Abstract
This analysis of nearly 10,000 hospital-associated urinary tract infection (UTI) episodes due to Escherichia coli showed that fluoroquinolone and third-generation-cephalosporin resistance rates were 34.5% and 8.6%, respectively; the rate of concurrent resistance to both agents was 7.3%. Fluoroquinolone resistance rates exceeded 25% regardless of geographic location or hospital characteristics. The findings suggest that fluoroquinolones should be reserved and third-generation cephalosporins be used with caution as empirical agents for hospitalized patients with UTIs due to E. coli.
TEXT
It is well established that Escherichia coli is the predominant cause of urinary tract infections (UTIs) (1, 2). In the hospital setting, fluoroquinolones or third-generation cephalosporins are commonly prescribed to provide empirical coverage against this pathogen among patients with UTIs. However, the effectiveness of these first-line agents has been compromised by the emergence of antibiotic resistance (3). The Infectious Diseases Society of America (IDSA) discourages empirical use of therapeutic agents for UTIs when resistance rates exceed 10 to 20% (1). While this is broadly recognized, few published contemporary surveillance studies among hospitalized patients have documented resistance rates for commonly used antibiotics for UTIs due to E. coli. This study was conducted to identify geographic locations and hospital characteristics associated with elevated resistance rates for fluoroquinolones and third-generation cephalosporins among hospitalized patients with UTIs due to E. coli.
This retrospective observational study used hospital discharge data from approximately 160 U.S. health care facilities in the Premier research database (1 January 2009 to 31 March 2013) with accessible microbiology results. Patients from the database were included if they had positive urine culture results for E. coli and had received an antibiotic with activity against Gram-negative organisms on the index culture date or within the 3-day period thereafter. For patients with multiple UTIs during a hospitalization, subsequent UTI episodes within 30 days after the first episode were excluded.
Nonsusceptibility was determined according to individual guidelines for susceptibility testing at each participating site. Fluoroquinolone resistance was defined as nonsusceptibility to levofloxacin, ciprofloxacin, or gatifloxacin. Third-generation-cephalosporin resistance, a surrogate marker of organisms that produce an extended-spectrum β-lactamase (ESBL), was classified as nonsusceptibility to ceftazidime, ceftriaxone, or cefotaxime. Fluoroquinolone and third-generation-cephalosporin resistance rates were stratified by U.S. geographic region (nine Centers for Disease Control and Prevention regions, namely, Mountain, Pacific, West North Central, West South Central, East North Central, East South Central, New England, Middle Atlantic, and South Atlantic) and hospital type (teaching versus nonteaching and urban versus rural).
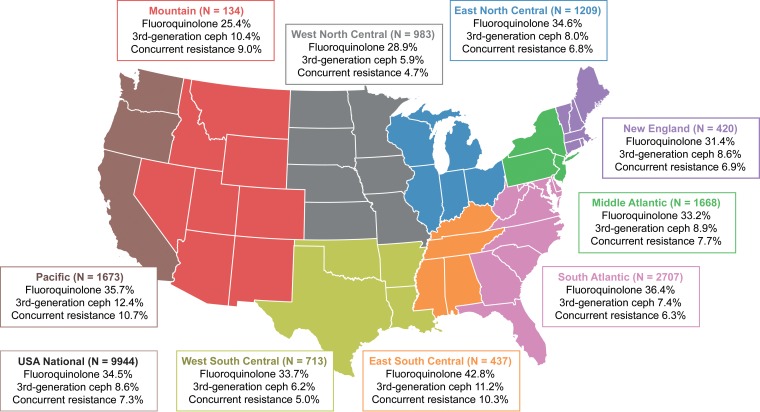
Descriptive statistics for patient demographics and characteristics of hospital visits, stratified by fluoroquinolone resistance, third-generation-cephalosporin resistance, and concurrent resistance, are provided in Table 1. A total of 9,944 UTI episodes due to E. coli were included for analysis. Overall rates of resistance to fluoroquinolones and third-generation cephalosporins were 34.5% and 8.6%, respectively. The rate of concurrent resistance to both fluoroquinolones and third-generation cephalosporins was 7.3%. Some variation in resistance phenotypes was seen upon stratification by geographic region (Fig. 1). Higher resistance rates were observed in the East South Central and Pacific areas, while lower rates were seen in the West North Central area. Stratification by hospital type showed stable fluoroquinolone resistance rates of around 35%, while ESBL and concurrent fluoroquinolone and ESBL-phenotype resistance rates were <10% (Table 1). Generally, the resistance rates for nonteaching and urban hospitals were slightly higher than the rates observed for their academic and rural counterparts.
TABLE 1.
Patient demographics and characteristics of hospital visits, stratified by fluoroquinolone resistance, third-generation-cephalosporin resistance, and concurrent resistance, for hospitalized patients with UTIs due to E. coli
| Characteristic | Overall no. of episodes (%) | No. of resistant episodes/no. of episodes per category (%) |
||
|---|---|---|---|---|
| Fluoroquinolone | Third-generation cephalosporin | Fluoroquinolone and third-generation cephalosporin | ||
| All | 9,944 (100) | 3,430 (34.5) | 853 (8.6) | 728 (7.3) |
| Age group | ||||
| 0–17 yr | 500 (5.0) | 21 (4.2) | 8 (1.6) | 6 (1.2) |
| 18–34 yr | 1,480 (14.9) | 183 (12.4) | 42 (2.8) | 28 (1.9) |
| 35–54 yr | 1,556 (15.6) | 470 (30.2) | 117 (7.5) | 92 (5.9) |
| 55–64 yr | 1,196 (12.0) | 484 (40.5) | 126 (10.5) | 105 (8.8) |
| ⩾65 yr | 5,212 (52.4) | 2,272 (43.6) | 560 (10.7) | 497 (9.5) |
| Sex | ||||
| Female | 6,531 (65.7) | 1,906 (29.2) | 476 (7.3) | 396 (6.1) |
| Male | 3,413 (34.3) | 1,524 (44.7) | 377 (11.0) | 332 (9.7) |
| Race/ethnicity | ||||
| Black/African American | 1,471 (14.8) | 494 (33.6) | 71 (4.8) | 53 (3.6) |
| Other | 1,334 (13.4) | 426 (31.9) | 170 (12.7) | 144 (10.8) |
| White | 7,139 (71.8) | 2,510 (35.2) | 612 (8.6) | 531 (7.4) |
| Blood culture | ||||
| No | 9,297 (93.5) | 3,227 (34.7) | 800 (8.6) | 687 (7.4) |
| Yes | 647 (6.5) | 203 (31.4) | 53 (8.2) | 41 (6.3) |
| Primary payer | ||||
| Charity | 42 (0.4) | 6 (14.3) | 1 (2.4) | 0 (0.0) |
| Commercial, indemnity | 369 (3.7) | 99 (26.8) | 22 (6.0) | 20 (5.4) |
| Direct employer contract | 21 (0.2) | 3 (14.3) | 0 (0.0) | 0 (0.0) |
| Indigent | 60 (0.6) | 9 (15.0) | 1 (1.7) | 1 (1.7) |
| Managed care capitated | 43 (0.4) | 13 (30.2) | 5 (11.6) | 5 (11.6) |
| Managed care noncapitated | 1,293 (13.0) | 280 (21.7) | 81 (6.3) | 65 (5.0) |
| Medicaid, managed care capitated | 67 (0.7) | 14 (20.9) | 5 (7.5) | 5 (7.5) |
| Medicaid, managed care noncapitated | 724 (7.3) | 160 (22.1) | 35 (4.8) | 22 (3.0) |
| Medicaid, traditional | 912 (9.2) | 197 (21.6) | 54 (5.9) | 45 (4.9) |
| Medicare, managed care capitated | 137 (1.4) | 58 (42.3) | 17 (12.4) | 15 (10.9) |
| Medicare, managed care noncapitated | 942 (9.5) | 353 (37.5) | 62 (6.6) | 54 (5.7) |
| Medicare, traditional | 4,625 (46.5) | 2,103 (45.5) | 537 (11.6) | 470 (10.2) |
| Other | 133 (1.3) | 41 (30.8) | 11 (8.3) | 10 (7.5) |
| Other government payer | 81 (0.8) | 24 (29.6) | 5 (6.2) | 3 (3.7) |
| Self-pay | 477 (4.8) | 63 (13.2) | 16 (3.4) | 12 (2.5) |
| Workers' compensation | 18 (0.2) | 7 (38.9) | 1 (5.6) | 1 (5.6) |
| Admission type | ||||
| Elective | 633 (6.4) | 215 (34.0) | 57 (9.0) | 51 (8.1) |
| Emergency | 7,543 (75.9) | 2,587 (34.3) | 606 (8.0) | 508 (6.7) |
| Other | 22 (0.2) | 1 (4.5) | 0 (0.0) | 0 (0.0) |
| Trauma center | 49 (0.5) | 10 (20.4) | 1 (2.0) | 1 (2.0) |
| Urgent | 1,697 (17.1) | 617 (36.4) | 189 (11.1) | 168 (9.9) |
| Admission source | ||||
| Home | 8,147 (81.9) | 2,711 (33.3) | 679 (8.3) | 581 (7.1) |
| Other | 378 (3.8) | 123 (32.5) | 22 (5.8) | 18 (4.8) |
| Skilled nursing facility transfer | 392 (3.9) | 254 (64.8) | 57 (14.5) | 48 (12.2) |
| Transfer from another hospital | 1,027 (10.3) | 342 (33.3) | 95 (9.3) | 81 (7.9) |
| Hospital type | ||||
| Nonteaching | 5,815 (58.5) | 2,060 (35.4) | 555 (9.5) | 473 (8.1) |
| Teaching | 4,129 (41.5) | 1,370 (33.2) | 298 (7.2) | 255 (6.2) |
| Hospital location | ||||
| Rural | 907 (9.1) | 313 (34.5) | 60 (6.6) | 50 (5.5) |
| Urban | 9,037 (90.9) | 3,117 (34.5) | 793 (8.8) | 678 (7.5) |
| Geographic region | ||||
| East North Central | 1,209 (12.2) | 418 (34.6) | 97 (8.0) | 82 (6.8) |
| East South Central | 437 (4.4) | 187 (42.8) | 49 (11.2) | 45 (10.3) |
| Middle Atlantic | 1,668 (16.8) | 554 (33.2) | 148 (8.9) | 128 (7.7) |
| Mountain | 134 (1.3) | 34 (25.4) | 14 (10.4) | 12 (9.0) |
| New England | 420 (4.2) | 132 (31.4) | 36 (8.6) | 29 (6.9) |
| Pacific | 1,673 (16.8) | 597 (35.7) | 208 (12.4) | 179 (10.7) |
| South Atlantic | 2,707 (27.2) | 984 (36.4) | 199 (7.4) | 171 (6.3) |
| West North Central | 983 (9.9) | 284 (28.9) | 58 (5.9) | 46 (4.7) |
| West South Central | 713 (7.2) | 240 (33.7) | 44 (6.2) | 36 (5.0) |
| Hospital size | ||||
| 0–100 beds | 273 (2.7) | 81 (29.7) | 19 (7.0) | 15 (5.5) |
| 100–199 beds | 1,273 (12.8) | 401 (31.5) | 84 (6.6) | 71 (5.6) |
| 200–299 beds | 2,256 (22.7) | 818 (36.3) | 191 (8.5) | 168 (7.4) |
| 300–499 beds | 3,601 (36.2) | 1,288 (35.8) | 370 (10.3) | 316 (8.8) |
| ≥500 beds | 2,541 (25.6) | 842 (33.1) | 189 (7.4) | 158 (6.2) |
| Large (≥500 beds) | 2,541 (25.6) | 842 (33.1) | 189 (7.4) | 158 (6.2) |
| Medium (200–499 beds) | 5,857 (58.9) | 2,106 (36.0) | 561 (9.6) | 484 (8.3) |
| Small (0–199 beds) | 1,546 (15.5) | 482 (31.2) | 103 (6.7) | 86 (5.6) |
FIG 1.
Fluoroquinolone and third-generation-cephalosporin resistance rates among E. coli isolates from hospitalized patients with UTIs, stratified by geographic area (U.S. Centers for Disease Control and Prevention regions), in 2009 to 2013. Ceph, cephalosporin.
Overall, there were several notable resistance patterns in this analysis of nearly 10,000 episodes of UTIs due to E. coli across approximately 160 U.S. hospitals. First, fluoroquinolone resistance exceeded 25% across all geographic and hospital type strata. The overall observed incidence of fluoroquinolone resistance (34.5%) was comparable to those described in a Study for Monitoring Antimicrobial Resistance Trends (SMART) analysis from 2009 to 2011 with fewer E. coli isolates (n = 1,045; ciprofloxacin and levofloxacin resistance rates of 35.2% and 34.8%, respectively) (4). These rates are considerably higher than those reported in surveillance studies of hospitalized patients with UTIs in North America from the early 2000s (3 to 5%) (5, 6). Other noteworthy findings were the observed rates of resistance to third-generation cephalosporins (a surrogate marker for ESBL producers) among patients with UTIs due to E. coli. The overall incidence of resistance to third-generation cephalosporins exceeded 5 to 10% in certain geographic regions. Interestingly, rates of concurrent resistance to fluoroquinolones and third-generation cephalosporins were similar to rates of resistance to third-generation cephalosporins (approximately 5 to 10%).
Given that resistance trends drive empirical therapy selection, our findings have important implications for clinical practice. Currently, fluoroquinolone antibiotics are frequently selected for empirical UTI treatment in hospital settings. In one study, fluoroquinolones were used for 42% of empirical therapy days (7). Although current IDSA UTI treatment guidelines identify fluoroquinolones as an appropriate empirical therapy option, this recommendation is contingent on local resistance rates being <10% (1, 2). Given the endemicity of fluoroquinolone-resistant E. coli found in our large population-based study, we propose that fluoroquinolones should not be used empirically for hospitalized patients with UTIs. Furthermore, our results suggest that caution should be exercised when using third-generation cephalosporins, such as ceftriaxone and ceftazidime, as empirical treatment for hospitalized patients with UTIs, as national resistance rates approach 10%. Institutions should be proactive and perform patient-level risk factor analyses to identify patient populations at greatest risk for third-generation-cephalosporin resistance. Given the risk for concurrent drug resistance among these patients, alternatives to both fluoroquinolones and third-generation cephalosporins should be considered empirically. Collectively, these data indicate that hospitalized patients with UTIs are candidates for empirical therapy that has a wider spectrum of activity than a fluoroquinolone or third-generation cephalosporin; institution-specific susceptibility rates should guide ultimate empirical therapy selection.
Several limitations affect the interpretation of these findings. We relied on susceptibility reporting by participating institutions. While it would have been preferable to employ a central clinical microbiology reference laboratory, this study provides “real-world” susceptibility data on consecutive isolates across a greater number of institutions than in a typical surveillance study. We defined UTIs by positive clinical urine culture results and receipt of antibiotic treatment only; differentiation between true infections and colonization was not possible without complete individual clinical information. However, our data clearly highlight major concerns regarding E. coli resistance among hospitalized patients. Although we stratified resistance rates by region and hospital characteristics, our findings may not apply to every institution in a given region. Local antibiograms should always drive empirical antibiotic selection. Although ESBL production was not confirmed with formal molecular testing, third-generation-cephalosporin resistance is a practical surrogate measure that is readily available on standard culture susceptibility reports. This study did not assess resistance rates among other uropathogens; empirical UTI treatment is primarily directed against E. coli, and its resistance patterns drive initial therapy selection. Additionally, we did not evaluate antibiotic resistance factors on a patient-specific level. Formal analysis of patient-specific risk factors for resistance is important, but this granularity was outside the scope of our objective to characterize E. coli resistance rates on the regional and institutional levels. When considering the potential impact of patient-specific factors on our findings, it is notable that only 3.9% of our cohort was from a skilled nursing facility. Greater inclusion of skilled nursing facility patients would likely result in even higher resistance rates than those observed in this study, but further evaluation is needed. Despite these limitations, we think that characterization of these resistance trends still provides valuable actionable information for clinicians. Furthermore, our methods and findings complement a growing interest in electronic medical record-based surveillance, particularly for antimicrobial stewardship benchmarking.
In conclusion, our findings underscore national public health concerns about increasing fluoroquinolone and third-generation-cephalosporin resistance among hospitalized patients with UTIs due to E. coli. Given that antimicrobial resistance patterns drive empirical therapy choices, our findings suggest that fluoroquinolones should be reserved and third-generation cephalosporins be used with caution among hospitalized patients with UTIs. While knowledge of resistance rates by geographic region and hospital type can provide a useful benchmark, clinicians should ultimately base empirical therapy decisions on institution-specific patterns and patient-specific data whenever possible.
ACKNOWLEDGMENTS
We thank Min Jung Yoon for outstanding assistance with data preparation and analysis and Zachary S. Henney for figure visualization support. Editorial support for the manuscript was provided by Jean Turner of Parexel and Meryl Mandle of ApotheCom and was funded by Merck & Co., Inc. (Kenilworth, NJ).
This study was funded by Merck & Co., Inc.
M. R. Bidell has no reported conflicts of interest. M. Palchak and J. Mohr were employees of Merck, Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc.) at the time of this study. T. P. Lodise has served as a consultant for Merck, Sharp & Dohme Corp.
The author contributions were as follows: study concept and design, M. Palchak, J. Mohr, and T. P. Lodise; analysis and interpretation of data, all authors; drafting of the manuscript, M. R. Bidell, M. Palchak, and T. P. Lodise; critical revision of the manuscript for important intellectual content, all authors; obtaining funding, J. Mohr and T. P. Lodise; study supervision, M. Palchak and T. P. Lodise; administrative, technical, or material support, J. Mohr.
REFERENCES
- 1.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 2.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 3.Pallett A, Hand K. 2010. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 65(Suppl 3):iii25–iii33. [DOI] [PubMed] [Google Scholar]
- 4.Bouchillon SK, Badal RE, Hoban DJ, Hawser SP. 2013. Antimicrobial susceptibility of inpatient urinary tract isolates of Gram-negative bacilli in the United States: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2011. Clin Ther 35:872–877. doi: 10.1016/j.clinthera.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Gordon KA, Jones RN. 2003. Susceptibility patterns of orally administered antimicrobials among urinary tract infection pathogens from hospitalized patients in North America: comparison report to Europe and Latin America: results from the SENTRY Antimicrobial Surveillance Program (2000). Diagn Microbiol Infect Dis 45:295–301. doi: 10.1016/S0732-8893(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 6.Mathai D, Jones RN, Pfaller MA. 2001. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn Microbiol Infect Dis 40:129–136. doi: 10.1016/S0732-8893(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 7.BioTrends Research Group. 2012. TreatmentTrends®: Gram negative infections (EU). BioTrends Research Group, Exton, PA. [Google Scholar]