EC50s toward macrophages and U2OS, respectively, at 3.5 ± 0.24 μM and 3 ± 0.5 μM. Indirubin 38 has an EC50 of 0.6 ± 0.1 with an SI above 17, whereas compound 42, which is more potent, has an EC50 of 0.06 ± 0.005 μM, with a high SI of 50 (see Table S1 in the supplemental material). For PP2, it was impossible to determine the exact EC50 because the parasite burden at 10 μM PP2 was 98% ± 8.5%, whereas it was only 54% ± 3% at 1 μM. This result could be explained by the detachment of noninfected macrophages. Indeed, we showed that the treatment of infected bone marrow-derived macrophages with 10 μM PP2 led to cell detachment, as judged by the percentage of total remaining cells in the well (60% ± 1%; see Table S1 in the supplemental material). We cannot completely exclude that cell detachment could be the consequence of cell mortality, but it seems unlikely, since the cells that remained attached were viable in the presence of the drug (VI = 83% ± 4%). This is consistent with what has been previously observed for other cell types, since PP2 is known to directly interfere with cell attachment (48). We estimated the EC50 of PP2 to be around 1 μM since the PB is 54.3% ± 3.2% at 1 μM (see Table S1 in the supplemental material), with an SI of >10. For step 4, we only selected 5′ITu, NSC699479, PP2, and compound 42 (Table 1).
TABLE 1.
Selected compounds against intracellular parasites tested for target deconvolutiona
Values are presented as means ± the standard deviations where applicable.
b Estimation of the EC50.
(Step 4) Target deconvolution.
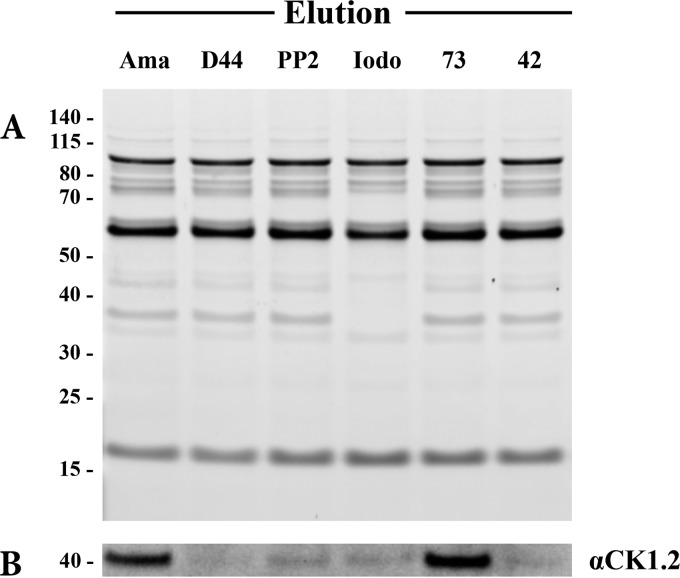
To confirm that 5′ITu, NSC699479, PP2, and compound 42 (Table 1) target Leishmania CK1.2 in the parasite and to estimate their affinity for this kinase, we investigated whether they could prevent the binding of CK1.2 to ATP-agarose (21). We treated amastigote lysates with PP2, 5′ITu, compound 42, NSC699479, or D4476 (positive control) before performing an affinity chromatography; we used an untreated sample as a negative control. The proteins eluted from the ATP-agarose were separated by SDS-PAGE and either stained with SYPRO-Ruby or analyzed by Western blotting with an anti-LmCK1.2 antibody (21). By comparing the protein elution profiles obtained with the untreated sample (Ama) to that obtained with the treated samples (D4476, PP2, Iodo, 73, and 42), we were able to assess compound selectivity (Fig. 7A). Most of the treated samples showed a profile similar to that of the untreated sample, except for the 5′ITu (Fig. 7A). As judged by the disappearance of several bands, 5′ITu could be targeting many ATP-binding proteins aside CK1.2. Because this absence of selectivity prevents any possibility for compound optimization, which could in turn lead to side effects, we eliminated 5′ITu. Based on the Western blot analysis, we also discarded NSC699479, since LmCK1.2 could still bind to the ATP agarose in the presence of this compound, suggesting that this kinase is not the primary target of NSC699479 (Fig. 7B). This is consistent with the fact that the IC50 of NSC699479 against LmCK1.2 is 8 μM, which is higher than that of the other compounds (see Table S1 in the supplemental material). However, because of its strong antileishmanial activity, it would be interesting to identify the primary target of NSC699479. As shown in Fig. 7B, similarly to D4476, only PP2 and compound 42 prevent the binding of LmCK1.2 to the ATP agarose without affecting the elution profile. This result suggests that LmCK1.2 could be one of the primary targets of these two compounds.
FIG 7.
PP2 and compound 42 are the most specific compounds toward CK1.2. Competitive ATP affinity chromatography assays were performed on amastigote cell lysates in presence or not of D4476, PP2, 5′ITu (Iodo), NSC699479 (compound 73), and compound 42. (A) ATP-binding proteins (elution) were eluted with an excess of ATP, resolved by SDS-PAGE electrophoresis and stained by SYPRO Ruby. (B) CK1.2 was revealed by Western blotting with an anti-LmCK1.2 antibody (SY3535).
DISCUSSION
The antileishmanial drugs currently available are compromised mostly because they lead to parasite resistance and have important side effects. Considering these parameters early in the process of drug development is therefore crucial to discover more efficient drugs. We established a pipeline consisting of four steps from target-based screening to target deconvolution. In order to integrate these parameters, we used LmCK1.2 (a Leishmania exokinase) as a target to address parasite resistance, and we excluded compounds based on their absence of antileishmanial activity, their lack of specificity, or their poor affinity for LmCK1.2 to address side effects. Our pipeline introduces two improvements compared to previous screening campaigns. First, since Leishmania and mammalian CK1s are closely related and to limit the possibility of cell toxicity, which leads to side effects, we screened, in parallel, leishmanial and mammalian CK1s to assess specificity. Using this approach, we could discriminate between compounds with low or high specificity. For instance, we showed that the purine derivatives displayed a better potency against mammalian CK1 than against LmCK1.2, which led to toxicity against mammalian host cells. In contrast, compounds of the indirubin family displayed better potency against LmCK1.2 than against mammalian CK1, which was subsequently confirmed by the absence of toxicity toward the mammalian host cell at 1 μM. This finding suggests a strong correlation between specificity toward the target and the subsequent effect on intracellular parasite survival. Moreover, our results also confirm that LmCK1.2 has an ATP binding pocket sufficiently divergent from that of mammalian CK1 to identify discriminating compounds (21). Indeed, more than 70% of the small molecules that we tested showed a differential potency against both kinases. We modeled the structure of Leishmania CK1.2 based on existing crystal structures of CK1s found in Protein Data Bank and noticed a few differences between the LmCK1.2 model (green; see Fig. S2 in the supplemental material) and the crystal structure of human CK1δ or of Schizosaccharomyces pombe CK1 (magenta or cyan [SpCK1], respectively; see Fig. S2 in the supplemental material) that could account for the specificity of LmCK1.2 toward certain compounds. Indeed, residues in the active site of LmCK1.2, such as F22 and K40, could be positioned differently, which could overall change the shape of the active site. Moreover, the structural alignment of LmCK1.2, human CK1δ, and SpCK1 (49), which is in complex with IC261 (a specific CK1 inhibitor), shows that the position of K40 in the active site of LmCK1.2 compared to that of K41 or K38 in the active sites of CK1δ and SpCK1, respectively, may account for the differential response to compound inhibition. Indeed, in contrast to K38 and K41, K40 could lead to conformational clash with IC261. This finding supports our previous results showing that the IC50 of IC261 toward LmCK1.2 is >10 μM, whereas it is 0.47 μM toward mammalian CK1 (21). Our results demonstrate that Leishmania CK1.2, which would have been rejected based on the strong identity to its mammalian orthologs, is a good drug target.
A second improvement was to assess whether compounds have multiple targets or low affinity for LmCK1.2. To address this issue, we performed target deconvolution. This strategy, which depends on affinity purification and competition, allowed the elimination of compounds based on their lack of specificity or their lack of affinity toward LmCK1.2. Indeed, we excluded 5′ITu that could be targeting many proteins, as revealed by their depletion after competition assay and ATP-affinity chromatography. This finding is consistent with recent publications showing that 5′ITu is a general kinase inhibitor due to its broad inhibitory activity (46). Although this compound could be a good lead compound purely based on its SI, its optimization could be difficult since the target responsible for the leishmanicidal activity is unknown. We also excluded NSC699479 because of its weak affinity toward LmCK1.2, suggesting that this kinase might not be its primary target. Based on previous results in mammalian cells, the primary target of NSC699479 could be topoisomerases in Leishmania, enzymes already known as good drug targets (50, 51, 52).
Using this pipeline, we screened 5,018 compounds in total and identified two lead compounds, PP2 and compound 42. PP2 is an inhibitor for which no antileishmanial activity against intracellular parasites in THP-1 cells in vitro has been previously described but appears to be efficient in an animal model (53). It has an EC50 on intracellular parasites of ∼1 μM but SIs of ∼10 for murine macrophages and >25 for human cell lines. The known targets of PP2 are tyrosine kinases, Src, Lck, Csk, Rip2, and Gak, for which there are no orthologs identified in Leishmania (54, 55). The two other kinases that are targeted by PP2 are p38 (52% inhibition at 1 μM [54]) and CK1δ (93% inhibition at 1 μM [54]), suggesting that the antileishmanial activity of PP2 is more likely mediated by the inhibition of CK1.2 in Leishmania. This finding is consistent with our experimental data showing that Leishmania CK1.2 is one of the primary targets of PP2. However, treatment with high concentrations of PP2 leads to macrophage detachment (40% at 10 μM) similar to what has been demonstrated previously for other cell types (48). Our results seem to indicate that most of the macrophages that detach from the slides are those that are not infected by Leishmania. This hypothesis is supported by a report by Tejle et al., which showed that the presence of L. donovani affect the detachment of monocyte-derived dendritic cells, suggesting that the presence of the parasite could promote cell adhesion (56).
Indirubins are particularly potent against both cultured and intracellular parasites and, among the 55 indirubin derivatives showing leishmanicidal activity at 10 μM, only 37% were cytotoxic against macrophages. Compound 42 is our best lead compound, with an EC50 on intracellular parasites of 60 ± 5 nM and an SI of 50. Although several authors have already described the antileishmanial effect of indirubins (57–59), this particular derivative has not been previously tested on intracellular parasites. From previous published work, we already know some of the targets of the indirubins, such as Leishmania CRK3 or GSK3 (57, 58). In our study, we reveal for the first time CK1.2 as a novel target for this family of compounds. This is particularly striking since in higher eukaryotes GSK3 and CK1 are often involved in similar signaling pathways, such as the Wnt/β-catenin or the Hedgehog pathways, where they act as priming kinases for one another (60–63). Using affinity purification, we found that GSK-3 is also a target of compound 42 (data not shown). We will determine precisely, using biochemical approaches, whether this compound targets other kinases and which one causes the antileishmanial effect.
In conclusion, we have established a comprehensive pipeline that identifies and selects LmCK1.2 inhibitors based on their specificity, antileishmanial activity, absence of cytotoxicity, and selectivity. As a proof of principle, we identified two lead compounds, PP2 and compound 42, that will be studied further to understand their mode of action and could also be used as pharmacological tools to study parasite-specific signal transduction. We will use this pipeline to screen diversified libraries that have not yet been screened against Leishmania kinases in order to identify lead compounds.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the 7th Framework Program of the European Commission through grants to the LEISHDRUG project (223414), by ANR-11-RPIB-0016 TRANSLEISH, and by the French Government's Investissements d'Avenir Program Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases (grant ANR-10-LABX-62-IBEID). The Imagopole-CiTech is part of the FranceBioImaging infrastructure supported by the French National Research Agency (ANR-10-INSB-04-01, Investments for the Future) and is supported by the Conseil de la Region Ile-de-France (program Sesame 2007, project Imagopole, S. Shorte) and the Fondation Française pour la Recherche Médicale (Programme Grands Equipements [N.A.]).
Acknowledgments
We thank all members of the FP7 LEISHDRUG consortium for fruitful discussions and, in particular, Geneviève Milon. We also thank Olivier Helynck and Hélène Munier-Lehmann for providing access to the TECAN Freedom EVOware platform for the automatic distribution of cells, parasites, and chemicals in a biosafety level 2 facility.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00021-16.
REFERENCES
- 1.Pace D. 2014. Leishmaniasis. J Infect 69(Suppl 1):S10–S18. doi: 10.1016/j.jinf.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Hussain H, Al-Harrasi A, Al-Rawahi A, Green IR, Gibbons S. 2014. Fruitful decade for antileishmanial compounds from 2002 to late 2011. Chem Rev 114:10369–10428. doi: 10.1021/cr400552x. [DOI] [PubMed] [Google Scholar]
- 3.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin Microbiol Rev 19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N, Kumar M, Singh RK. 2012. Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med 5:485–497. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 5.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Victoria FJ, Sanchez-Canete MP, Castanys S, Gamarro F. 2006. Phospholipid translocation and miltefosine potency require both Leishmania donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem 281:23766–23775. doi: 10.1074/jbc.M605214200. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Victoria JM, Bavchvarov BI, Torrecillas IR, Martinez-Garcia M, Lopez-Martin C, Campillo M, Castanys S, Gamarro F. 2011. Sitamaquine overcomes ABC-mediated resistance to miltefosine and antimony in Leishmania. Antimicrob Agents Chemother 55:3838–3844. doi: 10.1128/AAC.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P. 2002. Protein kinases–the major drug targets of the twenty-first century? Nat Rev Drug Discov 1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 9.Doerig C. 2004. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta 1697:155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Weinmann H, Metternich R. 2005. Drug discovery process for kinase inhibitors. Chembiochem 6:455–459. doi: 10.1002/cbic.200500034. [DOI] [PubMed] [Google Scholar]
- 11.Eglen RM, Reisine T. 2009. The current status of drug discovery against the human kinome. Assay Drug Dev Technol 7:22–43. doi: 10.1089/adt.2008.164. [DOI] [PubMed] [Google Scholar]
- 12.Eglen R, Reisine T. 2011. Drug discovery and the human kinome: recent trends. Pharmacol Ther 130:144–156. doi: 10.1016/j.pharmthera.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho TG, Doerig C, Reininger L. 2013. Nima- and Aurora-related kinases of malaria parasites. Biochim Biophys Acta 1834:1336–1345. doi: 10.1016/j.bbapap.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Yang SH, Sharrocks AD, Whitmarsh AJ. 2013. MAP kinase signaling cascades and transcriptional regulation. Gene 513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. 2010. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 16.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE. 2011. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 17.Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Allocco JJ, Donald R, Zhong T, Lee A, Tang YS, Hendrickson RC, Liberator P, Nare B. 2006. Inhibitors of casein kinase 1 block the growth of Leishmania major promastigotes in vitro. Int J Parasitol 36:1249–1259. doi: 10.1016/j.ijpara.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, Reiner NE. 2008. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol 9:R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paape D, Barrios-Llerena ME, Le Bihan T, Mackay L, Aebischer T. 2010. Gel-free analysis of the proteome of intracellular Leishmania mexicana. Mol Biochem Parasitol 169:108–114. doi: 10.1016/j.molbiopara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Rachidi N, Taly JF, Durieu E, Leclercq O, Aulner N, Prina E, Pescher P, Notredame C, Meijer L, Spath GF. 2014. Pharmacological assessment defines the Leishmania donovani casein kinase 1 as a drug target and reveals important functions in parasite viability and intracellular infection. Antimicrob Agents Chemother 58:1501–1515. doi: 10.1128/AAC.02022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Carvalho LP, Bhattachariya S, Carbone CJ, Kumar KG, Leu NA, Yau PM, Donald RG, Weiss MJ, Baker DP, McLaughlin KJ, Scott P, Fuchs SY. 2009. Mammalian casein kinase 1α and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol 29:6401–6412. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhardt J, Ferandin Y, Meijer L. 2007. Purification of CK1 by affinity chromatography on immobilised axin. Protein Expr Purif 54:101–109. doi: 10.1016/j.pep.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Saar Y, Ransford A, Waldman E, Mazareb S, Amin-Spector S, Plumblee J, Turco SJ, Zilberstein D. 1998. Characterization of developmentally regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol 95:9–20. doi: 10.1016/S0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 25.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. 2003. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol 130:31–42. doi: 10.1016/S0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 26.Morales MA, Watanabe R, Laurent C, Lenormand P, Rousselle J-C, Namane A, Späth GF. 2008. Phosphoproteomic analysis of Leishmania donovani pro- and amastigote stages. Proteomics 8:350–363. doi: 10.1002/pmic.200700697. [DOI] [PubMed] [Google Scholar]
- 27.Shimony O, Jaffe CL. 2008. Rapid fluorescent assay for screening drugs on Leishmania amastigotes. J Microbiol Methods 75:196–200. doi: 10.1016/j.mimet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Aulner N, Danckaert A, Rouault-Hardoin E, Desrivot J, Helynck O, Commere PH, Munier-Lehmann H, Spath GF, Shorte SL, Milon G, Prina E. 2013. High content analysis of primary macrophages hosting proliferating Leishmania amastigotes: application to anti-leishmanial drug discovery. PLoS Negl Trop Dis 7:e2154. doi: 10.1371/journal.pntd.0002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oumata N, Bettayeb K, Ferandin Y, Demange L, Lopez-Giral A, Goddard ML, Myrianthopoulos V, Mikros E, Flajolet M, Greengard P, Meijer L, Galons H. 2008. Roscovitine-derived, dual-specificity inhibitors of cyclin-dependent kinases and casein kinases 1. J Med Chem 51:5229–5242. doi: 10.1021/jm800109e. [DOI] [PubMed] [Google Scholar]
- 30.Polychronopoulos P, Magiatis P, Skaltsounis A-L, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L. 2004. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 47:935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 31.Vougogiannopoulou K, Ferandin Y, Bettayeb K, Myrianthopoulos V, Lozach O, Fan Y, Johnson CH, Magiatis P, Skaltsounis A-L, Mikros E, Meijer L. 2008. Soluble 3′,6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase-3 alter circadian period. J Med Chem 51:6421–6431. doi: 10.1021/jm800648y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferandin Y, Bettayeb K, Kritsanida M, Lozach O, Polychronopoulos P, Magiatis P, Skaltsounis A-L, Meijer L. 2006. 3′-Substituted 7-halogenoindirubins, a new class of cell death inducing agents. J Med Chem 49:4638–4649. doi: 10.1021/jm060314i. [DOI] [PubMed] [Google Scholar]
- 33.Schwede T, Kopp J, Guex N, Peitsch MC. 2003. Swiss-Model: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delano W. 2009. The PyMOL molecular graphics system, v1.01. DeLano Scientific, Palo Alto, CA: http://www.pymol.sourceforge.net. [Google Scholar]
- 35.Schnur L, Bachrach U, Bar-Ad G, Haran M, Tashma Z, Talmi M, Katzhendler J. 1983. The effect of diaminoalkyl-anthraquinone derivatives on the growth of the promastigotes of Leishmania tropica minor, L. major, L. donovani, and L. aethiopica. Biochem Pharmacol 32:1729–1732. doi: 10.1016/0006-2952(83)90117-X. [DOI] [PubMed] [Google Scholar]
- 36.Sittie AA, Lemmich E, Olsen CE, Hviid L, Kharazmi A, Nkrumah FK, Christensen SB. 1999. Structure-activity studies: in vitro antileishmanial and antimalarial activities of anthraquinones from Morinda lucida. Planta Med 65:259–261. doi: 10.1055/s-2006-960473. [DOI] [PubMed] [Google Scholar]
- 37.Bolognesi ML, Lizzi F, Perozzo R, Brun R, Cavalli A. 2008. Synthesis of a small library of 2-phenoxy-1,4-naphthoquinone and 2-phenoxy-1,4-anthraquinone derivatives bearing anti-trypanosomal and anti-leishmanial activity. Bioorg Med Chem Lett 18:2272–2276. doi: 10.1016/j.bmcl.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Conners R, Schambach F, Read J, Cameron A, Sessions RB, Vivas L, Easton A, Croft SL, Brady RL. 2005. Mapping the binding site for gossypol-like inhibitors of Plasmodium falciparum lactate dehydrogenase. Mol Biochem Parasitol 142:137–148. doi: 10.1016/j.molbiopara.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Montamat EE, Burgos C, Gerez de Burgos NM, Rovai LE, Blanco A, Segura EL. 1982. Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 218:288–289. doi: 10.1126/science.6750791. [DOI] [PubMed] [Google Scholar]
- 40.Padmanabhan PK, Mukherjee A, Singh S, Chattopadhyaya S, Gowri VS, Myler PJ, Srinivasan N, Madhubala R. 2005. Glyoxalase I from Leishmania donovani: a potential target for anti-parasite drug. Biochem Biophys Res Commun 337:1237–1248. doi: 10.1016/j.bbrc.2005.09.179. [DOI] [PubMed] [Google Scholar]
- 41.Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, Ruedi P. 2006. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother 50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen G, Mukhopadhyay S, Ray M, Biswas T. 2008. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. J Antimicrob Chemother 61:1066–1075. doi: 10.1093/jac/dkn053. [DOI] [PubMed] [Google Scholar]
- 43.da Silva ER, Maquiaveli Cdo C, Magalhaes PP. 2012. The leishmanicidal flavonols quercetin and quercitrin target Leishmania (Leishmania) amazonensis arginase. Exp Parasitol 130:183–188. doi: 10.1016/j.exppara.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Knockaert M, Gray N, Damiens E, Chang YT, Grellier P, Grant K, Fergusson D, Mottram J, Soete M, Dubremetz JF, Le Roch K, Doerig C, Schultz P, Meijer L. 2000. Intracellular targets of cyclin-dependent kinase inhibitors: identification by affinity chromatography using immobilised inhibitors. Chem Biol 7:411–422. doi: 10.1016/S1074-5521(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 45.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J Biol Chem 271:695–701. [DOI] [PubMed] [Google Scholar]
- 46.Massillon D, Stalmans W, van de Werve G, Bollen M. 1994. Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor. Biochem J 299(Pt 1):123–128. doi: 10.1042/bj2990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bento AP, Gaulton A, Hersey A, Bellis LJ, Chambers J, Davies M, Kruger FA, Light Y, Mak L, McGlinchey S, Nowotka M, Papadatos G, Santos R, Overington JP. 2014. The ChEMBL bioactivity database: an update. Nucleic Acids Res 42:D1083–D1090. doi: 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hishiki T, Saito T, Sato Y, Mitsunaga T, Terui E, Matsuura G, Saito E, Shibata R, Mise N, Yokoyama Y, Yoshida H. 2011. Src kinase family inhibitor PP2 induces aggregation and detachment of neuroblastoma cells and inhibits cell growth in a PI3 kinase/Akt pathway-independent manner. Pediatr Surg Int 27:225–230. doi: 10.1007/s00383-010-2775-2. [DOI] [PubMed] [Google Scholar]
- 49.Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J. 2000. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem 275:20052–20060. doi: 10.1074/jbc.M001713200. [DOI] [PubMed] [Google Scholar]
- 50.Balana-Fouce R, Alvarez-Velilla R, Fernandez-Prada C, Garcia-Estrada C, Reguera RM. 2014. Trypanosomatids topoisomerase re-visited: new structural findings and role in drug discovery. Int J Parasitol Drugs Drug Resist 4:326–337. doi: 10.1016/j.ijpddr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katayama H, Kiryu Y, Kaneko K, Ohshima R. 2000. Anti-cancer activities of pyrazolo[1,5-a]indole derivatives. Chem Pharm Bull 48:1628–1633. doi: 10.1248/cpb.48.1628. [DOI] [PubMed] [Google Scholar]
- 52.Das BB, Ganguly A, Majumder HK. 2008. DNA topoisomerases of Leishmania: the potential targets for anti-leishmanial therapy. Adv Exp Med Biol 625:103–115. doi: 10.1007/978-0-387-77570-8_9. [DOI] [PubMed] [Google Scholar]
- 53.Sanderson L, Yardley V, Croft SL. 2014. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. J Antimicrob Chemother 69:1888–1891. doi: 10.1093/jac/dku069. [DOI] [PubMed] [Google Scholar]
- 54.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. 2007. The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsons M, Worthey EA, Ward PN, Mottram JC. 2005. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei, and Trypanosoma cruzi. BMC Genomics 6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tejle K, Lindroth M, Magnusson KE, Rasmusson B. 2008. Wild-type Leishmania donovani promastigotes block maturation, increase integrin expression and inhibit detachment of human monocyte-derived dendritic cells–the influence of phosphoglycans. FEMS Microbiol Lett 279:92–102. doi: 10.1111/j.1574-6968.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 57.Grant KM, Dunion MH, Yardley V, Skaltsounis AL, Marko D, Eisenbrand G, Croft SL, Meijer L, Mottram JC. 2004. Inhibitors of Leishmania mexicana CRK3 cyclin-dependent kinase: chemical library screen and antileishmanial activity. Antimicrob Agents Chemother 48:3033–3042. doi: 10.1128/AAC.48.8.3033-3042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xingi E, Smirlis D, Myrianthopoulos V, Magiatis P, Grant KM, Meijer L, Mikros E, Skaltsounis AL, Soteriadou K. 2009. 6-Br-5methylindirubin-3′oxime (5-Me-6-BIO) targeting the leishmanial glycogen synthase kinase-3 (GSK-3) short form affects cell-cycle progression and induces apoptosis-like death: exploitation of GSK-3 for treating leishmaniasis. Int J Parasitol 39:1289–1303. doi: 10.1016/j.ijpara.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Efstathiou A, Gaboriaud-Kolar N, Smirlis D, Myrianthopoulos V, Vougogiannopoulou K, Alexandratos A, Kritsanida M, Mikros E, Soteriadou K, Skaltsounis AL. 2014. An inhibitor-driven study for enhancing the selectivity of indirubin derivatives towards leishmanial glycogen synthase kinase-3 over leishmanial cdc2-related protein kinase 3. Parasit Vectors 7:234. doi: 10.1186/1756-3305-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harwood AJ. 2002. Signal transduction in development: holding the key. Dev Cell 2:384–385. doi: 10.1016/S1534-5807(02)00156-9. [DOI] [PubMed] [Google Scholar]
- 61.Niehrs C, Shen J. 2010. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci 67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knippschild U, Kruger M, Richter J, Xu P, Garcia-Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J. 2014. The CK1 family: contribution to cellular stress response and its role in carcinogenesis. Front Oncol 4:96. doi: 10.3389/fonc.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beurel E, Grieco SF, Jope RS. 2015. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.