Abstract
Placental transfer of the HIV integrase inhibitor raltegravir (RLT) was investigated in term human cotyledons in the maternal-to-fetal (n = 3) and fetal-to-maternal (n = 6) directions. In the maternal-to-fetal direction, the mean ± standard deviation (SD) fetal transfer rate (FTR) was 9.1% ± 1.4%, and the mean ± SD clearance index (IC), i.e., RLT FTR/antipyrine FTR, was 0.28 ± 0.05. In the fetal-to-maternal direction, the mean ± SD CI was 0.31 ± 0.09. Placental transfer of RLT was high in both directions.
TEXT
Antiretroviral therapy (ART), in addition to its benefits for the health of people living with HIV, is effective for preventing mother-to-child transmission (MTCT) of HIV-1, which in industrialized countries has been reduced to <1% (1–3). Raltegravir (RLT) belongs to the integrase strand transfer inhibitor (INSTI) class of antiretroviral agents, which act by blocking the insertion of viral cDNA into human genomic DNA (4). RLT is classified in category C by the Food and Drug Administration (FDA), meaning that fetal risk has been detected in animal reproduction studies, but there have been no adequate studies conducted in pregnant women. The placental transfer of currently used antiretrovirals varies considerably between classes and individual molecules, and nucleoside reverse transcriptase inhibitors (NRTIs) have high placental transfer (5, 6), whereas protease inhibitors have lower placental transfer (7, 8). The purpose of this study was to investigate the bidirectional placental transfer of RLT by using the ex vivo model of the human perfused cotyledon.
Nine term placentas from normal pregnancies (from 38 to 42 weeks of gestational age) were obtained from Louis Mourier Hospital (Colombes, France). All mothers were seronegative for HIV infection and hepatitis B and C, and they had taken no medication other than oxytocin or epidural anesthesia during labor. Written informed consent was obtained for all placentas. RLT base was provided by Merck (Paris, France), antipyrine-phosphate-buffered saline (PBS) was purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France), and other chemicals were from Invitrogen (Cergy-Pontoise, France).
Placentas were perfused in an open double-circuit system, as previously described (9). Perfusion experiments were started within 20 min after delivery. After visual examination, an intact cotyledon was selected, and a truncal branch of the chorionic artery and the associated vein were cannulated; the establishment of venous flow after perfusion of the fetal artery flow confirmed the viability of the cotyledon. On the maternal side, the perfused area progressively whitened, allowing visualization of the cotyledon. The cotyledon was placed into the perfusion chamber and maintained at 37°C, with the maternal side upward. The intervillous space on the maternal side was perfused by two needles piercing the basal plate. The two circuits were pumped separately by peristaltic pumps. The fetal and maternal flows were 6 and 12 ml/min, respectively, and the perfusion length was 90 min. The pH levels of the maternal and fetal solutions, prepared with Earle medium, were adjusted to 7.4 ± 0.1 and 7.2 ± 0.1, respectively. A target RLT concentration of 2,000 ng/ml was used, corresponding to the mean peak value in patients receiving 400 mg of RLT with 100 mg of ritonavir twice a day (b.i.d.). Antipyrine, a marker that diffuses freely and is used to validate the experiments, was used at 20 mg/liter. RLT and antipyrine were perfused into maternal reservoir for maternal-to-fetal experiments and into the fetal artery for the fetal-to-maternal direction. Samples were collected every 5 and 15 min from the fetal and maternal compartments, respectively. RLT concentrations were determined using liquid chromatography coupled with tandem mass spectrometry, as previously described (10). Antipyrine concentrations were determined by liquid chromatography with UV detection, as previously described (11, 12). For maternal-to-fetal transfer, the fetal transfer rate (FTR) and clearance index (CI) were calculated under steady-state conditions as the ratios of fetal to maternal concentrations, and the FTR of RLT to that of antipyrine, respectively (13). An FTR of antipyrine of ≥20% was required to validate each experiment. In the fetal-to-maternal direction, inverse FTRs were calculated as the ratios of maternal to fetal concentrations.
Data were analyzed using R software version 3.2 (https://www.r-project.org/). A repeated-measures analysis of variance (ANOVA) was applied for the comparison of placental transfer in maternal-to-fetal and fetal-to-maternal directions. A P value of <0.05 was considered statistically significant.
In the maternal-to-fetal direction (n = 3 placentas), the mean ± standard deviation (SD) FTR and IC of RLT were 9.1% ± 1.4% and 0.28 ± 0.05, respectively. In the fetal-to-maternal direction (n = 6 placentas), the mean ± SD CI was 0.31 ± 0.09. The FTR did not differ significantly between the fetal-to-maternal and maternal-to-fetal directions (P = 0.07) (Fig. 1), nor did the CI (P = 0.63) (Fig. 2).
FIG 1.
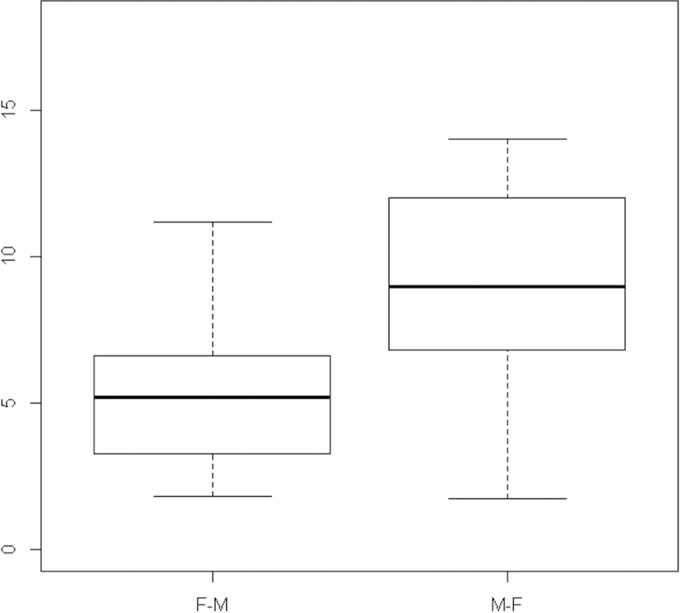
Fetal transfer rate (FTR) of RLT. Box plot of fetal transfer rate of RLT in the fetal-to-maternal (F-M) direction on the left (n = 6) and in the maternal-to-fetal (M-F) direction on the right (n = 3) (P = 0.07). Thick horizontal line indicates the mean, boxes indicate the interquartile range from 25th to 75th percentile, and whiskers indicate the maximum and minimum values.
FIG 2.

Clearance index (CI) of RLT. Box plot of CI of RLT in the fetal-to-maternal (F-M) direction on the left (n = 6) and in the maternal-to-fetal (M-F) direction on the right (n = 3) (P = 0.63). Thick horizontal line indicates the mean, boxes indicate the interquartile range from 25th to 75th percentile, and whiskers indicate the maximum and minimum values.
This study showed a high transfer of RLT across the human placenta in both the maternal-to-fetal and fetal-to-maternal directions. There are clinical data showing high cord-to-maternal (C/M) serum ratios at delivery on the order of 1 (14–18). Moreover, RLT penetrates well into the genital tract, with a cervicovaginal fluid-to-blood plasma ratio of 2.3 (19). This study is the first to investigate the bidirectional placental transfer of RLT using the ex vivo human perfusion model, which reproduces the conditions of the third trimester of pregnancy (20). The concordance between the results obtained with the ex vivo placental perfusion model and the C/M ratios has been shown for various antiretroviral agents (21). We are aware that our study lacks power due to the small number of experiments. RLT has characteristics that are known to favor transfer across the placenta: low molecular mass (444.4 Da), intermediate protein binding (83%), and low lipophilicity (oil/water partition coefficient at pH 7.4 of 2.80 [22]). Another important factor influencing the placental transfer of antiretroviral drugs is the ATP-binding cassette (ABC) transporters, which are present in trophoblast cells and thus can expel the drug into the maternal compartment or into the fetal compartment, thereby limiting placental transfer. RLT is substrate and inducer of P-glycoprotein (23–25). Since P-glycoprotein (P-gp) expression decreases between early and late pregnancy (26), it may be hypothesized that by the end of pregnancy, P-gp may not contribute significantly to the limitation of RLT transfer to the fetal compartment.
In conclusion, the ex vivo human cotyledon perfusion data confirm that RLT placental transfer is high. This has implications for ART in pregnant women. Current guidelines recommend starting ART early in order to obtain an undetectable maternal plasma HIV RNA level before delivery. While RLT is not licensed for use in pregnancy, there are increasing clinical observational data (27–33), and guidelines mention its use to prevent MTCT (34, 35). Although there are limited data regarding the use of RLT in pregnancy, there is increasing evidence of its efficacy to rapidly reduce maternal HIV-1 viral load in association with other antiretrovirals late in pregnancy (36). Its good placental transfer also allows for preexposure prophylaxis. Further investigations on the maternal, neonatal, and more long-term outcomes of RLT and other integrase inhibitors should be performed.
ACKNOWLEDGMENTS
We thank the patients for donating placentas for the study. We also thank Merck Corp. for donating RLT. We thank Dominique Duro for participating in the experiments.
REFERENCES
- 1.Mandelbrot L, Tubiana R, LeChenadec J, Dollfus C, Faye A, Pannier E, Matheron S, Khuong MA, Garrait V, Reliquet V, Devidas A, Berrebi A, Allisy C, Elleau C, Arvieux C, Rouzioux C, Warszawski J, Blanche S, ANRS-EPF Study Group. 2015. No perinatal transmission of HIV-1 from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 61:1715–1725. doi: 10.1093/cid/civ578. [DOI] [PubMed] [Google Scholar]
- 2.Sturt AS, Dokubo EK, Sint TT. 2010. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev 2010:CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, Taylor GP, Peckham CS, Tookey PA. 2014. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS 28:1049–1057. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 4.Prada N, Markowitz M. 2010. Novel integrase inhibitors for HIV. Expert Opin Investig Drugs 19:1087–1098. doi: 10.1517/13543784.2010.501078. [DOI] [PubMed] [Google Scholar]
- 5.Bawdon RE. 1998. The ex vivo human placental transfer of the anti-HIV nucleoside inhibitor abacavir and the protease inhibitor amprenavir. Infect Dis Obstet Gynecol 6:244–246. doi: 10.1155/S1064744998000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuntland T, Odinecs A, Pereira CM, Nosbisch C, Unadkat JD. 1999. In vitro models to predict the in vivo mechanism, rate, and extent of placental transfer of dideoxynucleoside drugs against human immunodeficiency virus. Am J Obstet Gynecol 180:198–206. doi: 10.1016/S0002-9378(99)70175-4. [DOI] [PubMed] [Google Scholar]
- 7.Forestier F, de Renty P, Peytavin G, Dohin E, Farinotti R, Mandelbrot L. 2001. Maternal-fetal transfer of saquinavir studied in the ex vivo placental perfusion model. Am J Obstet Gynecol 185:178–181. doi: 10.1067/mob.2001.113319. [DOI] [PubMed] [Google Scholar]
- 8.Gavard L, Gil S, Peytavin G, Ceccaldi PF, Ferreira C, Farinotti R, Mandelbrot L. 2006. Placental transfer of lopinavir/ritonavir in the ex vivo human cotyledon perfusion model. Am J Obstet Gynecol 195:296–301. doi: 10.1016/j.ajog.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Schneider H, Panigel M, Dancis J. 1972. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol 114:822–828. [DOI] [PubMed] [Google Scholar]
- 10.Jung BH, Rezk NL, Bridges AS, Corbett AH, Kashuba ADM. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 21:1095–1104. [DOI] [PubMed] [Google Scholar]
- 11.Mandelbrot L, Duro D, Belissa E, Peytavin G. 2015. Placental transfer of rilpivirine in an ex vivo human cotyledon perfusion model. Antimicrob Agents Chemother 59:2901–2903. doi: 10.1128/AAC.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelbrot L, Duro D, Belissa E, Peytavin G. 2014. Placental transfer of darunavir in an ex vivo human cotyledon perfusion model. Antimicrob Agents Chemother 58:5617–5620. doi: 10.1128/AAC.03184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challier JC, D'Athis P, Guerre-Millo M, Nandakumaran M. 1983. Flow-dependent transfer of antipyrine in the human placenta in vitro. Reprod Nutr Dev 23:41–50. doi: 10.1051/rnd:19830104. [DOI] [PubMed] [Google Scholar]
- 14.Belissa E, Benchikh A, Charpentier C, Valentin M, Bourgeois-Moine A, Lariven S, Damond F, Yazdanpanah Y, Matheron S, Peytavin G. 2015. RLT plasma concentrations on HIV-1 infected pregnant women. 22nd Conf Retroviruses Opportun Infect, 23 to 26 February 2015, Seattle, WA. [Google Scholar]
- 15.Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsäcker K, Haberl AE, Moltó J, Hawkins DA, van der Ende ME, Gingelmaier A, Taylor GP, Ivanovic J, Giaquinto C, Burger DM, Pharmacokinetics of Newly Developed Antiretroviral Agents in HIV-Infected Pregnant Women PANNA Network, PANNA Network. 2015. RLT in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis 61:809–816. doi: 10.1093/cid/civ366. [DOI] [PubMed] [Google Scholar]
- 16.Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, Aweeka F, Lizak P, Kreitchmann R, Burchett SK, Shapiro DE, Hawkins E, Smith E, Mirochnick M, IMPAACT 1026s Study Team. 2014. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr 67:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinnetti C, Baroncelli S, Villani P, Fantoni M, Tozzi V, De Luca A, Cauda R, Anzidei G, Cusato M, Regazzi M, Floridia M, Tamburrini E. 2010. Rapid HIV-RNA decline following addition of RLT and tenofovir to ongoing highly active antiretroviral therapy in a woman presenting with high-level HIV viraemia at week 38 of pregnancy. J Antimicrob Chemother 65:2050–2052. doi: 10.1093/jac/dkq264. [DOI] [PubMed] [Google Scholar]
- 18.Croci L, Trezzi M, Allegri MP, Carli T, Chigiotti S, Riccardi MP, Ricciardi B, Toti M, Nencioni C. 2012. Pharmacokinetic and safety of RLT in pregnancy. Eur J Clin Pharmacol 68:1231–1232. doi: 10.1007/s00228-012-1250-5. [DOI] [PubMed] [Google Scholar]
- 19.Clavel C, Peytavin G, Tubiana R, Soulié C, Crenn-Hebert C, Heard I, Bissuel F, Ichou H, Ferreira C, Katlama C, Marcelin A-G, Mandelbrot L. 2011. Raltegravir concentrations in the genital tract of HIV-1-infected women treated with a RLT-containing regimen (DIVA 01 study). Antimicrob Agents Chemother 55:3018–3021. doi: 10.1128/AAC.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. 2011. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther 90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- 21.Vinot C, Gavard L, Tréluyer JM, Manceau S, Courbon E, Scherrmann JM, Declèves X, Duro D, Peytavin G, Mandelbrot L, Giraud C. 2013. Placental transfer of maraviroc in an ex vivo human cotyledon perfusion model and influence of ABC transporter expression. Antimicrob Agents Chemother 57:1415–1420. doi: 10.1128/AAC.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger DM. 2010. Raltegravir: a review of its pharmacokinetics, pharmacology and clinical studies. Expert Opin Drug Metab Toxicol 6:1151–1160. doi: 10.1517/17425255.2010.513383. [DOI] [PubMed] [Google Scholar]
- 23.Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, Azrolan N, Iwamoto M, Wagner JA, Wenning LA. 2007. Metabolism and disposition in humans of RLT (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos 35:1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 24.Zembruski NCL, Büchel G, Jödicke L, Herzog M, Haefeli WE, Weiss J. 2011. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J Antimicrob Chemother 66:802–812. doi: 10.1093/jac/dkq501. [DOI] [PubMed] [Google Scholar]
- 25.Moss DM, Kwan WS, Liptrott NJ, Smith DL, Siccardi M, Khoo SH, Back DJ, Owen A. 2011. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob Agents Chemother 55:879–887. doi: 10.1128/AAC.00623-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil S, Saura R, Forestier F, Farinotti R. 2005. P-glycoprotein expression of the human placenta during pregnancy. Placenta 26:268–270. doi: 10.1016/j.placenta.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Hegazi A, Mc Keown D, Doerholt K, Donaghy S, Sadiq ST, Hay P. 2012. Raltegravir in the prevention of mother-to-child transmission of HIV-1: effective transplacental transfer and delayed plasma clearance observed in preterm neonates. AIDS 26:2421–2423. doi: 10.1097/QAD.0b013e32835a9aeb. [DOI] [PubMed] [Google Scholar]
- 28.McKeown DA, Rosenvinge M, Donaghy S, Sharland M, Holt DW, Cormack I, Hay P, Sadiq ST. 2010. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS 24:2416–2418. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 29.Taylor BJ, Olson DP, Ivy SP. 2001. Detection of P-glycoprotein in cell lines and leukemic blasts: failure of select monoclonal antibodies to detect clinically significant Pgp levels in primary cells. Leuk Res 25:1127–1135. doi: 10.1016/S0145-2126(01)00085-6. [DOI] [PubMed] [Google Scholar]
- 30.De Hoffer L, Di Biagio A, Bruzzone B, Sticchi L, Prinapori R, Gerbaldo D, Gotta C, Viscoli C. 2013. Use of raltegravir in a late presenter HIV-1 woman in advanced gestational age: case report and literature review. J Chemother 25:181–183. doi: 10.1179/1973947812Y.0000000066. [DOI] [PubMed] [Google Scholar]
- 31.Westling K, Pettersson K, Kaldma A, Navér L. 2012. Rapid decline in HIV viral load when introducing raltegravir-containing antiretroviral treatment late in pregnancy. AIDS Patient Care STDS 26:714–717. doi: 10.1089/apc.2012.0283. [DOI] [PubMed] [Google Scholar]
- 32.Jaworsky D, Thompson C, Yudin MH, Bitnun A, Brophy J, Samson L, Antoniou T, Loutfy MR. 2010. Use of newer antiretroviral agents, darunavir and etravirine with or without RLT, in pregnancy: a report of two cases. Antivir Ther (Lond) 15:677–680. doi: 10.3851/IMP1558. [DOI] [PubMed] [Google Scholar]
- 33.Nóbrega I, Travassos AG, Haguihara T, Amorim F, Brites C. 2013. Use of RLT in late-presenting HIV-infected pregnant women. AIDS Res Human Retroviruses 29:1451–1454. doi: 10.1089/aid.2013.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morlat P. 2013. Prise en charge médicale des personnes vivant avec le VIH. Recommandations du groupe d'experts rapport 2013. Ministère des Affaires Sociales et de la Santé, Paris, France. [Google Scholar]
- 35.U.S. Public Health Service. 2013. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. National Institutes of Health, Bethesda, MD: https://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. [Google Scholar]
- 36.Boucoiran I, Tulloch K, Pick N, Kakkar F, van Schalkwyk J, Money D, Boucher M. 2015. A case series of third-trimester RLT initiation: impact on maternal HIV-1 viral load and obstetrical outcomes. Can J Infect Dis Med Microbiol 26:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]


