Abstract
Benznidazole (Bz), the drug used for treatment of Chagas' disease (caused by the protozoan Trypanosoma cruzi), is activated by a parasitic NADH-dependent type I nitroreductase (NTR I). However, several studies have shown that other enzymes are involved. The aim of this study was to evaluate whether the aldo-keto reductase from T. cruzi (TcAKR), a NADPH-dependent oxido-reductase previously described by our group, uses Bz as the substrate. We demonstrated that both recombinant and native TcAKR enzymes reduce Bz by using NADPH, but not NADH, as a cofactor. TcAKR-overexpressing epimastigotes showed higher NADPH-dependent Bz reductase activity and a 50% inhibitory concentration (IC50) value for Bz 1.8-fold higher than that of the controls, suggesting that TcAKR is involved in Bz detoxification instead of activation. To understand the role of TcAKR in Bz metabolism, we studied TcAKR expression and NADPH/NADH-dependent Bz reductase activities in two T. cruzi strains with differential susceptibility to Bz: CL Brener and Nicaragua. Taking into account the results obtained with TcAKR-overexpressing epimastigotes, we expected the more resistant strain, Nicaragua, to have higher TcAKR levels than CL Brener. However, the results were the opposite. CL Brener showed 2-fold higher TcAKR expression and 5.7-fold higher NADPH-Bz reduction than the Nicaragua strain. In addition, NADH-dependent Bz reductase activity, characteristic of NTR I, was also higher in CL Brener than in Nicaragua. We conclude that although TcAKR uses Bz as the substrate, TcAKR activity is not a determinant of Bz resistance in wild-type strains and may be overcome by other enzymes involved in Bz activation, such as NADPH- and NADH-dependent reductases.
INTRODUCTION
Chagas' disease, caused by the protozoan Trypanosoma cruzi, affects 11 million people in Latin America (1). The parasite is usually transmitted to humans and other mammals by triatomine bugs. However, transmission can also be oral (through contaminated food), congenital, or transfusional or by organ transplantation, even in countries where the disease is not endemic (2). The course of infection includes an acute phase followed by an indeterminate phase without symptoms, which lasts throughout life in most infected people. Approximately 30% of patients can develop a chronic phase characterized by cardiac and/or digestive pathologies that can lead to death (3).
Benznidazole (Bz) and, to a lesser extent, nifurtimox (Nx) are the drugs currently used for treatment of T. cruzi infection (4). Although the mechanism of action of these nitroheterocyclic compounds is not clearly understood, it is known that, to exert their trypanocidal effects, they need to be activated through the reduction of their nitro group (5). Several studies have proposed that an NADH-dependent trypanosomal type I nitroreductase (NTR I) is a key enzyme which catalyzes Bz and Nx activation in vivo (6, 7, 8, 9). However, evidence indicates that although Bz is reduced to its anion radical, redox cycling may not be relevant for its mode of action, as is the case with Nx (8, 10, 11, 12, 13). Moreover, cells selected for Bz resistance display altered expression levels of several additional enzymes, indicating that other components of the parasite are involved (14, 15, 16). It has been proposed that Bz activity is mediated via highly toxic reduced intermediates that covalently bind macromolecules and cause deleterious effects, including DNA damage and thiol depletion (8, 17, 18).
We have previously described a novel NADPH-dependent aldo-keto reductase from T. cruzi, TcAKR, which reduces commonly used AKR substrates and ο-naphthoquinones (ο-NQ) with trypanocidal effects, such as β-lapachone (19). The generation of free radicals is concomitant with o-NQ reduction, suggesting that TcAKR participates in their metabolism (19, 20, 21). Trochine et al. recently reported that TcAKR interacts with an immobilized derivative of Bz, suggesting that it also participates in the Bz mode of action (22). Thus, the aim of this study was to examine TcAKR reductase activity using Bz as a substrate and the role of this enzyme in the metabolism of this drug. Here, we showed that both recombinant and native TcAKR enzymes can reduce Bz using NADPH as a cofactor. Studies with transfected epimastigotes overexpressing TcAKR suggest that this enzyme may contribute to Bz detoxification. In addition, experiments with two T. cruzi strains with differential susceptibilities to Bz indicated that TcAKR activity may not be relevant enough to determine Bz resistance in natural strains.
MATERIALS AND METHODS
Parasite culturing and processing.
The parasites used in this study were the CL Brener clone (discrete typing unit [DTU] VI), Nicaragua isolate (DTU I) (16), and Adriana (DTU I) transformed with the plasmid pLEW13 (23). Epimastigotes were cultured at 28°C in BHT medium (33 g/liter brain heart infusion medium, 3 g/liter Bacto tryptose, 0.3 g/liter glucose, 5.4 mM KCl, 28.2 mM Na2HPO4, 0.002% [wt/vol] hemin) supplemented with 10% (vol/vol) of fetal bovine serum (FBS). Transformed pLEW13 Adriana stock was cultured with 200 μg/ml G418 and hygromycin.
To obtain the soluble fraction of parasites, epimastigotes were suspended in 100 mM Tris-HCl, pH 7.4 (2 × 109 parasites in 1 ml of buffer), and ruptured by freeze-thawing with liquid N2; afterwards, lysates were centrifuged at 10,000 × g for 30 min at 4°C. The supernatant (soluble fraction) was used to measure enzymatic activities or purify native TcAKR.
Recombinant and native TcAKR purification.
Expression and purification of recombinant TcAKR (recTcAKR) were performed as previously described from Escherichia coli (M15) transformed with the plasmid pQE30-TcAKR (19). Native TcAKR was purified from the soluble fraction of CL Brener clone epimastigotes by Cibacron Blue–Sepharose CL-6B resin (Pharmacia Biotech) eluted with 0.7 mM NADPH (19).
Enzymatic assays.
Reducing activities were routinely assayed by measuring NADPH or NADH oxidation at 340 nm (ε = 6,270 M−1 cm−1) at 30°C in a Beckman Coulter DU-640UV instrument.
AKR activity was determined in reaction mixtures of 0.25 ml containing 5 to 150 μg of the soluble fraction from epimastigote lysates in 100 mM Tris-HCl buffer, pH 6.5, with 1 mM 4-nitrobenzaldehyde (4-NBA) and 0.2 mM NADPH.
Quinoneoxido-reductase (QOR) activity was evaluated using 0.025 mM 9,10-phenanthrenequinone (9,10-PQ) in a final volume of 0.1 ml containing 100 mM triethanolamine buffer, pH 7.7, 0.1 mM NADPH, 0.002 mM EDTA, and 25 to 120 μg of the soluble fraction.
Bz reductase activity was measured in the same reaction mixture used for AKR activity but with 4-NBA replaced by 0.1 mM Bz. In this case, 20 μg and 5 to 20 μg of recombinant and native TcAKR, respectively, or 25 to 250 μg of the soluble fraction was used. To assess NADH-dependent Bz reductase activity, NADPH was replaced by 0.1 mM NADH.
TcAKR subcloning, parasite transfection, and TcAKR overexpression.
The TcAKR gene was excised from the recombinant plasmid pGEM-TcAKR (19) with NotI and introduced into the inducible expression vector pTcIndex (23) excised with the same restriction enzyme to form pTcIndex-TcAKR. This plasmid was introduced by electroporation into Adriana epimastigotes previously transformed with plasmid pLEW13. For electroporation, parasites in the mid-log phase were washed and suspended in BHT medium at a final concentration of 5 × 108 cells per ml. Aliquots of 0.35 ml were dispensed into disposable 0.4-mm cuvettes containing 10 μg of plasmid DNA, and cells were electroporated by using a Bio-Rad gene pulser at 335 V and 1,400 mF, with two consecutive pulses. After 5 min on ice, cells were diluted 10-fold with BHT medium containing 10% tetracycline-free FBS (Natocor) and allowed to recover for 24 h. G418 and hygromycin B (200 μg/ml) were added, and parasites were incubated at 28°C. Transgenic parasites were obtained after 3 weeks of selection with both antibiotics. Epimastigote cultures were grown to reach a cell density of 5 × 106 parasites per ml, and protein expression was induced by the addition of 5 μg/ml tetracycline.
Adriana epimastigotes transfected with the green fluorescent protein (GFP) gene were used as controls in all the assays. GFP expression by tetracycline induction was evaluated by optical microscopy in a Leica DM 2500 fluorescence microscope.
Growth inhibition assays and IC50 calculation.
To determine the sensitivity to Bz, epimastigotes (5 × 106 cells/ml) were seeded into BHT medium–10% fetal calf serum (FCS) in 96-well culture flasks (200 μl/well) in the presence of increasing amounts of Bz (0 to 50 μM) solubilized in dimethyl formamide (DMFA) and cultured for 72 h. Transfected parasites were grown with 200 μg/ml G418 and hygromycin, and, to induce the overexpression of these proteins, 5 μg/ml of tetracycline was added 1 day before the starting of the assay. The final concentration of DMFA was fixed below the toxic level (1%). Parasite number was determined by optical microscopy counting in a Neubauer chamber. Untreated controls reached a cell density of about 33 × 106 parasites/ml after 72 h of culture, whereas untreated tetracycline-induced TcAKR-transfected parasites reached 18 × 106 parasites/ml. Parasite growth in the absence of Bz was considered 100%, and the concentration of drug that produced death in 50% of parasites (IC50) was calculated by a dose-response curve using nonlinear regression analysis carried out with Prism, version 5.0, software (GraphPad, San Diego, CA).
Intracellular ROS and ΔΨm detection.
Epimastigotes were grown as described in growth inhibition assays with Bz concentrations based on the IC50s. After 72 h of treatment, epimastigotes were washed, and pellets were resuspended in BHT medium. To evaluate intracellular reactive oxygen species (ROS), cells were incubated with 10 μM dichlorodihydrofluorescein diacetate (H2DCF-DA), and to evaluate the mitochondrial membrane potential (ΔΨm), cells were incubated with 10 μg/ml rhodamine 123 for 30 min at 28°C. Hydrogen peroxide (0.5 mM) and 20 μM carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were used as positive controls for ROS production and ΔΨm detection, respectively. After incubation with the probes, cells were collected and suspended in phosphate-buffered saline (PBS). Fluorescence was detected in a flow cytometer (FACSCalibur; Becton Dickinson and Co., NJ, USA), and Cyflogic software, version 1.2.1, was used for the data analyses. A total of 10,000 events were acquired in the region previously established as the one that corresponded to the parasites.
Western blot analysis of TcAKR expression.
Epimastigote pellets (2.5 × 105 parasites/lane) were separated by 12% SDS-PAGE and electroblotted onto nitrocellulose membranes. Blots were blocked with 10% (vol/vol) skim milk in PBS and incubated for 1 h at room temperature with mouse anti-recTcAKR serum (1:500). The membrane was washed with PBS and then incubated for 1 h at room temperature with biotinylated anti-mouse IgG (Vector) diluted 1:2,000. After washes, membranes were incubated with streptavidin-horseradish peroxidase (HRP) (Vector) diluted 1:1,000 for 30 min at room temperature (RT). To quantify TcAKR expression, TcCyp19, was used as a loading control and detected with rabbit anti-recTcCyp19 serum (1:1,000). Rabbit anti-T. cruzi old yellow enzyme (TcOYE) N-terminal peptide serum (1:500) was used to detect TcOYE expression. In both cases, after primary antibody treatment, membranes were incubated with mouse anti-rabbit IgG-HRP (Jackson) (1/2,000). Detection was performed with 4-chloro-1-naphthol, and bands were scanned and quantified using ImageJ software (version 1.410).
Statistical analysis.
Each experiment was performed in duplicate in at least three independent assays. Parameters are expressed as the mean values ± standard error of the mean (mean ± SEM). Results were analyzed by a Mann-Whitney test. Differences were considered to be statistically significant at a P value of <0.05.
RESULTS
Reductase activity of TcAKR using Bz as the substrate.
A recent publication reporting that TcAKR interacts with an immobilized derivative of Bz (22) led us to test the hypothesis that this enzyme may use Bz as a substrate. Both recTcAKR and the native enzyme purified from epimastigotes by Cibacron Blue affinity chromatography eluted with NADPH (19) showed reductase activity toward Bz using NADPH, but not NADH, as a cofactor, with specific activities of 90.58 ± 6.96 and 73.11 ± 3.65 nmol of NADPH/min/mg of protein, respectively. It is worth mentioning that native TcAKR did not coelute with trypanothione reductase (TR) or T. cruzi old yellow enzyme (TcOYE) since Western blot assays using specific sera against both enzymes did not react with the NADPH-eluted fraction (data not shown). When Nx was tested as the substrate of recTcAKR, no activity was detected with either NADH or NADPH as a cofactor.
Susceptibility to Bz of transfected epimastigotes overexpressing TcAKR.
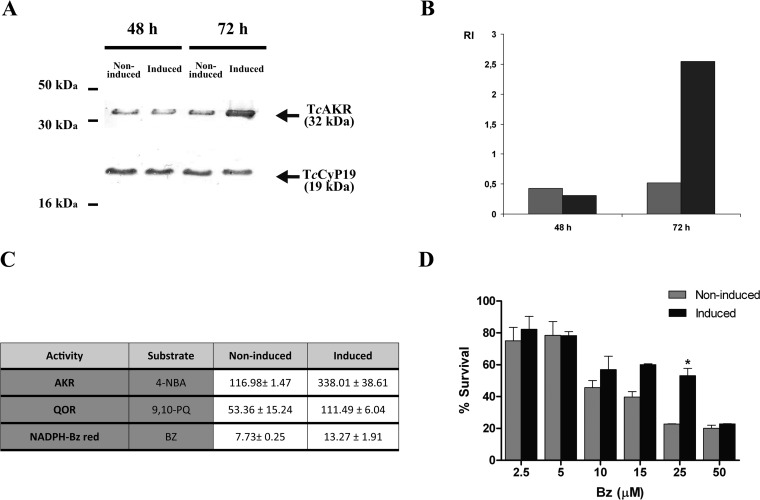
To understand the role of TcAKR in Bz metabolism, we genetically engineered epimastigotes for tetracycline-inducible overexpression of TcAKR. Time course evaluation of TcAKR expression by transfected parasites was performed by Western blotting using mouse anti-recTcAKR serum. Tetracycline-induced parasites showed about a 2.5-fold increase in TcAKR expression after 72 h of induction (Fig. 1A and B). Accordingly, AKR and QOR activities were increased to the same extents. NADPH-dependent Bz reductase activity was also increased after induction, confirming that TcAKR uses Bz as the substrate (Fig. 1C).
FIG 1.
Overexpression of TcAKR and Bz susceptibility in transfected T. cruzi epimastigotes. (A and B) Time course of TcAKR expression in transfected T. cruzi. Noninduced and tetracycline-induced transfected epimastigotes were collected at different times and fractionated by 12% SDS-PAGE, and Western blots were probed with a mouse anti-recTcAKR serum. Equivalence in protein loading was controlled by immunodetection of TcCyp19. A representative Western blot (A) and semiquantitative analysis of TcAKR levels (B) are shown. Relative intensity (RI) was calculated as follows: intensity of the signal obtained with anti-recTcAKR/intensity of the signal obtained with anti-TcCyp19. (C) AKR, QOR, and NADPH-dependent Bz reductase (red) activities of TcAKR-transfected epimastigotes. Enzymatic activities were measured in the soluble fraction of transfected epimastigotes, either noninduced or tetracycline induced for 72 h. AKR and QOR activities were evaluated using 4-NBA and 9,10-PQ as substrates, respectively. NADPH was used as a cofactor in all the reactions. Results are expressed as nanomoles of NADPH/minute/milligram of protein. (D) Effect of Bz on the survival of TcAKR-overexpressing T. cruzi. Noninduced and tetracycline-induced pTcIndex-TcAKR-transfected parasites were cultured for 72 h in the presence or absence of different Bz concentrations. A survival rate of 100% corresponds to the mean number of duplicate samples of untreated epimastigotes. *, P < 0.05, for results in noninduced versus tetracycline-induced epimastigotes.
When sensitivity to Bz of TcAKR-transfected epimastigotes was evaluated, TcAKR-overexpressing cells were more resistant to Bz, showing an IC50 value 1.8-fold higher than the values of the noninduced controls (Fig. 1D). TcAKR-transfected parasites shifted the IC50 from 9.90 ± 1.30 μM to 17.45 ± 1.25 μM after tetracycline induction. This suggests that TcAKR may not be involved in Bz activation but may participate in Bz detoxification.
TcAKR expression in two T. cruzi strains with differential susceptibilities to Bz.
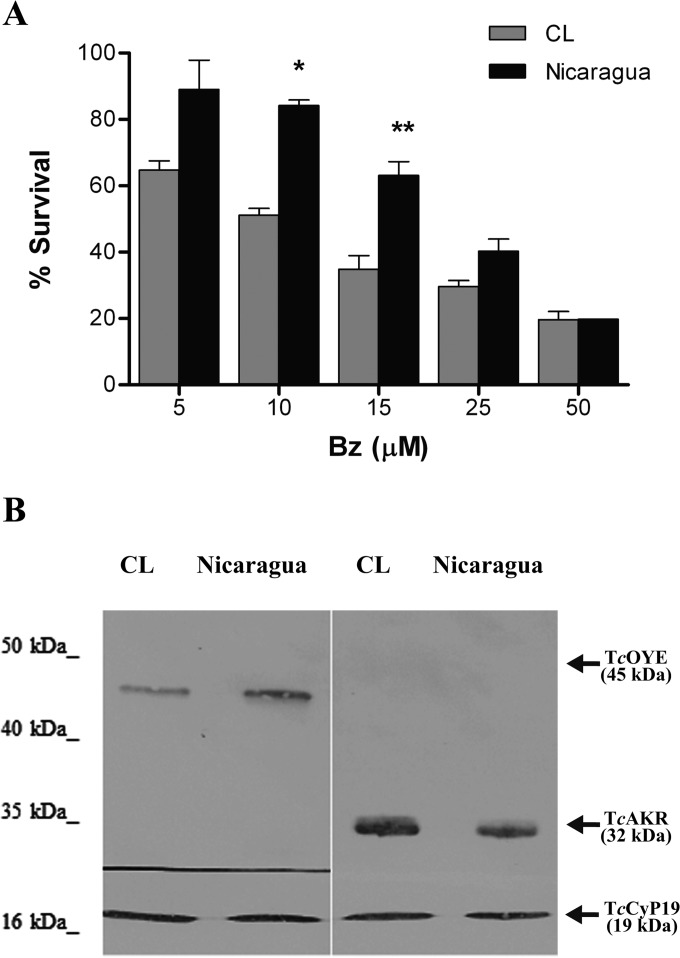
To evaluate the significance of TcAKR activity in Bz metabolism, we studied TcAKR expression and its related enzymatic activities in two T. cruzi strains with differential susceptibilities to Bz: CL Brener and Nicaragua (24). CL Brener was more susceptible to Bz than Nicaragua, presenting IC50s of 10 and 20.5 μM, respectively (Fig. 2A and Table 1), and showed higher TcAKR levels (Fig. 2B and Table 1), as evaluated by Western blotting using anti-recTcAKR serum. As a reference, the expression of TcOYE, an NAD(P)H oxidoreductase which does not reduce Bz (25), was also tested and found to be slightly higher in Nicaragua than in CL Brener (Fig. 2B). In correlation with TcAKR expression, CL Brener presented higher AKR, QOR, and NADPH-dependent Bz reductase activities than Nicaragua (Table 1). However, while TcAKR expression and AKR and QOR activities were about 2.8-fold higher in CL Brener than in the Nicaragua strain, NADPH-dependent Bz reductase activity was increased 5.7-fold, suggesting that enzymes other than TcAKR may reduce Bz using NADPH as a cofactor.
FIG 2.
Bz susceptibility and TcAKR expression in T. cruzi CL Brener and Nicaragua. (A) Effect of Bz on the survival of epimastigotes of T. cruzi CL Brener and Nicaragua. Parasite growth was monitored after 72 h of culture in the presence or absence of different Bz concentrations. A survival rate of 100% corresponds to the mean number of duplicate samples of untreated epimastigotes. *, P < 0.05; **, P < 0.01, for results with CL Brener versus those with Nicaragua. (B) Analysis of TcAKR protein expression by Western blotting in T. cruzi CL Brener and Nicaragua. Western blot analysis of proteins derived from epimastigotes of CL Brener and Nicaragua T. cruzi strains was performed, as indicated, with mouse anti-recTcAKR polyclonal serum (right panel), rabbit anti-TcOYE N-terminal peptide polyclonal serum (left panel), and rabbit anti-recTcCyp19 polyclonal serum (both panels). TcCyp19 protein was used as a loading control.
TABLE 1.
Relation between TcAKR activity and Bz susceptibility in epimastigotes of CL Brener and Nicaragua strains of T. cruzi
| Parameter | Value for the parameter by strain |
|
|---|---|---|
| CL Brener (DTU VI) | Nicaragua (DTU I) | |
| TcAKR expression (n-fold)a,i | 3.45 ± 0.46 | 1.86 ± 0.51 |
| NADPH-dependent enzymatic activity (nmol NADPH/min/mg) | ||
| AKRb,i | 365.37 ± 25.64 | 137.31 ± 8.72 |
| QORc | 140.92 ± 13.84 | 44.58 ± 7.53 |
| Bz reductased,i | 16.01 ± 0.65 | 2.80 ± 0.73 |
| NADH-dependent enzymatic activity (nmol NADH/min/mg) | ||
| Bz reductasee,i | 19.43 ± 1.44 | 8.24 ± 1.31 |
| Bz trypanocidal activity | ||
| IC50 (μM)f,i | 10 ± 0.095 | 20.50 ± 0.30 |
| ROS (RFU)g | 4.58 ± 0.88 | 3.63 ± 0.22 |
| ψmh | 0.28 ± 0.02 | 0.22 ± 0.02 |
Ratio of the densitometric values of TcAKR and TcCyp19 bands obtained in Western blot assays as shown in Fig. 2B.
AKR activity using 4-NBA as a substrate.
QOR activity using 9,10-PQ as a substrate.
Bz reduction using NADPH as a cofactor.
Bz reduction using NADH as a cofactor.
IC50s calculated by linear regression analysis of the plot of the growth constant versus drug concentration (Fig. 2A).
Detection of intracellular ROS production by flow cytometry using the H2DCF-DA probe of Bz-treated epimastigotes. Values correspond to the relative fluorescence units (RFU) calculated as the median fluorescence intensity (MFI) of Bz-treated parasites/MFI of untreated parasites.
Measurement of change in mitochondrial membrane potential (ΔΨm) by flow cytometry using rhodamine 123 of Bz-treated epimastigotes. Values correspond to the relative fluorescence units (RFU) calculated as the median fluorescence intensity (MFI) of Bz-treated parasites/MFI of untreated parasites.
P < 0.05%, for the difference between results with CL Brener and Nicaragua.
Based on the results of TcAKR-overexpressing epimastigotes, we expected TcAKR expression and activity to be lower in CL Brener (more susceptible) than in Nicaragua (more resistant). Therefore, we speculated that TcAKR activity might be overcome by other enzymes of the parasite involved in the activation of Bz, such as NADPH- or NADH-dependent reductases. In this context, we measured the NADH-dependent Bz reductase activity as an indication of the Bz-activating enzyme NTR I and found that it was higher in CL Brener than in Nicaragua (Table 1), indicating an association between the characteristic NTR I activity and Bz susceptibility.
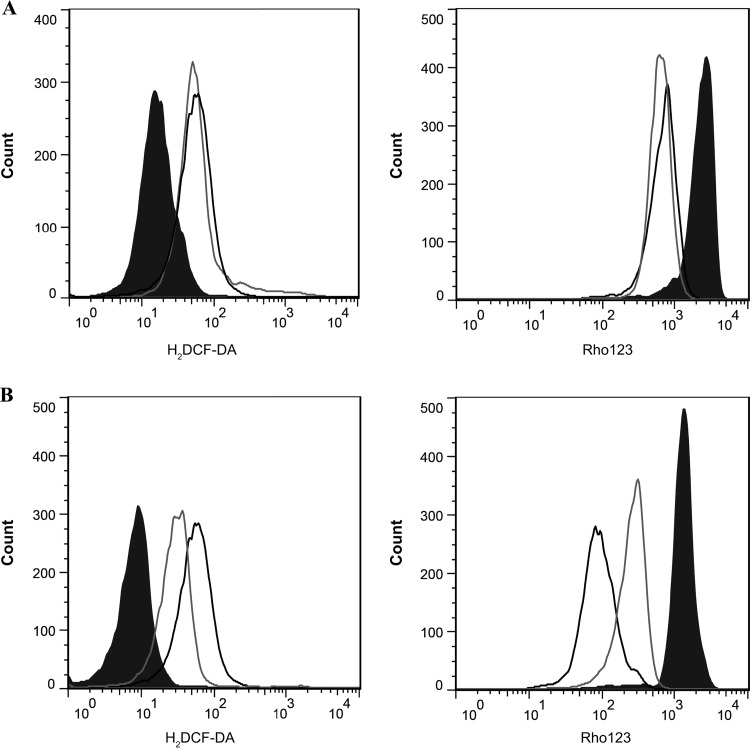
As the participation of ROS in the Bz mechanism of action is still controversial, ROS production and ΔΨm were evaluated by flow cytometry using specific probes in Bz-treated parasites of both lineages. Treatment with Bz induced significant ROS production and Ψm decreases in both CL Brener and Nicaragua compared to levels in untreated controls (Fig. 3). However, no differences between the two lineages were found when relative levels of ROS production and Ψm decrease were analyzed (Table 1), suggesting that Bz reduction by NADH and NADPH-dependent reductases may not generate ROS and, as stated by others (8, 10), that another mechanism different from ROS production may be responsible for Bz cytotoxicity.
FIG 3.
ROS production and mitochondrial membrane potential of CL Brener and Nicaragua epimastigotes treated with Bz. Shown are representative histograms corresponding to fluorescence analysis of epimastigotes of CL Brener (A) and Nicaragua (B) strains, either untreated or treated with 50 μM Bz and stained with H2DCF-DA (ROS) or rhodamine 123 (ΔΨm). The black histograms represent untreated parasites. The black line corresponds to a positive control performed with either 0.5 mM H2O2 for ROS production or 20 μM carbonyl cyanide m-chlorophenyl hydrazone (CCCP) for ΔΨm detection. The gray line corresponds to parasites treated with 50 μM Bz.
DISCUSSION
Here, we demonstrated that not only recombinant but also native TcAKR enzymes of T. cruzi reduce Bz using NADPH as a cofactor, with specific activity ranges in the same order of magnitude. These findings were supported by TcAKR-overexpressing parasites, which showed increased NADPH-dependent Bz reductase activity and higher resistance to this drug, suggesting that this enzyme may be involved in Bz detoxification. In addition, the NADPH-dependent reduction of Bz by TcAKR is consistent with the affinity of the natural enzyme to a Bz derivative coupled to a solid matrix found by Trochine et al. (22). It is important to note that the catalytic activities toward Bz are very difficult to measure because they are very poor and because Bz and the cofactor react nonenzymatically. Nevertheless, the Bz reductase activity of TcAKR was only slightly lower than that of recombinant NTR I (7, 26). However, unlike NTR I, a 2- to 4-fold increase in TcAKR expression modified the Bz resistance phenotype of transgenic parasites only mildly.
Throughout the work, TcAKR levels evaluated by Western blotting correlated with AKR, QOR, and NADPH-dependent Bz reductase activities. To our knowledge, besides TcAKR, no other enzymes of the AKR superfamily have been described in T. cruzi, and although several NADPH-dependent oxido-reductases have been identified (25, 27), we have shown that TcAKR has the highest abundance (19). Hence, we may assume that AKR activity, measured using 4-NBA as the substrate, reflects the amount of TcAKR present in the parasite lysate. In contrast, as other T. cruzi oxido-reductases display QOR activity (25, 26), this cannot be taken as a good indicator of TcAKR levels. The results reported here also indicate that we cannot ascribe NADPH-dependent Bz reductase activity solely to TcAKR.
To understand the importance of the NADPH-dependent reduction of Bz by TcAKR in nature, we studied this enzyme in two Bz-susceptible T. cruzi strains with significantly different IC50s. Taking into account the results obtained with TcAKR-overexpressing epimastigotes, we expected the more resistant strain, Nicaragua, to have higher TcAKR levels than CL Brener. However, the results were the opposite. As the only parasite enzyme that has been demonstrated to play a key role in Bz activation is NTR I, which uses NADH as a cofactor (7, 8), we evaluated the NADH-dependent Bz reductase activity in both strains. Since NTR I is a mitochondrial enzyme, before measuring its activity, we ensured the release of matrix material from mitochondria by checking the presence of cytochrome c in the soluble fraction by Western blotting (data not shown). In line with previous publications (8), the NADH-dependent Bz reductase activity was higher in CL Brener than in Nicaragua. In addition, that CL Brener showed higher NADPH-dependent Bz reductase activity than Nicaragua and that this activity was higher than that attributable to TcAKR suggest that Bz may be also activated through NADPH-dependent reductases.
The participation of ROS in the Bz mechanism of action is still controversial. It has been documented that Bz is reduced to its anion radical. However, the level of ROS detected seems to be insufficiently high to inhibit T. cruzi growth (5, 10, 12). To better understand the mechanism of action of Bz, we evaluated ROS production and ΔΨm in Bz-treated epimastigotes using a technique different from that used in previous studies: flow cytometry with the specific probes, H2DCF-DA and rhodamine 123, respectively. In order to determine whether there is a correlation between changes in these two parameters and susceptibility to Bz, the experiments were performed in two T. cruzi strains with not only differential susceptibilities to Bz but also different TcAKR expression levels and NADH and NADPH-dependent Bz reductase activities. We demonstrated that treatment with Bz produces ROS and ΔΨm. However, since these effects were observed to the same extent in CL Brener and Nicaragua, we may infer (i) that the IC50 differences between the two strains are not due to these alterations and (ii) that Bz reduction by NADH and NADPH-dependent reductases may not generate ROS. This result is in agreement with previous reports demonstrating that the trypanocidal effect of Bz is exerted by reduced metabolites of the drug interacting with macromolecules from the parasite rather than by ROS formation and ΔΨm (17, 18, 28).
Approaches to understand the Bz mechanism of action have yielded a wide range of proteins (14, 15, 16, 22, 29), but regulation of neither NTR I nor TcAKR has been detected. There is no documentation regarding Bz detoxification pathways, and no enzymes have so far been demonstrated to be involved in this mechanism. However, our results suggest that TcAKR is one of the enzymes participating in this process. Future research about the catalytic mechanism used by TcAKR with Bz as the substrate may elucidate this unknown metabolism. Our studies with TcAKR-overexpressing epimastigotes and T. cruzi strains with differential susceptibilities to Bz allow the conclusion that although TcAKR uses Bz as a substrate, the Bz reductase activity of TcAKR is not a determinant of Bz resistance.
ACKNOWLEDGMENTS
This work was supported by FOCANLIS 2011 and 2013 from the Instituto Nacional de Parasitología “Dr. Mario Fatala Chaben”—ANLIS “Dr. Carlos G. Malbrán.” M.L. had fellowships from AMSUD-PASTEUR, ANPCyT, and the National Research Council of Argentina (CONICET), and his work was done in the laboratory of J. J. Cazzulo (Instituto de Investigaciones Biotecnologicas IIB-INTECH). G.A.G. is a member of the Research Career of CONICET.
We thank John Kelly and Martin Taylor (London School of Tropical Medicine, London, United Kingdom) for kindly providing the pTcINDEX vector.
REFERENCES
- 1.Dias JC, Silveira AC, Schofield CJ. 2002. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz 97:603–612. doi: 10.1590/S0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 2.Schmunis GA, Yadon ZE. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Rassi A Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 4.Sosa-Estani S, Viotti R, Segura EL. 2009. Therapy, diagnosis and prognosis of chronic Chagas disease: insight gained in Argentina. Mem Inst Oswaldo Cruz 104:167–180. doi: 10.1590/S0074-02762009000900023. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson SR, Bot C, Kelly JM, Hall BS. 2011. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr Top Med Chem 11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 6.Baker N, Alsford S, Horn D. 2011. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol Biochem Parasitol 176:55–57. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. 2008. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci U S A 105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall BS, Wilkinson SR. 2012. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56:115–123. doi: 10.1128/AAC.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos MC, Leon LL, Taylor MC, Kelly JM. 2014. Benznidazole-resistance in Trypanosoma cruzi: evidence that distinct mechanisms can act in concert. Mol Biochem Parasitol 193:17–19. doi: 10.1016/j.molbiopara.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno SN, Docampo R, Mason RP, León W, Stoppani AO. 1982. Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi microsomal preparations. Arch Biochem Biophys 218:585–591. doi: 10.1016/0003-9861(82)90383-6. [DOI] [PubMed] [Google Scholar]
- 11.Docampo R, Moreno SN. 1984. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis 6:223–238. doi: 10.1093/clinids/6.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Moreno SN. 1988. The reductive metabolism of nifurtimox and benznidazole in Crithidia fasciculata is similar to that in Trypanosoma cruzi. Comp Biochem Physiol C 91:321–325. doi: 10.1016/0742-8413(88)90036-9. [DOI] [PubMed] [Google Scholar]
- 13.Maya JD, Bollo S, Núñez-Vergara LJ, Squella JA, Repetto Y, Morello A, Perie J, Chauvière G. 2003. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem Pharmacol 65:999–1006. doi: 10.1016/S0006-2952(02)01663-5. [DOI] [PubMed] [Google Scholar]
- 14.Villarreal D, Nirdé P, Hide M, Barnabé C, Tibayrenc M. 2005. Differential gene expression in benznidazole-resistant Trypanosoma cruzi parasites. Antimicrob Agents Chemother 49:2701–2709. doi: 10.1128/AAC.49.7.2701-2709.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murta SM, Nogueira FB, dos Santos PF, Campos FM, Volpe C, Liarte DB, Nirdé P, Probst CM, Krieger MA, Goldenberg S, Romanha AJ. 2008. Differential gene expression in Trypanosoma cruzi populations susceptible and resistant to benznidazole. Acta Trop 107:59–65. doi: 10.1016/j.actatropica.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Andrade HM, Murta SM, Chapeaurouge A, Perales J, Nirdé P, Romanha AJ. 2008. Proteomic analysis of Trypanosoma cruzi resistance to benznidazole. J Proteome Res 7:2357–2367. doi: 10.1021/pr700659m. [DOI] [PubMed] [Google Scholar]
- 17.Trochine A, Creek DJ, Faral-Tello P, Barrett MP, Robello C. 2014. Benznidazole biotransformation and multiple targets in Trypanosoma cruzi revealed by metabolomics. PLoS Negl Trop Dis 8:e2844. doi: 10.1371/journal.pntd.0002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz de Toranzo EG, Castro JA, Franke de Cazzulo BM, Cazzulo JJ. 1988. Interaction of benznidazole reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia 44:880–881. doi: 10.1007/BF01941187. [DOI] [PubMed] [Google Scholar]
- 19.Garavaglia PA, Cannata JB, Ruiz AM, Maugeri D, Duran R, Galleano M, García GA. 2010. Identification, cloning and characterization of an aldo-keto reductase from Trypanosoma cruzi with quinone oxido-reductase activity. Mol Biochem Parasitol 173:132–141. doi: 10.1016/j.molbiopara.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Boveris A, Docampo R, Turrens JF, Stoppani AO. 1978. Effect of beta-lapachone on superoxide anion and hydrogen peroxide production in Trypanosoma cruzi. Biochem J 175:431–439. doi: 10.1042/bj1750431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goijman SG, Stoppani AO. 1985. Effects of beta-lapachone, a peroxide-generating quinone, on macromolecule synthesis and degradation in Trypanosoma cruzi. Arch Biochem Biophys 240:273–280. doi: 10.1016/0003-9861(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 22.Trochine A, Alvarez G, Sandra Corre S, Paula Faral-Tello P, Durán R, Batthyany CI, Cerecetto H, González M, Robello C. 2014. Trypanosoma cruzi chemical proteomics using immobilized benznidazole. Exp Parasitol 140:33–38. doi: 10.1016/j.exppara.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MC, Kelly JM. 2006. pTcINDEX: a stable tetracycline-regulated expression vector for Trypanosoma cruzi. BMC Biotechnol 6:32. doi: 10.1186/1472-6750-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosso NL, Bua J, Perrone AE, González MN, Bustos PL, Postan M, Fichera LE. 2010. Trypanosoma cruzi: biological characterization of an isolate from an endemic area and its susceptibility to conventional drugs. Exp Parasitol 126:239–244. doi: 10.1016/j.exppara.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubata BK, Kabututu Z, Nozaki T, Munday CJ, Fukuzumi S, Ohkubo K, Lazarus M, Maruyama T, Martin SK, Duszenko M, Urade Y. 2002. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J Exp Med 196:1241–1251. doi: 10.1084/jem.20020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall BS, Meredith EL, Wilkinson SR. 2012. Targeting the substrate preference of a type I nitroreductase to develop anti-trypanosomal quinone-based prodrugs. Antimicrob Agents Chemother 56:5821–5830. doi: 10.1128/AAC.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumenstiel K, Schoneck R, Yardley V, Croft SL, Krauth-Siegel RL. 1999. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem Pharmacol 58:1791–1799. doi: 10.1016/S0006-2952(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 28.Maya JD, Rodríguez A, Pino L, Pabon A, Ferreira J, Pavani M, Repetto Y, Morello A. 2004. Effects of buthionine sulfoximine nifurtimox and benznidazole upon trypanothione and metallothionein proteins in Trypanosoma cruzi. Biol Res 37:61–69. [DOI] [PubMed] [Google Scholar]
- 29.Murta SM, Krieger MA, Montenegro LR, Campos FF, Probst CM, Avila AR, Muto NH, de Oliveira RC, Nunes LR, Nirdé P, Bruna-Romero O, Goldenberg S, Romanha AJ. 2006. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol 146:151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]