Abstract
We studied the pharmacokinetics and efficacy of the broad-spectrum triazole isavuconazole for the treatment of experimental invasive pulmonary aspergillosis (IPA) in persistently neutropenic rabbits. Treatment started 24 h after endotracheal administration of Aspergillus fumigatus inoculum; study subjects included rabbits receiving orally administered prodrug isavuconazonium sulfate (BAL8557) equivalent to active moiety isavuconazole (ISA; BAL4815) at 20 (ISA20), 40 (ISA40), and 60 (ISA60) mg/kg (of body weight)/day, with an initial loading dose of 90 mg/kg (ISA90), and untreated rabbits (UC). There were significant concentration-dependent reductions of residual fungal burden (log CFU/gram) and of organism-mediated pulmonary injury, lung weights, and pulmonary infarct scores in ISA40- and ISA60-treated rabbits in comparison to those of UC (P < 0.001). ISA20-treated (P < 0.05), ISA40-treated, and ISA60-treated (P < 0.001) rabbits demonstrated significantly prolonged survival in comparison to that of UC. ISA40- and ISA60-treated animals demonstrated a significant decline of serum (1→3)-β-d-glucan levels (P < 0.05) and galactomannan indices (GMIs) during therapy following day 4 in comparison to progressive GMIs of UC (P < 0.01). There also were significantly lower concentration-dependent GMIs in bronchoalveolar lavage (BAL) fluid from ISA40- and ISA60-treated rabbits (P < 0.001). There was a direct correlation between isavuconazole plasma area under the concentration-time curve from 0 to 24 h (AUC0–24) and residual fungal burdens in lung tissues, pulmonary infarct scores, and total lung weights. In summary, rabbits treated with isavuconazole at 40 and 60 mg/kg/day demonstrated significant dose-dependent reduction of residual fungal burden, decreased pulmonary injury, prolonged survival, lower GMIs in serum and BAL fluid, and lower serum (1→3)-β-d-glucan levels.
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) is a life-threatening infection in immunosuppressed patients, particularly in those with severe and prolonged neutropenia as a consequence of aplastic anemia, those undergoing myelotoxic chemotherapy for the treatment of cancer, and those receiving immunosuppressive medication for rejection prophylaxis after organ transplantation or treatment of graft-versus-host disease in allogeneic bone marrow transplantation (1–5).
Invasive pulmonary aspergillosis is the most common cause of infectious pneumonic death in bone marrow transplant recipients, accounting for 45% of all such lethal cases. Mortality rates of IPA in cancer patients have varied between 13% and 100% depending on the recovery from neutropenia and status of underlying disease.
Current treatment of IPA in immunosuppressed hosts relies on the administration of voriconazole as primary therapy. Unfortunately, the overall rate of response of invasive aspergillosis to voriconazole remains at approximately 50% to 60%, with responses as low as nearly 30% in hematopoietic stem cell transplantation recipients (6). Although voriconazole is an important therapeutic advance against IPA, the problems of visual hallucinations, cutaneous solar hypersensitivity, hepatotoxicity, drug interactions, variable plasma pharmacokinetics (PK), and need for therapeutic drug monitoring warrant the development of new antifungal agents against Aspergillus spp. Clearly, new strategies are needed for the treatment of IPA.
Isavuconazole is a new broad-spectrum triazole antifungal agent that has been recently approved by the FDA for primary treatment of invasive aspergillosis and mucormycosis (7–9). Isavuconazole in vitro demonstrates superior hyphal growth inhibition and MICs against Aspergillus fumigatus in comparison to those of voriconazole (10–14). However, there is a paucity of laboratory animal data for isavuconazole against Aspergillus spp. The pharmacodynamics of isavuconazole was explored in a murine neutropenic IPA model by Lepak and colleagues (15) and also in an invasive aspergillosis model of immunocompetent mice by Seyedmousavi et al. (16). We therefore studied the efficacy and pharmacokinetics of isavuconazole in treatment of experimental IPA in persistently neutropenic rabbits.
MATERIALS AND METHODS
Animals.
Healthy female New Zealand White rabbits (Covance Research Products, Inc., Denver, PA) weighing 2.6 to 3.5 kg at the time of endotracheal inoculation were used in our experiments. All rabbits were monitored under humane care and use of standards in facilities, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and according to the guidelines of the National Research Council (17) for the care and use of laboratory animals and under the approval of the Animal Care and Use Committee of the Weill Cornell Medical Center, New York, NY. Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum. Atraumatic vascular access was established by modified surgical placement of a Silastic tunneled central venous catheter as previously described (18). The Silastic catheter permitted nontraumatic venous access for administration of parenteral agents and for repeated blood sampling for study of plasma pharmacokinetics, serum galactomannan and (1→3)-β-d-glucan levels, and biochemical and hematological parameters.
Organism, inoculum, and inoculation.
A well-characterized clinical isolate of Aspergillus fumigatus, NIH 4215 (ATCC MYA-1163), initially obtained from a neutropenic patient with autopsy-proven pulmonary aspergillosis, was used for preparation of the inoculum, as previously described (19). All rabbits received predetermined inocula of 1 × 108 to 1.25 × 108 conidia of A. fumigatus in a volume of 250 μl to 350 μl administered endotracheally under general anesthesia on day 2 of the experiments, as previously described (19). The MIC of isavuconazole against A. fumigatus, determined according to Clinical and Laboratory Standards Institute (CLSI) standard M38-A microdilution methods (20), was 1 μg/ml.
Immunosuppression and maintenance of neutropenia.
Immunosuppression and profound persistent neutropenia (a neutrophil concentration of <100 neutrophils/μl) were established and maintained with intravenous (i.v.) administration of cytarabine (ara-C) (Zydus Hospira Oncology Private Ltd., Gujarat, India for Hospira, Inc., Lake Forest, IL) at an initial dosage of 525 mg/m2 for five consecutive days (days 1 to 5), and methylprednisolone (Solu-Medrol, Pfizer for Pharmacia & Upjohn Co., New York, NY) at a dosage of 5 mg/kg/day on days 1 and 2, as described elsewhere (19). Maintenance doses of ara-C at 484 mg/m2 were administered on days 8, 9, 13, and 14 of the study. Ceftazidime (75 mg/kg i.v. twice daily; Glaxo Pharmaceuticals, Research Triangle Park, NC), gentamicin (5 mg/kg i.v. every other day; Elkins-Sinn, Inc., Cherry Hill, NJ), and vancomycin (15 mg/kg i.v. daily; Abbott Laboratories, North Chicago, IL) were administered from day 4 of immunosuppression for the prevention of opportunistic bacterial infections during neutropenia. For prevention of antibiotic-associated diarrhea due to Clostridium spiroforme, all rabbits received 50 mg of vancomycin per liter of drinking water.
Antifungal compounds and treatment regimens.
Prodrug isavuconazonium sulfate (BAL8557) powder, provided by Astellas Pharma Global Development, Inc., was dissolved in sterile water, and the same amount of 5% dextrose injection solution (D5W; Baxter Healthcare Corp., Deerfield, IL) was then added prior to oral administration. All of the dosages used in the study were based upon the active moiety isavuconazole (BAL4815). All concentration measurements (i.e., PK data and calculated pharmacokinetics) are based upon the active drug. A conversion factor is necessary to compare equivalent prodrug and active drug amounts on a milligram-per-kilogram basis. This conversion factor was determined on the basis of a prodrug-to-drug equivalency rating of 1.863 (provided by the sponsor) and the 89% purity of the prodrug powder. Thus, the conversion factor for determining the equivalent isavuconazole dose from the prodrug dose was 0.48 (i.e., for every 1 mg/kg of prodrug administered orally, the equivalent in vivo isavuconazole dose would be 0.48 mg/kg).
Treatment started with an oral loading dose of isavuconazole at 90 mg/kg (ISA90) administered 24 h after endotracheal inoculation and continued thereafter once daily at dosages of 20 (ISA20) (n = 8), 40 (ISA40) (n = 8), and 60 (ISA60) (n = 8) mg/kg/day for up to 12 days. The plasma pharmacokinetics of isavuconazole in rabbits are similar to those observed in human volunteers. The near steady-state plasma exposure of isavuconazole in rabbits correlates with the plasma exposure achieved in the dose regimen used in the phase III clinical trial of invasive aspergillosis. The control group consisted of rabbits not receiving antifungal therapy (UC) (n = 8).
Outcome variables.
The following panel of outcome variables was used to assess antifungal efficacy: survival, measures of organism-mediated pulmonary injury (as pulmonary infarct score and lung weight), residual fungal burden (log CFU/gram), burden of circulating galactomannan in serum and bronchoalveolar lavage (BAL) fluid, and serum (1→3)-β-d-glucan levels. The outcome variable panel was applied to all experimental rabbits when possible.
Survival.
The survival time, in days postinoculation, was recorded for each rabbit in each group. Following humane endpoints, rabbits were euthanized by i.v. administration of pentobarbital (65 mg of pentobarbital sodium/kg of body weight; Beuthanasia-D Special [euthanasia solution]; Schering-Plough Animal Health Corp., Union, NJ) on day 13 postinoculation, 24 h after the last dose of study drug.
Organism-mediated pulmonary injury and residual fungal burden.
Postmortem examination of carefully resected lungs at necropsy was performed as previously described elsewhere (21). Lung weights and pulmonary lesion scores, as measures of organism-mediated pulmonary injury, were assessed. The number of positive lobes for hemorrhagic infarcts was added, and the mean value for all positive lobes was calculated for each group (pulmonary lesion score). Lung tissue from each rabbit was sampled and cultured for quantitative counts, the number of CFU of A. fumigatus was recorded for each lobe, and the CFU/gram were calculated.
BAL.
BAL was performed postmortem on each lung preparation, as described elsewhere (22), by the instillation of 10 ml of sterile normal saline (NaCl) into the clamped trachea with a sterile 12-ml syringe and subsequent withdrawal. The instillations were repeated twice. The lavage fluid was then centrifuged for 10 min at 400 × g. The upper portion of the supernatant was carefully removed (BAL fluid supernatant), leaving the pellet and 2 ml of supernatant (BAL fluid). BAL fluid was then vortexed, and the aliquots of 100 μl and 100 μl of a dilution (101) were cultured on 5% Sabouraud glucose agar (SGA) plates. The remaining BAL fluid was stored at −80°C until it was tested for galactomannan antigen.
Galactomannan detection.
Serum samples from each rabbit were collected every other day and stored at −80°C before analysis. Galactomannan antigen levels were determined in serial serum samples and postmortem-obtained BAL fluid by a one-stage immunoenzymatic sandwich microplate assay method (23) (Platelia Aspergillus enzyme immunoassay [EIA]; Bio-Rad, Marnes la Coquette, France) according to the manufacturer's instructions and as described elsewhere (22). Enzyme immunoassay data were expressed as a serum galactomannan index (GMI) plotted over time. The GMI for each test serum or BAL fluid sample was equal to the absorbance of a standard sample divided by the absorbance of a threshold serum provided by the manufacturer. A GMI of less than 0.5 was considered negative.
Detection of (1→3)-β-d-glucan levels.
Serum from each rabbit was collected every other day for determination of (1→3)-β-d-glucan levels by using a colorimetric assay (Fungitell; Associates of Cape Cod, Inc.) read at 405 nm (with 490-nm background subtraction), based upon para-nitroanilide absorption at that wavelength, performed according to the manufacturer's instructions and described in detail elsewhere (24). The (1→3)-β-d-glucan levels were determined by taking the mean optical density of the duplicate readings and comparing with the standard curve of predetermined concentrations. Interpretation of (1→3)-β-d-glucan values, according to the manufacturer's instructions, was as follows: <60 pg/ml, negative; 60 to 79 pg/ml, indeterminate; and ≥80 pg/ml, positive. The median correlation coefficient (r) of the standard curves performed in these studies was ≥0.9992 (range, 0.9982 to 0.9998).
Pharmacokinetic studies. (i) Single-dose pharmacokinetics of isavuconazole.
The plasma pharmacokinetics of isavuconazole was investigated for each dosage group of four noninfected healthy rabbits. Isavuconazole was administered as a single dose orally at dosages of 20, 40, 60, and 90 (ISA90) mg/kg/day. Following withdrawal of a baseline predose, blood samples were collected into heparinized syringes from each rabbit at the following time points from the beginning of isavuconazole administration: 1, 2, 4, 8, 12, 18, 24, and 48 h. Plasma was immediately separated by centrifugation at 400 × g, and samples were stored in 2-ml Sarstedt microtubes at −80°C prior to analysis.
(ii) Optimal sampling pharmacokinetics of isavuconazole.
The plasma pharmacokinetics of isavuconazole was studied in five to eight infected animals each per dosage cohort. Time points for sampling were determined by inspection of full plasma concentration profiles obtained in normal rabbits following administration of similar dosages based upon previous plasma pharmacokinetic studies. Plasma sampling was performed on day 6 of antifungal therapy. Blood samples were drawn predose and at 1, 4, 8, and 24 h postdosing. Plasma was immediately separated by centrifugation and stored at −80°C until assayed.
LC-MS/MS assay. (i) Plasma samples.
Paraoxon at 0.1 M (10 μl per 1 ml of plasma) was added to the blank EDTA/K3 and blank Li-heparin rabbit plasma to inhibit esterases. Spiking solutions of BAL0004815 and BAL0008728 were prepared by serial dilution in dimethyl sulfoxide (DMSO) and acetonitrile (ACN)–0.05% trifluoroacetate (TFA) out of a 2-mg/ml stock solution with a range of 0.5 μg/ml to 500 μg/ml. Calibration of samples was performed by spiking 1 μl of each DMSO (or ACN–0.05% TFA) solution in 99 μl of blank EDTA/K3 plasma followed by 300 μl of ACN–0.05% TFA containing 1 μg/ml of BAL0004815-d4 and pyridooxazinone as internal standards. After centrifugation, 10 μl of the supernatant was injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS) instrument (QTRAP; Applied Biosystems) (25). The quality control (QC) samples were prepared like the calibrators but using blank Li-heparin plasma instead of EDTA/K3 plasma. For the samples, 50 μl of plasma was mixed with 150 μl of ACN–0.05% TFA containing 1 μg/ml of BAL0004815-d4 and pyridooxazinone as internal standards and then treated like the calibrators.
(ii) Lung tissue samples.
Two samples were prepared from each lung tissue. One equivalent of rabbit lung piece was mixed with two equivalents of water by using the Ultra-Turrax (IKA) dispenser. Fifty microliters of this lung-water solution was quenched with 150 μl of ACN–0.05% TFA containing 1 μg/ml of BAL0004815-d4 and pyridooxazinone as internal standards. Samples were vortexed and centrifuged, and 10 μl of the supernatant was injected into the LC-MS/MS. For quantification, standard curves of BAL0004815 and BAL0008728 were prepared with a range of 2.5 to 5,000 ng/ml in lung-water solution. This “lung-water matrix” was made by mixing one equivalent of rabbit lung with two equivalents of water. The lung-water solution was spiked (1 μl of spiking solutions in 99 μl of lung-water matrix) and treated like the samples.
(iii) BAL fluid samples.
BAL fluid and BAL fluid spiking solutions of BAL0004815 and BAL0008728 were prepared by serial dilution, respectively, in DMSO and ACN–0.05% TFA out of a 2-mg/ml stock solution with a range of 0.5 μg/ml to 500 μg/ml. Calibration of samples was performed by spiking 1 μl of each DMSO (or ACN–0.05% TFA) solution in 99 μl of 0.9% NaCl solution followed by 300 μl of ACN–0.05% TFA containing 1 μg/ml of BAL0004815-d4 and pyridooxazinone as internal standards. After centrifugation, 10 μl of the supernatant was injected into the LC-MS/MS. The QC samples were prepared like the calibrators but using normal rabbit BAL fluid supernatant instead of 0.9% NaCl solution. For the samples, 50 μl of rabbit BAL fluid or BAL fluid supernatant was mixed with 150 μl of ACN–0.05% TFA containing 1 μg/ml of BAL0004815-d4 and pyridooxazinone as internal standards and then treated like the calibrators.
Determination of pharmacokinetic parameters.
Pharmacokinetic parameters for isavuconazole were determined from plasma concentration data using noncompartmental methods (WinNonlin Professional version 4.1; Pharsight Corp., Mountain View, CA). Pharmacokinetic measures were maximum observed plasma concentration (Cmax), area under the plasma concentration-time curve (AUC) through 24 h after the first dose (AUC0–24) or at steady state (AUCss) calculated by the linear trapezoidal rule, clearance (CL) calculated by dividing dose by AUC, volume of distribution at steady state (Vss) calculated by multiplying the dose by the ratio of the area under the first moment curve to the square of AUC, and terminal elimination half-life (t1/2) calculated from a linear regression of the log-linear portion of the log concentration-time curve.
Statistical analysis.
Comparisons between the groups were performed by analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons or the Mann-Whitney U test, as appropriate. The central hypothesis of this analysis was based upon the response of isavuconazole in comparison to that of untreated controls. A two-tailed P value of ≤0.05 was considered to be statistically significant. Survival was plotted by Kaplan-Meier analysis. Differences in survival of treatment groups and untreated controls were analyzed by log rank test. Values are expressed as means ± standard errors of the means (SEMs). Pharmacokinetic parameters were compared using ANOVA or Student's t test, as appropriate. Correlation of AUC0–24 and outcome variables was performed using Pearson's correlation method.
RESULTS
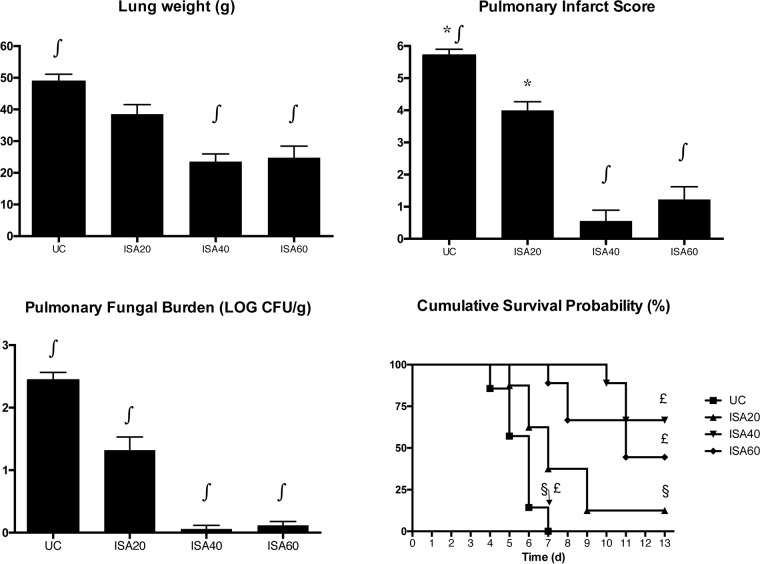
Rabbits treated with ISA20 (1.32 ± 0.21 log CFU/g; mean ± SEM), ISA40 (0.06 ± 0.06 log CFU/g), and ISA60 (0.12 ± 0.06 log CFU/g) showed significant decreases (P ≤ 0.001) in residual fungal burden in comparison to that of UC (2.44 ± 0.12 log CFU/g) (Fig. 1). There also was a significant reduction in organism-mediated pulmonary injury as measured by total lung weight and infarct score. Rabbits treated with ISA40 and ISA60 showed significantly decreased (P ≤ 0.001) total lung weights in comparison to that of UC (23.5 ± 2.4 and 24.8 ± 3.6 g, respectively, versus 48.9 ± 2.2 g). Rabbits treated with ISA40 and ISA60 also demonstrated significant decreases (P ≤ 0.001) in pulmonary infarct score in comparison to that of UC (0.56 ± 0.34 and 1.22 ± 0.40, respectively, versus 5.71 ± 0.18). ISA20-treated rabbits had significantly lower infarct scores (P ≤ 0.01) (4.00 ± 0.27) in comparison to that of UC (Fig. 1). There also was significantly prolonged (P ≤ 0.001) survival of rabbits treated with ISA40 (6 of 9 [66.7%] surviving) and ISA60 (4 of 9 [44.4%]) in comparison to that of UC (0 of 8 [0%]) (Fig. 1).
FIG 1.
Response of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy measured by mean pulmonary tissue residual fungal burden (log CFU/gram), mean lung weight, mean pulmonary infarct score, and survival in untreated controls (UC) and rabbits receiving oral isavuconazole (BAL4815). An initial loading dose of 90 mg/kg of isavuconazole was administered orally, and thereafter the drug was administered daily at 20 mg/kg (ISA20), 40 mg/kg (ISA40), and 60 mg/kg (ISA60). Values are means ± SEMs. For the measure of survival, the values on the y axis are probability of survival. Survival was plotted by Kaplan-Meier analysis. Differences in survival of treatment groups and untreated controls were analyzed by log rank test. P values are indicated as follows: *, P < 0.05, decreased infarct score in ISA20-treated rabbits in comparison to that of UC; ∫, P < 0.001, decreased residual fungal burden, lung weight, and infarct score in ISA40- and ISA60-treated rabbits in comparison to that of UC; * and ∫, P values were obtained by comparison to UC by ANOVA with Bonferroni's correction for multiple comparisons; £, P < 0.001, prolonged survival of rabbits treated with ISA40 and ISA60 in comparison to that of UC; §, P < 0.05, prolonged survival of rabbits treated with ISA20 in comparison to that of UC.
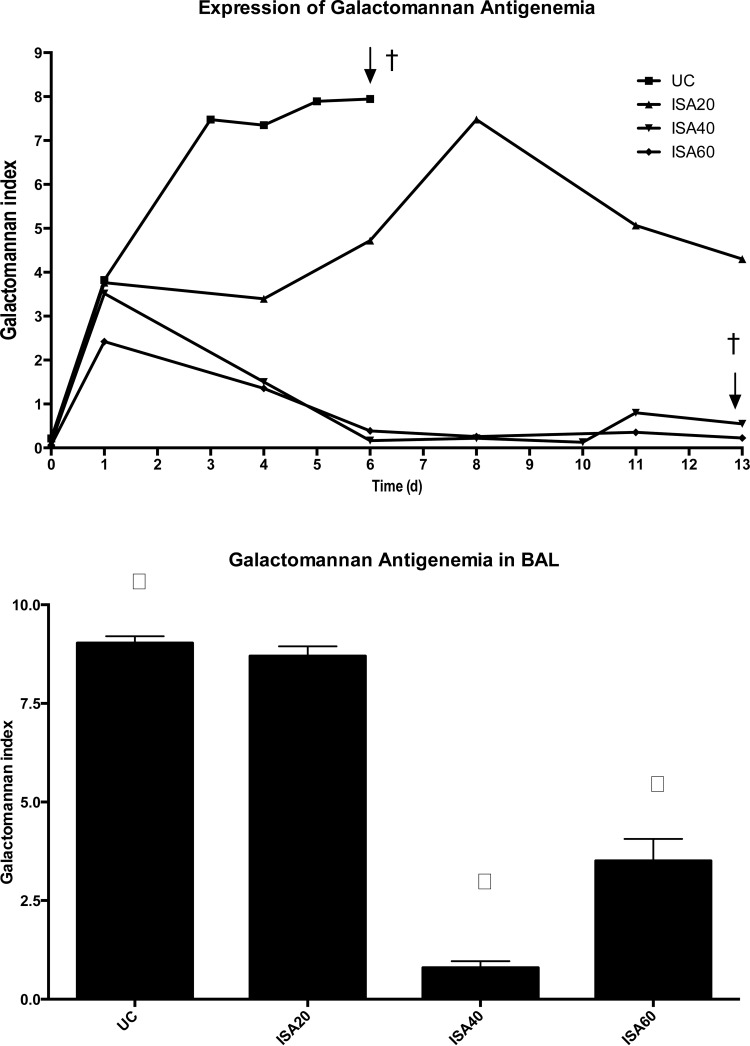
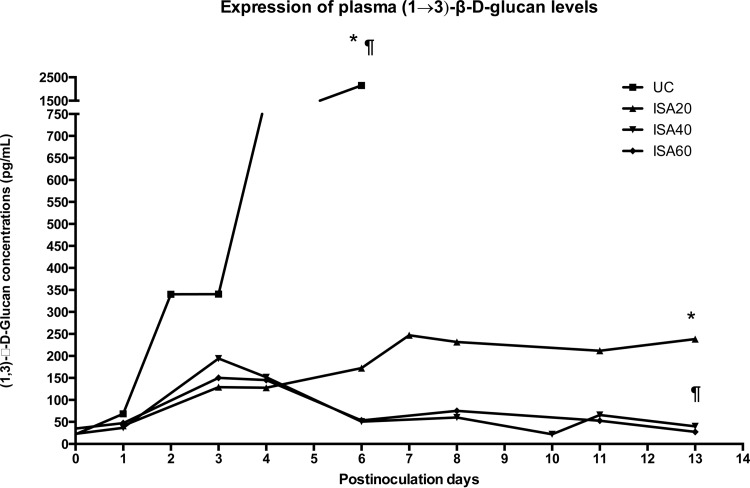
Rabbits treated with ISA40 and ISA60 had lower GMIs during the study. ISA40- and ISA60-treated rabbits showed significantly lower (P < 0.01) GMIs than that of UC rabbits (Fig. 2, top). Consistent with the lower serum GMI, there were significantly lower (P < 0.001) GMIs in BAL fluid from rabbits treated with ISA40 and ISA60 than that of UC rabbits (Fig. 2, bottom). There were significantly lower (P ≤ 0.05) plasma (1→3)-β-d-glucan levels during experiments in ISA20-, ISA40-, and ISA60-treated rabbits (P ≤ 0.01) than for UC (Fig. 3).
FIG 2.
(Top) Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis in untreated controls and rabbits receiving oral dosing of isavuconazole (BAL4815). An initial loading dose of 90 mg/kg of isavuconazole was administered orally, and thereafter the drug was administered daily at 20, 40, and 60 mg/kg. Values are means ± SEMs. (Bottom) BAL fluid galactomannan antigen levels in persistently neutropenic rabbits with pulmonary aspergillosis in untreated controls and rabbits treated with isavuconazole at 20, 40, and 60 mg/kg/day p.o. Values are means ± SEMs. P values are indicated as follows: †, P < 0.01, lower GMI in ISA40- and ISA60-treated rabbits than in UC; ∫, P < 0.001, lower GMI in BAL from rabbits treated with ISA40 and ISA60 than in that of UC (P values were obtained by comparison to UC by ANOVA with Bonferroni's correction for multiple comparisons).
FIG 3.
Serum (1→3)-β-d-glucan levels in persistently neutropenic rabbits of the experimental pulmonary aspergillosis model in groups of untreated controls and rabbits receiving oral doses of isavuconazole (BAL4815). An initial loading dose of 90 mg/kg of isavuconazole was administered orally, and thereafter the drug was administered daily at 20, 40, and 60. Values are (1→3)-β-d-glucan concentrations. P values are indicated as follows: *, P < 0.05, decrease of plasma (1→3)-β-d-glucan concentrations in ISA20-treated rabbits in comparison to that of UC; ¶, P < 0.01, decrease of plasma (1→3)-β-d-glucan concentrations in ISA40- and ISA60-treated rabbits in comparison to that of UC.
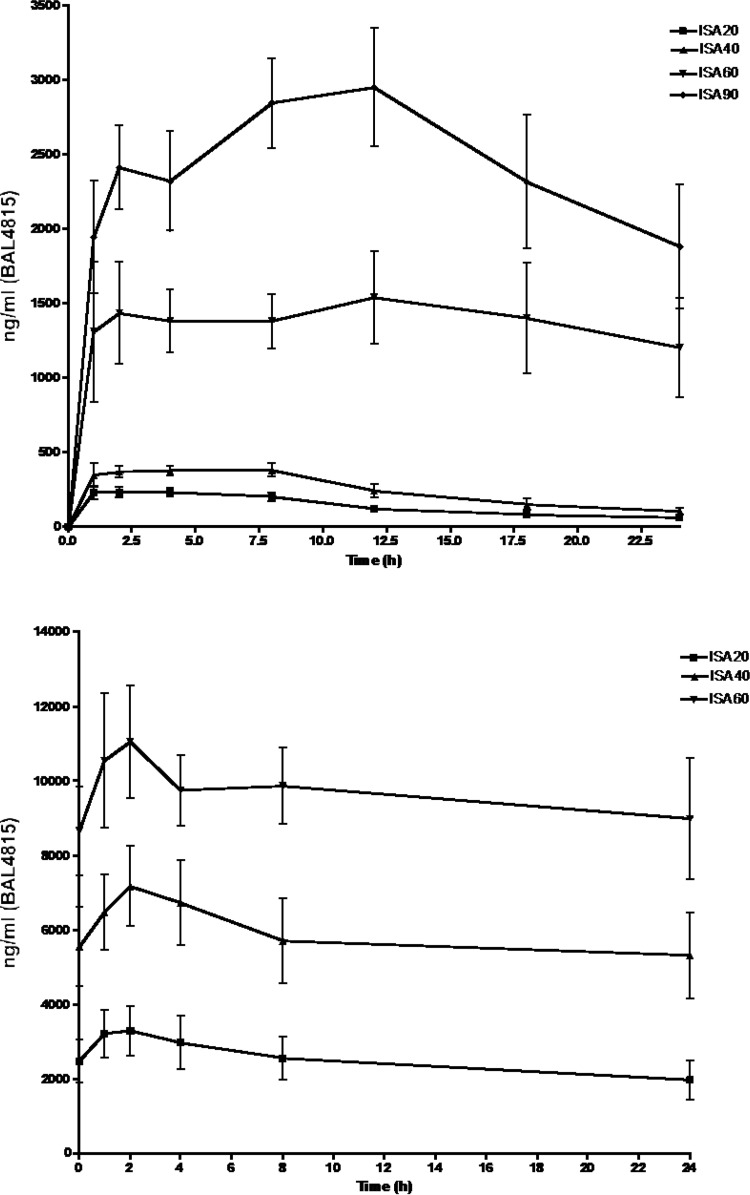
AUC0–24 values after a single oral dose in normal animals for ISA20, ISA40, ISA60, and ISA90 were 3.3 × 103 ± 0.4 × 103, 5.9 × 103 ± 0.8 × 103, 32.8 × 103 ± 6.7 × 103, and 58.2 × 103 ± 7.4 × 103 ng · h/ml, respectively, and for clearance were 15.7 ± 3.1, 17.8 ± 2.3, 3.4 ± 0.9, and 2.0 ± 0.6 ml/h (Fig. 4; Table 1). On the other hand, AUC0–24 values after per os (p.o.) administration of BAL8557 for 6 days to the infected animals for ISA20, ISA40, and ISA60 were 59.7 × 103 ± 13.8 × 103, 141 × 103 ± 32.9 × 103, and 197 × 103 ± 27.8 × 103 ng · h/ml, respectively, consistent with linear kinetics (Fig. 4; Table 1).
FIG 4.
(Top) Mean plasma profiles of BAL4815 after single oral-dose administration of BAL8557 prodrug equivalent to active compound of 20, 40, 60, and 90 mg/kg/day. (Bottom) Mean plasma profiles of isavuconazole after oral dose administration of prodrug isavuconazonium sulfate for 6 days to the infected animals. A loading oral dose of 90 mg/kg of isavuconazole was administered, followed by once-daily maintenance doses of 20, 40, and 60 mg/kg.
TABLE 1.
Pharmacokinetic parameters of isavuconazole (BAL4815) after oral administration of isavuconazonium (BAL8557) prodrug equivalent to active compound of 20, 40, 60, and 90 mg/kg/day
| Dose (mg/kg p.o.) | AUC0–24 (ng · h/ml) |
CL (ml/h/kg) | |
|---|---|---|---|
| Single dose | 6 doses to infected rabbits | ||
| 20 | 3.3 × 103 ± 0.4 × 103 | 59.7 × 103 ± 13.8 × 103 | 15.7 ± 3.1 |
| 40 | 5.9 × 103 ± 0.8 × 103 | 141 × 103 ± 32.9 × 103 | 17.8 ± 2.3 |
| 60 | 32.8 × 103 ± 6.7 × 103 | 197 × 103 ± 27.8 × 103 | 3.4 ± 0.9 |
| 90 | 58.2 × 103 ± 7.4 × 103 | —a | 2.0 ± 0.6 |
—, 90 mg/kg/day was administered only on day 1 to measure the exposure of a single loading dose.
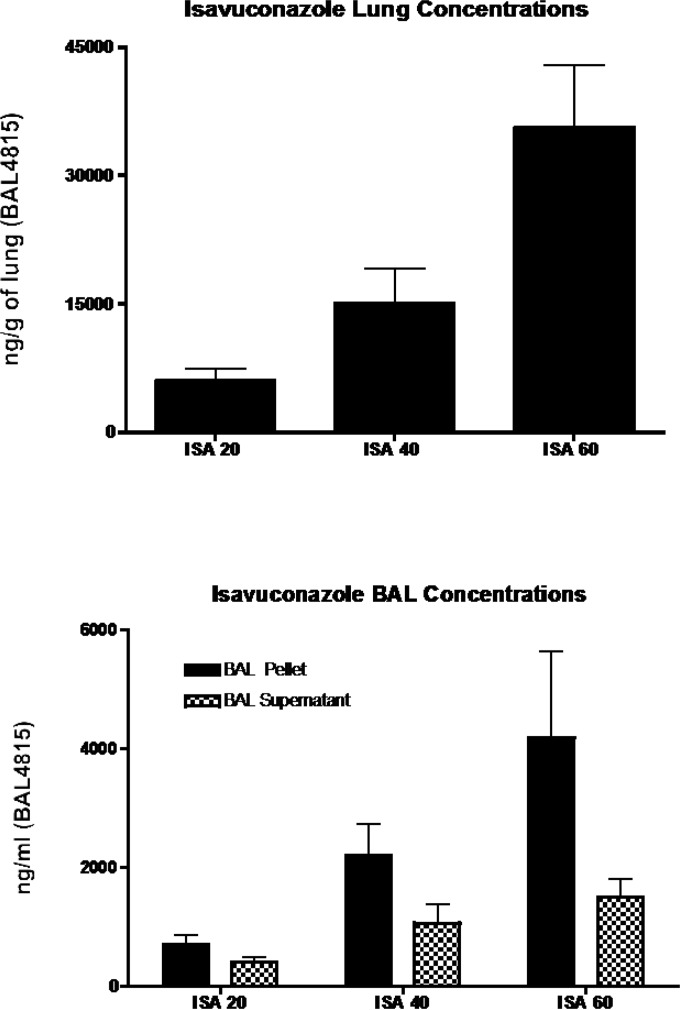
Figure 5 depicts the pulmonary distribution of isavuconazole. There was a nonlinear concentration-dose relation between isavuconazole concentrations in lung tissue and the dose of drug, with concentrations being disproportionally higher in animals treated with 60 mg/kg/day than in those treated with 40 mg/kg/day. In testing the hypothesis that isavuconazole may accumulate in pulmonary alveolar macrophages (PAMs), we found that the BAL fluid containing PAMs also had high concentrations of the study drug. At 60 mg/kg/day, isavuconazole distributed significantly more highly into BAL fluid than into the BAL supernatant.
FIG 5.
(Top) Concentrations of isavuconazole (BAL4815) in the lung tissue after oral administration of BAL8557 for 12 days to the infected animals. An initial loading dose of 90 mg/kg of isavuconazole was administered orally, and thereafter the drug was administered daily at 20, 40, and 60. (Bottom) Concentrations of isavuconazole in the BAL fluid and BAL fluid supernatant after oral administration of prodrug isavuconazonium sulfate for 12 days to the infected animals. An initial loading dose of 90 mg/kg of isavuconazole was administered orally, and thereafter the drug was administered daily at 20, 40, and 60 mg/kg.
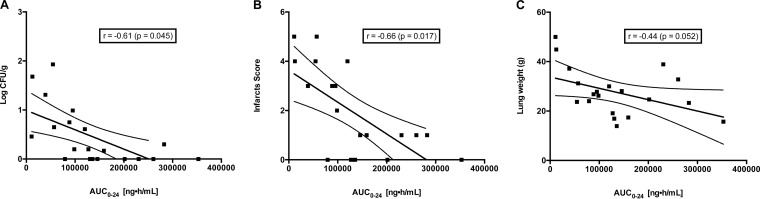
There was a significant pharmacodynamic correlation between AUC and key outcome variables of residual fungal burden (r = 0.61; P = 0.045), pulmonary infarct score (r = 0.66; P = 0.017), and lung weight (r = 0.44; P = 0.052) (Fig. 6). The maximum effect on residual fungal burden and on pulmonary infarct score appears to coincide with an AUC of approximately 20,000 ng · h/ml.
FIG 6.
Correlation of AUC0–24 and outcome variables. (A) Residual fungal burden in the lung tissues; (B) infarct score; (C) total lung weight. Calculations of r and P values were performed using Pearson's correlation method.
DISCUSSION
Isavuconazole in this study was highly effective in treatment of experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits as measured by a panel of outcome variables: residual fungal burden, survival, markers of pulmonary injury (lung weights and infarct scores), serum GMI, BAL fluid GMI, and serum (1→3)-β-d-glucan. The dosages of 40 mg/kg/day and 60 mg/kg/day yielded AUCs of 141 × 103 ± 32 × 103 ng · h/ml and 197 × 103 ± 27 × 103 ng · h/ml and were comparable in antifungal activity by all parameters. Isavuconazole at 20 mg/kg (59.7 × 103 ± 14 × 103 ng · h/ml) was less active than at the higher dosages. These findings might suggest that administration of higher dosages of isavuconazole for some isolates of A. fumigatus with elevated MICs may be beneficial.
Comparison of these effects of isavuconazole to previous studies in the same animal model system of persistently neutropenic rabbits with invasive pulmonary aspergillosis reveals antifungal activity similar to those of liposomal amphotericin B (26, 27) and posaconazole (22). The antifungal activity of isavuconazole was similar across all major outcome variables that were used to study these antifungal agents. These dosages demonstrated a robust response to isavuconazole in significantly increased survival, resolution of circulating serum biomarkers [galactomannan and (1→3)-β-d-glucan], and reduction of markers of organism-mediated pulmonary injury (lung weight and pulmonary infarct score).
The plasma pharmacokinetics of isavuconazole in the experimental rabbit model showed dose proportionality in near steady state. This is similar to that which is observed in human volunteers (25, 28, 29) receiving as much as 600 mg. The near steady-state plasma exposure of isavuconazole in the rabbit model where maximal antifungal activity was achieved ranged from 59.7 × 103 to 141 × 103 ng · h/ml, which also correlates with the plasma exposure achieved in the dose regimen used in the phase III clinical trial of invasive aspergillosis (30). Following a loading regimen of 372 mg of isavuconazonium sulfate (equivalent to 200 mg isavuconazole, active moiety) given every 8 h over 48 h and a maintenance dose of 372 mg/day, Desai and colleagues reported a mean plasma AUC in patients of 97.9 μg · h/ml for this dosing regimen (31), which falls just under the AUC of the 40-mg/kg/day isavuconazole maintenance dosage achieved in the rabbit model. Consistent with human plasma pharmacokinetic data, clearance of isavuconazole is relatively low in single- and multiple-dosing studies of the rabbits and is compatible with an extended plasma half-life.
Our data indicate that dosages above 20 mg/kg/day in rabbits may confer greater survival and microbiological resolution. A pharmacodynamic study of isavuconazole in a murine model of invasive aspergillosis by Seyedmousavi et al. found that an AUC/MIC ratio of produced a survival rate of 50% when the effective AUC0–24/MIC (EUCAST) ratio for isavuconazole total drug was 24.73 (95% confidence interval, 22.50 to 27.18) (16). Lepak and colleagues found a similar dose-dependent response across a range of MICs for isolates of A. fumigatus in a murine model of IPA (15). With the emergence of increasing MICs in A. fumigatus clinical isolates, this property of isavuconazole may prove to be clinically important in treatment of invasive aspergillosis caused by these isolates.
The patterns of serum galactomannan in these experiments demonstrate a rapid resolution of circulating antigen at the higher dosages of 40 and 60 mg/kg/day within the first 6 days of the experiments. This rapid resolution of galactomannan antigenemia also correlated with reduction in residual fungal burden and markers of pulmonary injury, suggesting that exposures higher than those achieved in rabbits treated at a dosage of 20 mg/kg/day (AUC = 59 × 103 ng · h/ml) may be beneficial in patients with aggressive or advanced infection for which stabilization is critical. These data may provide a rationale for developing a clinical trial for higher dosages in the subpopulation with rapidly progressive or advanced IPA. Whether this antifungal activity of isavuconazole may be further augmented when used in combination with an echinocandin (32) merits further laboratory investigation.
ACKNOWLEDGMENTS
We are grateful to Brigitte T. Huertas and Egle Petraityte for their laboratory technical assistance in this work.
This research was supported by an Investigator-Initiated grant from Astellas. T.J.W. is a Scholar of the Henry Schueler Foundation and a Scholar of Pediatric Infectious Diseases of the Sharp Family Foundation and receives support from the Save our Sick Kids Foundation, as well as research grants for experimental and clinical antimicrobial pharmacotherapeutics from Novartis, Merck, ContraFect, Pfizer, and Cubist. He has served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. W.W.H. has acted as a consultant and/or received research support from Gilead, Astellas, Pfizer, and F2G. L.L.K. is an employee of Astellas Pharma Global Development, Inc.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. 2015. The clinical spectrum of pulmonary aspergillosis. Thorax 70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF. 2009. Risk stratification for invasive aspergillosis: early assessment of host susceptibility. Med Mycol 47(Suppl 1):S255–S260. [DOI] [PubMed] [Google Scholar]
- 4.Rubio PM, Sevilla J, Gonzalez-Vicent M, Lassaletta A, Cuenca-Estrella M, Diaz MA, Riesco S, Madero L. 2009. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: a retrospective single centre study. J Pediatr Hematol Oncol 31:642–646. doi: 10.1097/MPH.0b013e3181acd956. [DOI] [PubMed] [Google Scholar]
- 5.Valdez JM, Scheinberg P, Young NS, Walsh TJ. 2009. Infections in patients with aplastic anemia. Semin Hematol 46:269–276. doi: 10.1053/j.seminhematol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration Anti-Infective Drug Advisory Committee. 22 January 2015. AstellasPharma. Isavuconazole. Food and Drug Administration, Washington, DC: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM431846.pdf. [Google Scholar]
- 7.Miceli MH, Kauffman CA. 15 November 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 8.Livermore J, Hope W. 2012. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol 8:759–765. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]
- 9.Ananda-Rajah MR, Kontoyiannis D. 2015. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol 10:693–708. doi: 10.2217/fmb.15.34. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2013. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol 51:2608–2616. doi: 10.1128/JCM.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Florl C, Martin-Mazuelos E, Meis J, Pelaez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. 2015. Isavuconazole, micafungin, and 8 comparator antifungal agents' susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis 82:303–313. doi: 10.1016/j.diagmicrobio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Guinea J, Hagen F, Meis JF, Thompson GR III, Turnidge J. 2015. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27-A3 broth microdilution method. Antimicrob Agents Chemother 59:666–668. doi: 10.1128/AAC.04055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Box H, Livermore J, Johnson A, McEntee L, Felton TW, Whalley S, Goodwin J, Hope WW. 26 October 2015. Pharmacodynamics of isavuconazole in a dynamic in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother doi: 10.1128/AAC.01364-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57:6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyedmousavi S, Bruggemann RJ, Meis JF, Melchers WJ, Verweij PE, Mouton JW. 2015. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob Agents Chemother 59:2855–2866. doi: 10.1128/AAC.04907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 18.Walsh TJ, Bacher J, Pizzo PA. 1988. Chronic Silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci 38:467–471. [PubMed] [Google Scholar]
- 19.Petraitis V, Petraitiene R, Sarafandi AA, Kelaher AM, Lyman CA, Casler HE, Sein T, Groll AH, Bacher J, Avila NA, Walsh TJ. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J Infect Dis 187:1834–1843. doi: 10.1086/375420. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Reference method for the broth dilution antifungal susceptibility testing of filamentous fungi: approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 21.Petraitis V, Petraitiene R, Groll AH, Bell A, Callender DP, Sein T, Schaufele RL, McMillian CL, Bacher J, Walsh TJ. 1998. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 42:2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petraitiene R, Petraitis V, Groll AH, Sein T, Piscitelli S, Candelario M, Field-Ridley A, Avila N, Bacher J, Walsh TJ. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother 45:857–869. doi: 10.1128/AAC.45.3.857-869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stynen D, Sarfati J, Goris A, Prevost MC, Lesourd M, Kamphuis H, Darras V, Latge JP. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun 60:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petraitiene R, Petraitis V, Hope WW, Mickiene D, Kelaher AM, Murray HA, Mya-San C, Hughes JE, Cotton MP, Bacher J, Walsh TJ. 2008. Cerebrospinal fluid and plasma (1→3)-beta-d-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 52:4121–4129. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis P, Lee JW, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo PA, Walsh TJ. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J Infect Dis 169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 27.Hope WW, Petraitis V, Petraitiene R, Aghamolla T, Bacher J, Walsh TJ. 2010. The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1→3) beta-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother 54:4879–4886. doi: 10.1128/AAC.00673-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornely OA, Bohme A, Schmitt-Hoffmann A, Ullmann AJ. 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother 59:2078–2085. doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR III, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 31.Desai A, Kovanda L, Kowalski D, Lu Q, Townsend RW. 2014. Isavuconazole (ISA) population pharmacokinetic modeling from phase 1 and phase 3 clinical trials and target attainment analysis, abstr A-697 Abstr 45th Intersci Conf Antimicrob Agents Chemother, Washington DC. [Google Scholar]
- 32.Petraitis V, Petraitiene R, Hope WW, Meletiadis J, Mickiene D, Hughes JE, Cotton MP, Stergiopoulou T, Kasai M, Francesconi A, Schaufele RL, Sein T, Avila NA, Bacher J, Walsh TJ. 2009. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlations of the concentration- and dose-dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob Agents Chemother 53:2382–2391. doi: 10.1128/AAC.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]