Abstract
The increasing number of infections produced by beta-lactam–resistant Gram-positive bacteria and the morbidity secondary to these infections make it necessary to optimize the use of vancomycin. In 2009, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Disease Pharmacists published specific guidelines about vancomycin dosage and monitoring. However, these guidelines have not been updated in the past 6 years. This review analyzes the new available information about vancomycin published in recent years regarding pharmacokinetics and pharmacodynamics, serum concentration monitoring, and optimal vancomycin dosing in special situations (obese people, burn patients, renal replacement therapy, among others). Vancomycin efficacy is linked to a correct dosage which should aim to reach an area under the curve (AUC)/MIC ratio of ≥400; serum trough levels of 15 to 20 mg/liter are considered a surrogate marker of an AUC/MIC ratio of ≥400 for a MIC of ≤1 mg/liter. For Staphylococcus aureus strains presenting with a MIC >1 mg/liter, an alternative agent should be considered. Vancomycin doses must be adjusted according to body weight and the plasma trough levels of the drug. Nephrotoxicity has been associated with target vancomycin trough levels above 15 mg/liter. Continuous infusion is an option, especially for patients at high risk of renal impairment or unstable vancomycin clearance. In such cases, vancomycin plasma steady-state level and creatinine monitoring are strongly indicated.
INTRODUCTION
Vancomycin has traditionally been used as a first-line agent for treating methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive beta-lactam–resistant bacteria (1), which are frequent etiologies of severe health-related infections (2, 3).
Although the effectiveness of vancomycin is supported by more than 5 decades of use and multiple studies, the clinical and microbiological scenario in which it is used is always changing. Attaining an appropriate dosage of vancomycin for S. aureus infections might be difficult due to the clinical impact of the creep in the MIC of vancomycin and heteroresistance among MRSA strains, or due to complex pharmacokinetic and pharmacodynamic (PK/PD) situations. In this context, the American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) introduced a practice guideline in 2009 (4), marking a milestone in vancomycin therapy. However, several questions, such as the optimal dosing in some special clinical situations (e.g., with renal replacement therapies or in burn patients or obese patients), the role of continuous infusion, or the renal toxicity when the suggested vancomycin serum levels are achieved, remain unanswered. The present review mainly focuses on these issues.
VANCOMYCIN PHARMACODYNAMICS AND ITS IMPLICATIONS FOR DRUG MONITORING
The killing effect of vancomycin is characterized by a slow mode of action and is further hampered by a large bacterial inoculum, a stationary growth phase, and anaerobic conditions (5, 6). Although several pharmacodynamic parameters have been proposed to determine vancomycin activity, data from experimental and clinical studies have selected the area under the curve (AUC)/MIC ratio as the best parameter to predict the effectiveness of vancomycin (4, 7–9). The target consensus of an AUC/MIC ratio of ≥ 400 for MRSA infections is supported by in vitro data, animal models, and clinical studies that have related an AUC/MIC ratio of 350 to 400 to successful outcome (7–18). However, it must be noted that the vancomycin MIC for S. aureus varies depending on the testing method used. Etest yields MICs of 0.5- to 1.5-log2 dilutions higher than those determined by broth microdilution (BMD) (10, 14–19). In this review, unless otherwise noted, all of the AUC/MIC ratios are Etest measures; Etest is the recommended method for measuring the MIC for MRSA bloodstream infection isolates (18).
Using an experimental murine model of MRSA pneumonia, our group found that an optimized dose of vancomycin (AUC/MIC ratio ≥ 400) was more efficacious than lower doses of vancomycin in the clearance of bacteria from lungs and blood, even though it could not demonstrate a higher survival rate (20). In clinical studies, an AUC/MIC ratio around 400 is related to the best survival rate or to clinical success in patients with S. aureus bacteremia, although other thresholds have been observed (10, 17, 18, 21). In a cohort of 182 patients with S. aureus bacteremia, an AUC/MIC ratio of >373 was identified as the breakpoint significantly associated with lower 30-day mortality (odds ratio [OR], 0.44) using the BMD method. Zelenitsky et al. (21) found that, in a group of 35 patients with MRSA-associated septic shock, the survival rate was greater in those with higher AUC/MIC values, reaching 70% when the AUC/MIC ratio was ≥451 (P = 0.006) and 81.8% when the AUC/MIC ratio was ≥578 (P = 0.012). Nonetheless, these results should be interpreted with caution: the AUC was constructed with a population PK model using just one serum level determination, and a MIC of 1 mg/liter was assumed based on surveillance BMD data. A recent retrospective cohort study (18), which used Bayesian methods to estimate the vancomycin exposure profile in 123 patients with MRSA bacteremia, showed that failure (defined as 30-day mortality, bacteremia for ≥ 7 days, or recurrence) was less in those cases achieving an AUC/MICEtest ratio of ≥ 303 and ≥320 (relative risk [RR], 0.5) on day 1 and day 2, respectively or an AUC/MICBMD ratio of ≥ 521 (RR = 0.6) and ≥650 (RR = 0.5) on day 1 and day 2, respectively.
Peak vancomycin serum levels do not correlate to toxicity or efficacy (22). Instead, trough serum levels at steady-state conditions have been proposed as a more accurate and practical method to monitor vancomycin. Guidelines for vancomycin therapy are clear in stressing the importance of therapeutic drug monitoring and the use of the trough concentration as a surrogate for the target AUC. The main guidelines recommendations are to administer dosages of 15 to 20 mg/kg of body weight every 8 to 12 h to achieve target trough levels of 15 to 20 mg/liter and to start monitoring the vancomycin trough concentration before the fourth dose (4). This strategy is based in several premises. First, vancomycin efficacy and toxicity are both related to the AUC, with a quite narrow therapeutic ratio. Second, determining the AUC requires multiple serum vancomycin samples, and a different strategy is needed in the clinical setting to make monitoring easier. However, whether trough levels are an optimal surrogate of AUC is still a source of controversy. In the PK/PD study with a series of Montecarlo simulations performed by Patel et al. (23), a wide range of AUC values from several dosage regimens yielding isometric Cmin values, and vice versa, was found. The simulations also showed that the likelihood of achieving an AUC/MIC ratio of >400 was virtually 100% with different dosage regimens when the trough was 15 to 20 mg/liter and the MIC was ≤1 mg/liter, but the likelihood gradually decreases with the MIC growth. Neely et al. (24) have published the largest population PK model, which is based on three previous data sets from 47 richly sampled adults receiving vancomycin. Their results bear out a noteworthy interpatient variability of AUC, trough, and peak values. These authors performed a two-compartmental model based on the full data set that fitted the observed concentrations well (R2 = 0.902). They found that the AUCs estimated from the trough and the peak-trough data sets were lower than the AUCs from the full data sets, with a difference of 341.9 mg/liter (P < 0.001) and 159.3 mg/liter (P < 0,001), respectively. Notwithstanding, up to 60% of adults who achieved a therapeutic AUC of >400 mg · h/liter would have had a trough concentration below 15 mg/liter (24). This stresses that, for strains with a MIC of ≤1 mg/liter, trough concentrations of 15 mg/liter might usually be enough to achieve the target AUC/MIC ratio of ≥400. Otherwise, if the vancomycin MIC is >1 mg/liter, an alternative agent should be considered. It must be stressed that this recommendation could not apply to S. aureus strains showing heteroresistance to vancomycin. Heterogeneous vancomycin-intermediate S. aureus (hVISA) strains exhibit a vancomycin MIC in the susceptible range but include up to 1/105 to 1/106 bacterial subpopulations with increased MIC (84). The real prevalence of hVISA is unknown; notwithstanding, current data indicates that it is growing. In addition, the proportion of hVISA increases as the vancomycin MIC are >1 mg/liter (85).
Due to this relevant interindividual variability correlating vancomycin trough levels with the AUC/MIC ratio, guiding vancomycin dosing exclusively based on trough levels may be insufficient. A more accurate approach has been provided by linear regression analysis, population PK models, and Bayesian estimation procedures (25).
Linear regression analysis estimates dosing based in two serum determinations, assuming a one-compartment model. It is an easy method but is not particularly accurate in a changing situation (e.g., renal function) (25).
Population methods use population PK parameters to design nomograms for calculating dosages, but these methods have several drawbacks. First, they assume a linear correlation between renal function and vancomycin clearance. Second, they usually seek to ensure target trough levels, not a target AUC. In addition, only a few nomograms have been developed to achieve the current target endpoints. The studies from Wesner et al. (26) and Kullar et al. (27) targeted different trough levels, and those from Revilla et al. (28) constructed nomograms to achieve an AUC/MIC ratio of ≥400. In all cases, application to populations of patients excluded from the studies should be avoided.
The third method, Bayesian estimation procedures, combines optimized population information with PK information from the patient for calculating doses. It is the most accurate procedure when correctly used. By Bayesian techniques, vancomycin dosages can be calculated to achieve a target AUC/MIC; therefore, they avoid the use of trough serum levels as a surrogate target (18). The main drawback is that Bayesian techniques require exact information about many parameters, such as age, weight, renal function, and previous therapeutic regimen, among others. Another disadvantage is the need for trained personnel with specialized pharmacokinetics knowledge (25).
LOADING DOSE
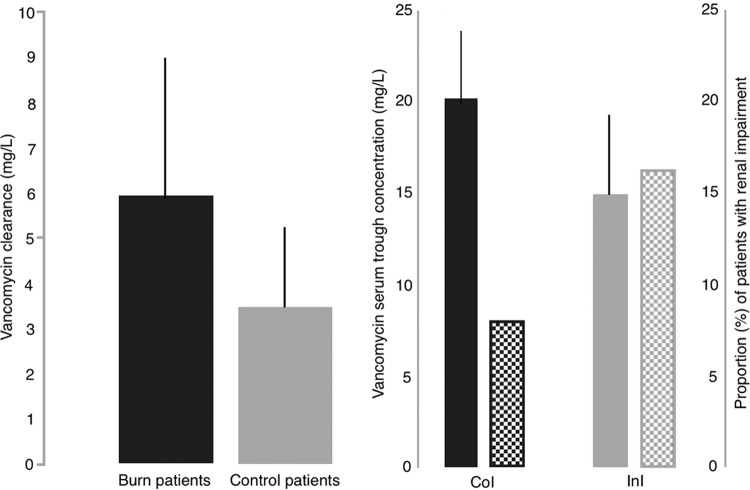
A loading dose of 25 to 30 mg/kg has been proposed as an appropriate strategy in order to avoid subtherapeutic vancomycin levels in the initial stages of therapy. This recommendation is based on one randomized clinical trial (RCT) (29) and on other studies evaluating trough serum vancomycin levels after a loading dose on different types of patients (Fig. 1) (30–32). The previously mentioned RCT (29) assayed a loading dose of 25 to 30 mg/kg in critically ill patients. Regrettably, it presented the caveat that the authors only determined peak vancomycin levels, despite peak levels not correlating to efficacy (29). Recently, Rossini et al. (30) performed an RCT on 99 patients receiving a loading dose of 30 mg/kg of vancomycin or the standard therapy with 15 mg/kg. After 12 h, the proportion of patients achieving a trough level of 15 mg/liter was higher in the group with loading dose (34% versus 3%; P < 0.01), without toxicity differences between them. This study included both critically and noncritically ill patients. Truong et al. (31) failed to find differences in the proportion of patients with trough vancomycin levels of ≥15 mg/liter in a pre- and postintervention study when comparing standard therapy with a fixed loading dose of 2 g in 52 critically ill patients. Despite that, the mean (± standard deviation [SD]) trough plasma concentrations were higher in the postintervention group (9.8 ± 6.6 versus 14.9 ± 6.3 mg/liter). However, the sample is lacking statistical power, with just 11 patients receiving the loading dose. Vandecasteele et al. (32) proposed a loading dose for patients undergoing hemodialysis. It was calculated according to dry body weight and the period to the next dialysis session. The usefulness of a loading dose to achieve the targeted trough levels early in other groups of patients has not been assessed. In summary, selected patients with severe disease may benefit from a vancomycin loading dose with the aim of achieving early steady-state levels. Further studies are needed to clarify the clinical impact of applying a loading dose in all kinds of patients.
FIG 1.
Recommendations of a vancomycin loading dose (LD), to achieve early therapeutic levels, have been established after several studies evaluating trough serum vancomycin concentrations after an LD. In critically ill patients (CIP), trough vancomycin levels (mean ± SD) after a fixed LD of 2 g (≈30 mg/kg, n = 21 patients) was higher than in patients without an LD (n = 31) (P = 0.01) in an intervention observational study (31) (evidence level IIB [83]). In patients presenting to an emergency department (EDP), trough levels (mean ± SD) after an LD of 30 mg/kg (n = 50) were higher than without an LD (n = 49) (P < 0.001) in a randomized clinical trial (30) (evidence level IA [83]). Finally, in patients on hemodialysis (HD) (n = 15), trough serum levels (mean ± SD) after an LD of 20 mg/kg (32) were similar to those found in the above-mentioned two studies carried out in patients with normal renal function (evidence level IIC [83]).
INTERMITTENT VERSUS CONTINUOUS INFUSION THERAPY
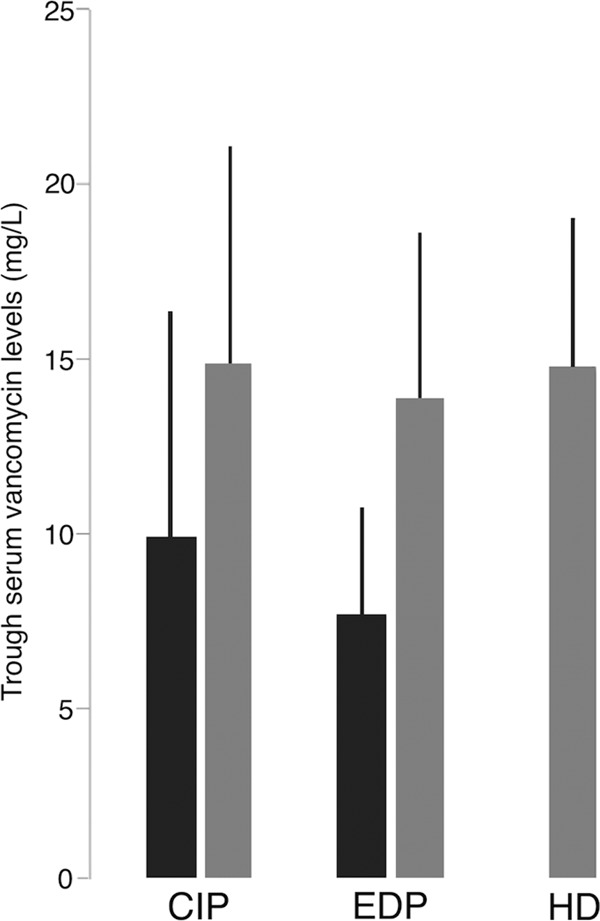
All efforts to demonstrate differences in the effectiveness of continuous infusion (CoI) and intermittent infusion (InI) have failed (33–35). In contrast, there are many reports of a reduced toxicity of CoI with respect to InI (36). Cataldo et al. (34) performed a meta-analysis comparing these two dosing approaches and concluded that CoI achieved a similar overall mortality rate and less renal impairment (Fig. 2). Of note, only six studies, quite heterogeneous (I2 of 90% for vancomycin exposure, I2 of 0 for nephrotoxicity and mortality), could be included, and just one was a randomized clinical trial, so results cannot be considered conclusive. Subsequently, Hanrahan et al. (37) found a significant association between InI and nephrotoxicity in a retrospective cohort of 1,430 critically ill patients (OR, 8.2). Thus, the available information encourages using CoI when a high risk of nephrotoxicity exists, such as in the coadministration of other nephrotoxic agents (aminoglycosides or loop diuretics), in the presence of septic shock, or in patients needing high vancomycin doses (those with central nervous system infection or obese patients). Stronger evidence would be welcome.
FIG 2.
Continuous (CoI) versus intermittent (InI) vancomycin infusion impact on mortality and nephrotoxicity has been evaluated through a systematic review and meta-analysis of vancomycin for the treatment of Gram-positive infections (34). The global mortality was not different between patients on CoI versus those on InI (RR, 1.03; 95% CI, 0.7 to 1.6; P = 0.9). On the contrary, nephrotoxicity was higher in patients receiving vancomycin InI than in those with CoI (P = 0.02).
In addition, CoI has other advantages, such as easier monitoring and lower price (33). It is an attractive option for the treatment and monitoring of outpatients, in which an efficacy similar to InI has been shown (35), and on busy nursing wards. Continuous vancomycin infusion might require less therapeutic drug monitoring, and samples can be obtained anytime after the first 18 to 24 h (38). This could be useful for patients with unstable vancomycin clearance (burns patients, patients under continuous renal replacement therapy). The solution of vancomycin for CoI should be stable for at least 72 h, although careful attention must be paid to incompatibilities with other medications in the same infusion (39).
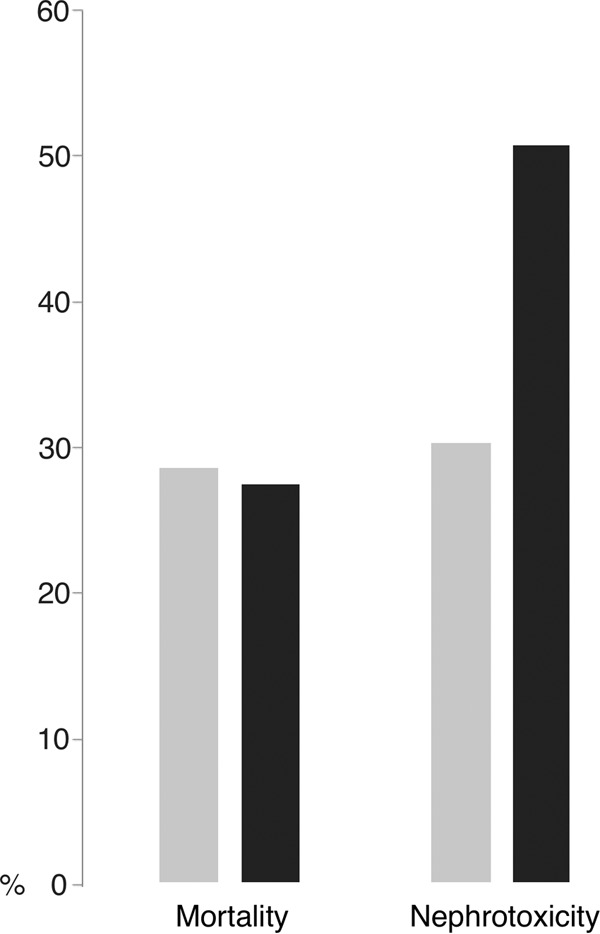
Different target steady-state levels have been proposed. A PD study reasserted that bacterial killing and development of diminished vancomycin susceptibility during CoI therapy were dependent on the AUC/MIC ratio, with values of 480 being bactericidal and suppressing emerging resistance; this target would be achieved with CoI at a steady-state concentration of 20 mg/liter when vancomycin pathogen MICs are 1 mg/liter (40). Other authors have suggested that, given the tendency toward increasing vancomycin MICs in clinical isolates of S. aureus in some centers, a target concentration of 25 mg/liter may be more appropriate (41). However, in this case, careful monitoring must be carried out. Norton et al. (42) and Spapen et al. (43) refer to the fact that steady-state concentrations of ≥32 mg/liter and >30 mg/liter, respectively, which are close to 25 mg/liter, were related to higher risks of nephrotoxicity in patients receiving CoI of vancomycin (Fig. 3).
FIG 3.
The suggested steady-state concentrations to be reached in vancomycin continuous infusion (CoI) should be between 20 and 30 mg/liter (evidence level IIB [83]), to avoid nephrotoxicity. Spapen et al. (43), in a retrospective cohort study carried out in critically ill patients, found high acute kidney injury frequency in patients with vancomycin levels of >30 mg/liter (P < 0.01). Norton et al. (42), in a retrospective outpatient cohort, found high nephrotoxicity in patients with vancomycin levels of ≥32 mg/liter (P < 0.01). The diverse types of patients and the nonhomogeneous criteria to determine nephrotoxicity may explain the difference in the proportions of nephrotoxicity between the two studies.
The largest PK study on CoI of vancomycin showed that dosages need to be individualized according to the actual body weight (ABW) and creatinine clearance (CrCl) of the patient (44). To achieve a steady-state concentration of ≥20 mg/liter, these authors propose a minimum loading dose of 35 mg/kg, followed by a daily dose adjusted to CrCl, based on a population PK analysis of retrospective data from critically ill patients. However, the efficacy and safety of this scheme have not been prospectively validated in clinical studies. Several administration protocols and nomograms validated in a few patient cohorts are available. They used different loading doses and also used diverse daily doses, for which calculations were based on estimates of CrCl by the Cockcroft-Gault formula, in order to achieve target steady-state concentrations of 15 or 20 mg/liter (45), 25 mg/liter (46), and 27.5 mg/liter (47). All of these schemes were developed in critically ill patient samples, so implementation in other patients may require closer monitoring.
RENAL REPLACEMENT THERAPIES
Many critically ill patients with sepsis need both vancomycin and a continuous renal replacement therapy (CRRT). A proper vancomycin dosage is crucial in order to achieve therapeutic levels without worsening renal function. However, some PK factors must be taken into consideration. Volume of distribution (V) may change rapidly in patients on continuous venovenous hemofiltration, and it is also related to the serum albumin, which can be unstable as well (48). The type of technique (hemofiltration, hemodialysis, or hemodiafiltration), the filter used, the effluent flow rate, the blood flow rate, and the pre- or postfilter volume reposition are the main factors influencing the PK of vancomycin in patients receiving CRRT (49). According to the regression analysis of published PK data performed by Jamal et al. (50), effluent flow rate seems to be the most reliable predictor of extracorporeal vancomycin clearance in patients with CRRT.
Different dosages, either for continuous or intermittent vancomycin therapy, have been proposed in recent PK observational studies (51–56) to understand the best dosages in patients receiving CRRT. CoI seems to be more successful under these conditions to achieve the PK/PD targets. The weight-based CoI proposed by Beumier et al. (52) achieved an AUC/MIC ratio of >400 in all patients with a MIC of ≤1 mg/liter and for 72% of patients when the MIC was 1.5 mg/liter. In contrast, the largest vancomycin population PK analysis conducted in patients undergoing CRRT and receiving CoI of vancomycin was not able to quantify the effect of CRRT on vancomycin pharmacokinetics (54). Escobar et al. (55) propose continuous infusion as the most useful alternative to maintain stable vancomycin levels during high-volume hemofiltration (HVHF). They performed a population PK study and estimated that, after a loading dose of at least 20 mg/kg, maintaining daily doses between 1 and 2 g/24 h (depending on the HVHF intensity) was required to achieve steady-state levels of vancomycin between 20 and 30 mg/liter. Probably, until prospective, multicenter studies provide robust information, weight-based loading dose, CoI, and frequent monitoring of vancomycin levels are best practices in patients on CRRT.
Vancomycin is very useful in patients undergoing intermittent hemodialysis, since it allows outpatients to be treated with intravenous therapy. Nonetheless, classic therapeutic schemes of fixed doses rarely achieve the target trough of 15 to 20 mg/liter. Lin et al. (57) described that an individualized loading dose of 15 to 20 mg/kg followed by a maintaining dose of 500 mg after each hemodialysis session provided trough serum concentrations of 10 to 20 mg/liter in 87% of patients. With a similar loading dose, Rymarz et al. (58) found that 72.7% of patients reached these concentrations. Nonetheless, in the reports by Lin et al. and Rymarz et al., just 68.1% and 27.2% of patients, respectively, achieved trough levels between 15 and 20 mg/liter. In the study by Vandecasteele et al. (32), fixed loading doses achieved vancomycin levels below 15 mg/liter in 87.2% of patients undergoing intermittent hemodialysis. These authors identified predialysis vancomycin trough levels, dry body weight, and time period to the next dialysis session as the parameters best related to vancomycin trough levels in these patients, and they subsequently developed a vancomycin dose calculator and validated it prospectively in an 18-patient cohort; in the cohort, up to 77.9% of patients achieved appropriate therapeutic levels of vancomycin. In a report by Jeremiah et al. (59), calculating the maintaining dose with a nomogram based only on the current trough levels, just 54.5% of patients obtained 15- to 20-mg/liter trough concentrations. Although the dosing of vancomycin in patients undergoing intermittent hemodialysis, to achieve early trough levels between 15 and 20 mg/liter, should generally include a loading dose of ≈20 mg/kg (Fig. 4), the above-cited studies point out that vancomycin therapy for patients undergoing intermittent dialysis must be individualized, taking into consideration as many relevant variables as possible. Moreover, to our knowledge, there is no data to confirm that trough levels of 15 to 20 mg/liter are surrogate markers of fixed AUC in patients on hemodialysis.
FIG 4.
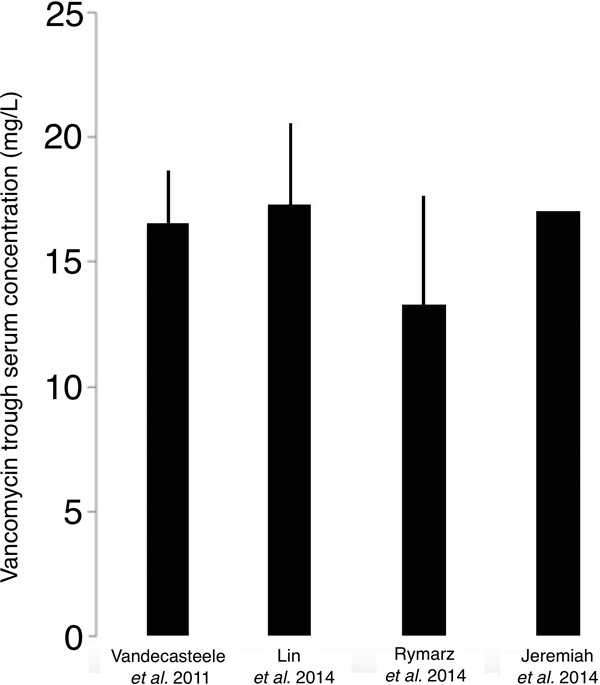
The dosing vancomycin in patients undergoing intermittent hemodialysis, to achieve vancomycin trough concentrations between 15 and 20 mg/liter, must include a loading dosage of ≈20 mg/kg (evidence level IIB [83]), as suggested by a study carried out to develop a vancomycin dose calculator (Vandecasteele et al. [32]) and by several observational studies (Lin et al. [57], Rymarz et al. [58], and Jeremiah et al. [59]) (data expressed as mean ± SD). Thereafter, using pharmacokinetics data from 41 patients (32), the estimated doses for different intervals to the next hemodialysis may be 15, 25, and 35 mg/kg for 1-day, 2-day, and 3-day elapse times, respectively.
VANCOMYCIN AND OBESITY
There is a lack of available data about the dosing of antimicrobials in obese patients, and, focusing on vancomycin, these patients frequently receive insufficient dosages (60). Obesity produces an increased volume of distribution for most antibiotics and different hepatic metabolism and renal excretion. Besides, it is associated with an increase in certain circulating proteins, which results in altered free serum vancomycin concentrations. Increased blood flow secondary to increased cardiac output and blood volume also occurs, resulting in increased vancomycin clearance (61). Achieving adequate vancomycin serum levels and similar efficacy without a high risk of toxicity is a current challenge (62). For obese patients, the most widespread recommendation is an initial dose based on ABW, not exceeding 2 g for each dose, and adjusting the subsequent doses based on serum vancomycin concentrations to achieve therapeutic levels (4). However, Reynolds et al. (63) found that these dosages are associated with a high risk of above-target trough levels. They compared the IDSA-based protocol with a revised one in 74 and 64 obese patients, respectively, reporting that the revised protocol better enabled them to reach trough concentrations of 10 to 20 mg/liter (36% versus 59% in IDSA-based versus revised protocol; P = 0.006). Nonetheless, the higher risk of low concentrations, the absence of demonstrated impact on toxicity, and the low target levels chosen for the study limit its application. Wesner et al. (26) developed a nomogram based on the dry weight to calculate dosages in obese patients. However, the achievement of therapeutic levels was 39% in obese patients, and the study excluded patients with a weight of >120 kg. Therefore, there is not enough data currently to make a statement to guide vancomycin dosing in obese patients.
BURN PATIENTS AND VANCOMYCIN
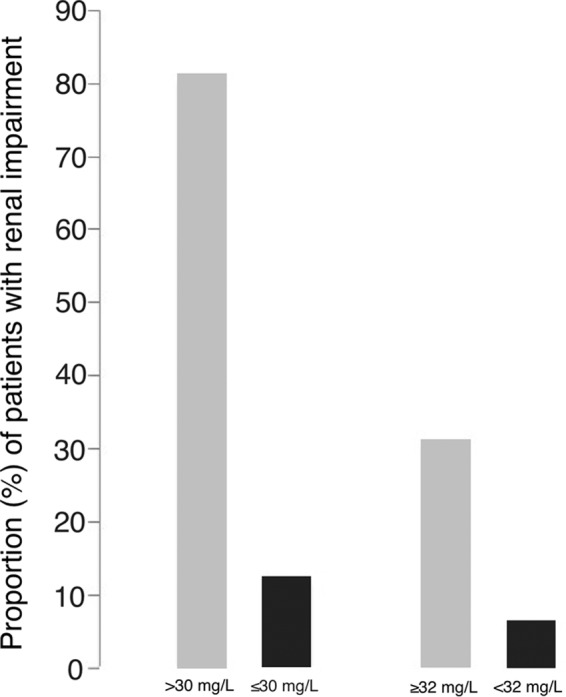
Burn patients present a higher vancomycin total body clearance (64). This leads to a low likelihood of achieving therapeutic vancomycin levels (65). Moreover, vancomycin PK changes with time after the incident, which makes it even more difficult to give fixed patterns of dosing (49). Therefore, there are no data about weight-adjusted vancomycin dosing in burn patients. Close monitoring of vancomycin serum concentrations is recommended to ensure targets are met and maintained. In a recent retrospective cohort study, burn patients receiving continuous infusion of vancomycin more frequently had levels in the therapeutic range and were less likely to have serum trough levels of <10 μg/ml, without differences in overall clinical outcome or toxicity, than were burn patients receiving intermittent infusion (65) (Fig. 5).
FIG 5.
Burn patients have higher vancomycin clearance and lower serum trough levels than control patients, as found by Dolton et al. (64). In this context, vancomycin continuous infusion (CoI) may improve the achievement of the target serum trough levels (mean ± SD, solid bars) without increasing nephrotoxicity (gridded bars) (65), compared with patients receiving vancomycin intermittent infusion (InI) (evidence level IIC [83]).
VANCOMYCIN IN CYSTIC FIBROSIS
Pharmacokinetics of antibiotics in patients with cystic fibrosis (CF) are altered in comparison to healthy people. The main differences reported are higher volume of distribution and total body clearance (66). However, specific information about the PK of vancomycin in this setting is very scarce.
Pleasants et al. (67) performed a PK study of vancomycin in CF patients. The values of several PK parameters (volume of distribution, total body clearance, and terminal elimination rates constant) were similar to those obtained in previous studies of healthy individuals. In contrast, Fung (68) described difficulties in achieving target trough levels with the current recommended dosages in three CF patients. In these cases, treatment administration was switched to continuous infusion, with successful clinical improvement, therapeutic steady-state level achievement, and absence of nephrotoxicity. The absence of further information does not allow specific recommendations for CF patients.
VANCOMYCIN TOXICITY
Vancomycin has been associated with several adverse events. Nephrotoxicity causes the most concern. Since it is related to serum vancomycin concentrations, the safety of current recommendations to target higher serum trough levels has been questioned. Some authors have proposed that AUC, not trough level, is the parameter best related to nephrotoxicity (24). However, to date, trough concentration is the most validated parameter to describe the drug exposure-toxicity relationship.
Vancomycin-induced nephrotoxicity is usually mild to moderate and reversible. It is defined in most publications as an increase of >0.5 mg/dl (or a >50% increase) in serum creatinine over baseline, in consecutively obtained daily serum creatinine values in the absence of an alternative justification (4). Even so, some authors have suggested that the new criteria of the Acute Kidney Injury Network, which include the reduction of urine output in the definition of renal function impairment, should be used (69). These criteria seem better to evaluate renal impairment in the clinical setting; thus, considering them, the real incidence of vancomycin-induced nephrotoxicity may differ from that previously reported.
Whether high vancomycin levels are a cause or an effect of renal function impairment has been a point of debate. A multicenter prospective clinical trial, including 288 adult patients (70), and a recent meta-analysis (71) have reaffirmed the high probability of nephrotoxicity with vancomycin trough levels of >15 mg/liter. This threshold is not uniform in all studies, and other authors have found that recommended dosages of vancomycin with target troughs of 15 to 20 mg/liter are not an independent risk factor for nephrotoxicity (72). Davies et al. (73) found that vancomycin was related to a rise in creatinine levels only with trough levels of >20 mg/liter. Additionally, Hanrahan et al. (37) showed that the risk of renal impairment among critically ill patients was related to higher serum levels, ranging from a RR of 1.07 for a trough of 15 mg/liter to a RR of 2.2 for a trough of >30 mg/liter. Other identified risk factors for renal function impairment during vancomycin therapy were duration of therapy, especially ≥7 days (37, 71, 74), previous renal insufficiency (75), and concomitant administration of nephrotoxic agents (37, 76, 77). Aminoglycosides gave specific cause for concern, and many studies described a higher rate of renal function impairment (up to 22%) when administered along with vancomycin (78, 79). With this combination, nephrotoxicity typically occurs after at least 5 days of therapy (80). Prevention of vancomycin-induced nephrotoxicity by using several antioxidant substances has shown beneficial effects in animal models but has not been confirmed by clinical trials yet (81).
How to manage vancomycin dosing in patients with renal failure has received little attention, and most clinicians are inclined to change the antibiotic. Nonetheless, the alternative drugs might not be tolerated and might not even available in some situations. In a small prospective study in patients receiving doses of vancomycin to achieve drug trough levels of 15 to 20 mg/liter and developing renal function impairment, Teng et al. (82) reported successful therapies and reversibility of renal impairment just by adjusting vancomycin dosages.
Vancomycin ototoxicity is controversial (4). The verifiable risk of vancomycin-induced hearing loss is low, and it does not seem to correlate to serum vancomycin concentration (76). Thus, monitoring serum vancomycin levels to prevent ototoxicity is not recommended (4).
CONCLUDING REMARKS
Vancomycin efficacy is related to its correct dosing according to optimal PK/PD parameters. An AUC/MIC ratio around 400 has been related to increased survival rates in patients with S. aureus bacteremia. Although trough vancomycin levels are not a perfect surrogate of AUC, achieving a trough concentration of 15 to 20 mg/liter would be enough to treat infections produced by S. aureus with a MICEtest of ≤1 mg/liter. Due to relevant interindividual variability, individualized doses are the best option, and Bayesian estimation procedures are the most accurate method to calculate them. Trials about special pharmacokinetic situations, such as obese patients or renal replacement therapy (RRT), are necessary. Vancomycin therapy for patients undergoing intermittent RRT should be individualized according to validated nomograms, and patient weight, dialyzer type, residual renal function, and interdialysis interval, helped by monitoring levels, should be considered. Continuous infusion of vancomycin may be helpful in those clinical situations, in which a high risk of toxicity and/or an increased variability in vancomycin levels are expected. Monitoring trough serum levels remains the best way to prevent nephrotoxicity. Clinicians must decide in each case, when nephrotoxicity occurs, whether to replace vancomycin with another active drug, knowing that continuing vancomycin under close surveillance is a valid option.
ACKNOWLEDGMENTS
Supported by Plan Nacional de I+D+i and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0001), and cofinanced by the European Development Regional Fund “A way to achieve Europe.”
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Moellering RC., Jr 2006. Vancomycin: a 50-year reassessment. Clin Infect Dis 42(Suppl 1):S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Baño J, Millán AB, Domínguez MA, Borraz C, González MP, Almirante B, Cercenado E, Padilla B, Pujol M; GEIH/GEMARA/REIPI. 2009. Impact of inappropriate empirical therapy for sepsis due to health care-associated methicillin-resistant Staphylococcus aureus. J Infect 58:131–137. doi: 10.1016/j.jinf.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 4.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 5.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 48:4665–4672. doi: 10.1128/AAC.48.12.4665-4672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamp KC, Rybak MJ, Bailey EM, Kaatz GW. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother 36:2709–2714. doi: 10.1128/AAC.36.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybak MJ. 2006. Pharmacodynamics: relation to antimicrobial resistance. Am J Med 119(Suppl 1):S37–S44. doi: 10.1016/j.amjmed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 9.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 10.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Howden BP, Johnson PD. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harigaya Y, Bulitta JB, Forrest A, Sakoulas G, Lesse AJ, Mylotte JM, Tsuji BT. 2009. Pharmacodynamics of vancomycin at simulated epithelial lining fluid concentrations against methicillin-resistant Staphylococcus aureus (MRSA): implications for dosing in MRSA pneumonia. Antimicrob Agents Chemother 53:3894–3901. doi: 10.1128/AAC.01585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob Agents Chemother 52:2156–2162. doi: 10.1128/AAC.01046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubenko IY, Strukova EV, Smirnova MV, Vostrov SN, Portnoy YA, Zinner SH, Firsov AA. 2008. Telavancin and vancomycin pharmacodynamics with Staphylococcus aureus in an in vitro dynamic model. J Antimicrob Chemother 62:1065–1069. doi: 10.1093/jac/dkn288. [DOI] [PubMed] [Google Scholar]
- 14.Jung Y, Song K-H, Cho JE, Kim H-S, Kim N-H, Kim TS, Choe PG, Chung JY, Park WB, Bang JH, Kim ES, Park KU, Park SW, Kim HB, Oh MD. 2014. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteremia. Int J Antimicrob Agents 43:179–183. doi: 10.1016/j.ijantimicag.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 16.Brown J, Brown K, Forrest A. 2012. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother 56:634–638. doi: 10.1128/AAC.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 18.Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, McNutt LA. 2014. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 59(5):666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 19.Lepe JA, Domínguez-Herrera J, Pachón J, Aznar J. 2014. Determining accurate vancomycin MIC values for methicillin-resistant Staphylococcus aureus by the microdilution method. J Antimicrob Chemother 69:136–138. doi: 10.1093/jac/dkt308. [DOI] [PubMed] [Google Scholar]
- 20.Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jiménez-Mejias ME, Pichardo C, Ibáñez-Martínez J, Pachón J. 2012. Efficacy of linezolid versus a pharmacodynamically optimized vancomycin therapy in an experimental pneumonia model caused by methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 67:1961–1967. doi: 10.1093/jac/dks142. [DOI] [PubMed] [Google Scholar]
- 21.Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, Kumar A. 2013. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents 41:255–260. doi: 10.1016/j.ijantimicag.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I, Itoh H, Hiramatsu K, Takeyama M, Kadota J. 2012. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 58:308–312. doi: 10.1159/000343162. [DOI] [PubMed] [Google Scholar]
- 23.Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. 2011. Vancomycin: we can't get there from here. Clin Infect Dis 52:969–974. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 24.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avent ML, Vaska VL, Rogers BA, Cheng AC, van Hal SJ, Holmes NE, Howden BP, Paterson DL. 2013. Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J 43:110–119. doi: 10.1111/imj.12036. [DOI] [PubMed] [Google Scholar]
- 26.Wesner AR, Brackbill ML, Coyle LL, Kidd RS. 2013. Prospective trial of a novel nomogram to achieve updated vancomycin trough concentrations. Interdiscip Perspect Infect Dis 2013:839456. doi: 10.1155/2013/839456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kullar R, Leonard SN, Davis SL, Delgado G, Pogue JM, Wahby KA, Falcione B, Rybak MJ. 2011. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15 to 20 mg/liter suggested by the vancomycin consensus guidelines. Pharmacotherapy 31:441–448. doi: 10.1592/phco.31.5.441. [DOI] [PubMed] [Google Scholar]
- 28.Revilla N, Martín-Suárez A, Pérez MP, González FM, Fernández de Gatta MDM. 2010. Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol 70:201–212. doi: 10.1111/j.1365-2125.2010.03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JT, Fang CT, Chen YC, Chang SC. 2001. Necessity of a loading dose when using vancomycin in critically ill patients. J Antimicrob Chemother 47:246. doi: 10.1093/jac/47.2.246. [DOI] [PubMed] [Google Scholar]
- 30.Rossini JM, Laughner J, Levine BJ, Papas MA, Reinhardt JF, Jasani NB. 2015. A randomized trial of loading vancomycin in the emergency department. Ann Pharmacother. 49:6–13. doi: 10.1177/1060028014556813. [DOI] [PubMed] [Google Scholar]
- 31.Truong J, Levkovich BJ, Padiglione AA. 2012. Simple approach to improving vancomycin dosing in intensive care: a standardized loading dose results in earlier therapeutic levels. Intern Med J 42:23–29. doi: 10.1111/j.1445-5994.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- 32.Vandecasteele SJ, De Bacquer D, De Vriese AS. 2011. Implementation of a dose calculator for vancomycin to achieve target trough levels of 15 to 20 μg/ml in persons undergoing hemodialysis. Clin Infect Dis 53:124–129. doi: 10.1093/cid/cir337. [DOI] [PubMed] [Google Scholar]
- 33.Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 45:2460–2467. doi: 10.1128/AAC.45.9.2460-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24. doi: 10.1093/jac/dkr442. [DOI] [PubMed] [Google Scholar]
- 35.Verrall AJ, Llorin R, Tam VH, Lye DC, Sulaiman Z, Zhong L, Archuleta S, Fisher DA. 2012. Efficacy of continuous infusion of vancomycin for the outpatient treatment of methicillin-resistant Staphylococcus aureus infections. J Antimicrob Chemother 67:2970–2973. doi: 10.1093/jac/dks328. [DOI] [PubMed] [Google Scholar]
- 36.Vuagnat A, Stern R, Lotthe A, Schumahmacher H, Duong M, Hoffmeyer P, Bernard L. 2004. High dose vancomycin for osteomyelitis: continuous versus intermittent infusion. J Clin Pharm Ther 29:351–357. doi: 10.1111/j.1365-2710.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 37.Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, Roberts J. 2014. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med 42:2527–2536. doi: 10.1097/CCM.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 38.Saugel B, Nowack MCM, Hapfelmeier A, Umgelter A, Schultheiss C, Thies P, Phillip V, Eyer F, Schmid RM, Huber W. 2013. Continuous intravenous administration of vancomycin in medical intensive care unit patients. J Crit Care 28:9–13. doi: 10.1016/j.jcrc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Raverdy V, Ampe E, Hecq J-D, Tulkens PM. 2013. Stability and compatibility of vancomycin for administration by continuous infusion. J Antimicrob Chemother 68:1179–1182. doi: 10.1093/jac/dks510. [DOI] [PubMed] [Google Scholar]
- 40.Zelenitsky S, Alkurdi N, Weber Z, Ariano R, Zhanel G. 2011. Preferential emergence of reduced vancomycin susceptibility in health care-associated methicillin-resistant Staphylococcus aureus isolates during continuous-infusion vancomycin therapy in an in vitro dynamic model. Antimicrob Agents Chemother 55:3627–3630. doi: 10.1128/AAC.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, Hindler JF, Ward KW, Bruckner DA. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 44:3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norton K, Ingram PR, Heath CH, Manning L. 2014. Risk factors for nephrotoxicity in patients receiving outpatient continuous infusions of vancomycin in an Australian tertiary hospital. J Antimicrob Chemother 69:805–808. doi: 10.1093/jac/dkt402. [DOI] [PubMed] [Google Scholar]
- 43.Spapen HD, Janssen van Doorn K, Diltoer M, Verbrugghe W, Jacobs R, Dobbeleir N, Honoré PM, Jorens PG. 2011. Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care 1:26. doi: 10.1186/2110-5820-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts JA, Taccone FS, Udy AA, Vincent JL, Jacobs F, Lipman J. 2011. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother 55:2704–2709. doi: 10.1128/AAC.01708-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pea F, Furlanut M, Negri C, Pavan F, Crapis M, Cristini F, Viale P. 2009. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother 53:1863–1867. doi: 10.1128/AAC.01149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeurissen A, Sluyts I, Rutsaert R. 2011. A higher dose of vancomycin in continuous infusion is needed in critically ill patients. Int J Antimicrob Agents 37:75–77. doi: 10.1016/j.ijantimicag.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Ampe E, Delaere B, Hecq J-D, Tulkens PM, Glupczynski Y. 2013. Implementation of a protocol for administration of vancomycin by continuous infusion: pharmacokinetic, pharmacodynamic, and toxicological aspects. Int J Antimicrob Agents 41:439–446. doi: 10.1016/j.ijantimicag.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Golper TA, Marx MA. 1998. Drug dosing adjustments during continuous renal replacement therapies. Kidney Int Suppl 66:S165–S168. [PubMed] [Google Scholar]
- 49.Elligsen M, Walker SAN, Walker SE, Simor A. 2011. Optimizing initial vancomycin dosing in burn patients. Burns 37:406–414. doi: 10.1016/j.burns.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Jamal JA, Udy AA, Lipman J, Roberts JA. 2014. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: an analysis of published literature and dosing regimens. Crit Care Med 42:1640–1650. doi: 10.1097/CCM.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 51.Chaijamorn W, Jitsurong A, Wiwattanawongsa K, Wanakamanee U, Dandecha P. 2011. Vancomycin clearance during continuous venovenous hemofiltration in critically ill patients. Int J Antimicrob Agents 38:152–156. doi: 10.1016/j.ijantimicag.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Beumier M, Roberts JA, Kabtouri H, Hites M, Cotton F, Wolff F, Lipman J, Vincent JL, Taccone FS. 2013. A new regimen for continuous infusion of vancomycin during continuous renal replacement therapy. J Antimicrob Chemother 68:2859–2865. doi: 10.1093/jac/dkt261. [DOI] [PubMed] [Google Scholar]
- 53.Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, de Backer D, Vincent JL, Taccone FS. 2013. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agents 41:261–266. doi: 10.1016/j.ijantimicag.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Udy AA, Covajes C, Taccone FS, Jacobs F, Vincent J-L, Lipman J, Roberts JA. 2013. Can population pharmacokinetic modelling guide vancomycin dosing during continuous renal replacement therapy in critically ill patients? Int J Antimicrob Agents 41:564–568. doi: 10.1016/j.ijantimicag.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Escobar L, Andresen M, Downey P, Gai MN, Regueira T, Bórquez T, Lipman J, Roberts JA. 2014. Population pharmacokinetics and dose simulation of vancomycin in critically ill patients during high-volume hemofiltration. Int J Antimicrob Agents 44:163–167. doi: 10.1016/j.ijantimicag.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Petejova N, Martinek A, Kahalkova J, Duricova J, Brozmannova H, Urbanek K, Grundmann M, Plasek J, Kacirova I. 2014. Vancomycin pharmacokinetics during high-volume continuous venovenous hemofiltration in critically ill septic patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 158:65–72. doi: 10.5507/bp.2012.092. [DOI] [PubMed] [Google Scholar]
- 57.Lin SY, Shen MC, Hwang SJ, Chen TC, Chiu YW, Lu PL. 2014. Evaluation of vancomycin dosing protocols to achieve serum concentrations in patients receiving high-flux hemodialysis. Int J Antimicrob Agents 43:384–385. doi: 10.1016/j.ijantimicag.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Rymarz A, Brodowska-Kania D, Gomolka M, Jozefczac-Bergier E, Dzierzanowska M, Niemczyk S. 2014. Vancomycin dosing in patients undergoing maintenance hemodialysis. Int Urol Nephrol 46:1681–1682. doi: 10.1007/s11255-014-0707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeremiah CJ, Wills C, Bayly A, Perry GJ, Davis JS, Tong SY, Currie BJ. 2014. Vancomycin dosing nomogram for hemodialysis patients. Nephrology 19:513–516. doi: 10.1111/nep.12270. [DOI] [PubMed] [Google Scholar]
- 60.Hall RG 2nd, Payne KD, Bain AM, Rahman AP, Nguyen ST, Eaton SA, Busti AJ, Vu SL, Bedimo R. 2008. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med 121:515–518. doi: 10.1016/j.amjmed.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grace E. 2012. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J Antimicrob Chemother 67:1305–1310. doi: 10.1093/jac/dks066. [DOI] [PubMed] [Google Scholar]
- 62.Janson B, Thursky K. 2012. Dosing of antibiotics in obesity. Curr Opin Infect Dis 25:634–649. doi: 10.1097/QCO.0b013e328359a4c1. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds DC, Waite LH, Alexander DP, DeRyke CA. 2012. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm 69:944–950. doi: 10.2146/ajhp110324. [DOI] [PubMed] [Google Scholar]
- 64.Dolton M, Xu H, Cheong E, Maitz P, Kennedy P, Gottlieb T, Buono E, McLachlan AJ. 2010. Vancomycin pharmacokinetics in patients with severe burn injuries. Burns 36:469–476. doi: 10.1016/j.burns.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Akers KS, Cota JM, Chung KK, Renz EM, Mende K, Murray CK. 2012. Serum vancomycin levels resulting from continuous or intermittent infusion in critically ill burn patients with or without continuous renal replacement therapy. J Burn Care Res 33:e254–e262. doi: 10.1097/BCR.0b013e31825042fa. [DOI] [PubMed] [Google Scholar]
- 66.Touw DJ. 1998. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm World Sci 20:149–160. doi: 10.1023/A:1008634911114. [DOI] [PubMed] [Google Scholar]
- 67.Pleasants RA, Michalets EL, Williams DM, Samuelson WM, Rehm JR, Knowles MR. 1996. Pharmacokinetics of vancomycin in adult cystic fibrosis patients. Antimicrob Agents Chemother 40:186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fung L. 2012. Continuous infusion vancomycin for treatment of methicillin-resistant Staphylococcus aureus in cystic fibrosis patients. Ann Pharmacother 46:e26. doi: 10.1345/aph.1R272. [DOI] [PubMed] [Google Scholar]
- 69.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. 2011. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 55:5475–5479. doi: 10.1128/AAC.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 mg/liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall RG 2nd, Hazlewood KA, Brouse SD, Giuliano CA, Haase KK, Frei CR, Forcade NA, Bell T, Bedimo RJ, Álvarez CA. 2013. Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol 14:12. doi: 10.1186/2050-6511-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies SW, Guidry CA, Petroze RT, Hranjec T, Sawyer RG. 2013. Vancomycin and nephrotoxicity: just another myth? J Trauma Acute Care Surg 75:830–835. doi: 10.1097/TA.0b013e3182a74b70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. 2010. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med 123:1143–1149. doi: 10.1016/j.amjmed.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 75.Panwar B, Johnson VA, Patel M, Balkovetz DF. 2013. Risk of vancomycin-induced nephrotoxicity in the population with chronic kidney disease. Am J Med Sci 345:396–369. doi: 10.1097/MAJ.0b013e318268023d. [DOI] [PubMed] [Google Scholar]
- 76.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 77.Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. 2007. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther 29:1107–1115. doi: 10.1016/j.clinthera.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. 1990. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 25:679–687. doi: 10.1093/jac/25.4.679. [DOI] [PubMed] [Google Scholar]
- 79.Wood CA, Kohlhepp SJ, Kohnen PW, Houghton DC, Gilbert DN. 1986. Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother 30:20–24. doi: 10.1128/AAC.30.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pannu N, Nadim MK. 2008. An overview of drug-induced acute kidney injury. Crit Care Med 36(Suppl 4):S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 81.Elyasi S, Khalili H, Hatamkhani S, Dashti-Khavidaki S. 2013. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur J Clin Pharmacol 69:747–754. doi: 10.1007/s00228-012-1406-3. [DOI] [PubMed] [Google Scholar]
- 82.Teng CB, Rezai K, Itokazu GS, Xamplas RC, Glowacki RC, Rodvold KA, Weinstein RA, Schawrtz DN. 2012. Continuation of high-dose vancomycin despite nephrotoxicity. Antimicrob Agents Chemother 56:3470–3471. doi: 10.1128/AAC.00240-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canadian Task Force on the Periodic Health Examination. 1979. The periodic health examination. Can Med Assoc J 121:1193–1254. [PMC free article] [PubMed] [Google Scholar]
- 84.Ruef C. 2004. Epidemiology and clinical impact of glycopeptide resistance in Staphylococcus aureus. Infection 32:315–327. [DOI] [PubMed] [Google Scholar]
- 85.Tenover FC, Moellering RC Jr. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 44:1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]