Abstract
Little has been done during the past 100 years to develop new antileishmanial drugs. Most infected individuals live in poor countries and have a low cash income to be attractive targets to pharmaceutical corporations. Two heterosidic steroids, solamargine and solasonine, initially identified as major components of the Brazilian plant Solanum lycocarpum, were tested for leishmanicidal activity. Both alkaloids killed intracellular and extracellular Leishmania mexicana parasites more efficiently than the reference drug sodium stibogluconate. A total of 10 μM each individual alkaloid significantly reduced parasite counts in infected macrophages and dendritic cells. In vivo treatment of C57BL/6 mice with a standardized topical preparation containing solamargine (45.1%) and solasonine (44.4%) gave significant reductions in lesion sizes and parasite counts recovered from lesions. Alkaloids present different immunochemical pathways in macrophages and dendritic cells. We conclude that this topical preparation is effective and a potential new and inexpensive treatment for cutaneous leishmaniasis.
INTRODUCTION
Parasitic infections represent a global health problem, particularly in the developing world (1). It has been reported that infection and parasitic diseases contribute to ∼25% of the total global disease burden (1–3). The World Health Organization (WHO) estimates that 350 million people live in countries where at least one of the different forms of leishmaniasis is endemic and that 12 million people are infected worldwide, with an annual mortality rate of ∼60,000 people (1–3). There are ∼2 million new cases each year, and the disease significantly contributes to the propagation of poverty due to loss of wages and because available treatment is expensive and represents an unaffordable economic burden. Leishmania species are obligate intracellular protozoan parasites that, in the amastigote form, live in macrophages and dendritic cells of infected hosts. Leishmania mexicana causes mostly localized cutaneous leishmaniasis (LCL) and in just a few cases causes diffuse cutaneous leishmaniasis (DCL) (1–3). L. mexicana infections are prevalent mostly in Mexico and Central America (1–3). L. mexicana promastigotes live in the sandfly vector, which includes several identified species of the genus Lutzomyia (1). Leishmaniasis is mostly zoonotic in nature, but an anthroponotic mode of transmission also exists (1). Leishmania species in different parts of the world have developed resistance to currently used antileishmanial drugs. For example, parasites infecting the human population in India, in particular in the city of Bihar, present an alarmingly high level of resistance to antimonials (4). Therefore, the development of novel treatments for this disease is considered a priority worldwide (2–5). Natural products represent an invaluable source of drugs to treat various diseases, and in many areas of the world where the disease is endemic, local populations rely on medicinal plant material to treat different forms of leishmaniasis. The New World plants Pentalinon andrieuxii Muell. Arg. (Apocynaceae) and Pera benensis (Euphorbiaceae) have been a source of valuable leishmanicidal components (2, 6–8). Fruits of the plant Solanum lycocarpum St. Hil. (Solanaceae), also known as wolf fruit (“fruto-do-lobo”), have been used to treat a number of medical conditions. Preparations from this plant also display cytotoxic, antimicrobial, immunomodulatory, and antimutagenic activities (9–18). We showed that alkaloids from this plant are lethal to Leishmania amazonensis promastigotes (9). In this work, we studied the role of the alkaloidal extract of S. lycocarpum in the in vitro and in vivo destruction of L. mexicana parasites. We found that two alkaloids, solamargine and solasonine, isolated from this plant present considerable leishmanicidal activity in vitro and induce different immunochemical pathways in macrophages and dendritic cells.
MATERIALS AND METHODS
Pure heterosidic steroids and preparation of the topical formulation for animal treatment.
Pure solamargine and solasonine (heterosidic steroids, each >95% pure) were provided by J.D.M., and J.K.B. prepared the topical formulation containing the steroid extract. The plant extract containing these two steroids (∼45% each) was analyzed by high-performance liquid chromatography using an ultraviolet detector (HPLC-UV) as described previously (10). Briefly, 250 mg of dried alkaloidic extract was dissolved in 20 ml of a mixture of water and ethanol (80%) containing 3 μg/ml veratraldehyde as an internal standard (IS). We used a Shimadzu (Kyoto, Japan) high-performance (pressure) liquid chromatograph with an isocratic system of elution, and the mobile phase contained acetonitrile and sodium phosphate buffer (pH 7.2; 0.01 M) in a ratio of 36.5:63.5 (vol/vol), using a flow rate of 1 ml/min. The detector reading was set at 200 nm, and a sample of 20 μl was injected and ran for 20 min. Stems, leaves, and ripe and unripe fruits of S. lycocarpum originally collected in Cajurú, SP, Brazil, were deposited in the Department of Botany University of São Paulo, SP, Brazil, with the accession number SPFR:11638. Fruits were chopped, dried in an air-circulating oven at 40°C, powdered by using a knife mill, and stored until use in a −20°C freezer. A standardized extract was prepared as reported previously (19). Briefly, 100 g of powdered biomass was extracted overnight with hydrochloric acid (0.2 M). The acid extract was then filtered, and the pH was adjusted to 12 with NaOH (6 M). The supernatant was removed by centrifugation, and the pellet was suspended in ethanol with careful shaking. This ethanolic extract was concentrated under a vacuum, followed by lyophilization furnishing the glycoalkaloid standardized extract. The contents of solamargine and solasonine in the topical formulation were 45.1 and 44.4%, respectively (Fig. 1B). Next, the topical preparation was formulated for animal and human treatment of cutaneous infections with L. mexicana parasites. A known amount of glycoalkaloid extract was mixed with 5% 2,3-dihydroxypropyl (9Z)-9-octadecenoate (monoolein) and 5% propylene glycol in hydroxyethylcellulose (HEC) (Natrosol gel, a nonionic, water-soluble polymer [pH 6.5]). The control topical preparation was prepared by mixing 5% monoolein and 5% propylene glycol in HEC (Natrosol gel, a nonionic, water-soluble polymer [pH 6.5]).
FIG 1.
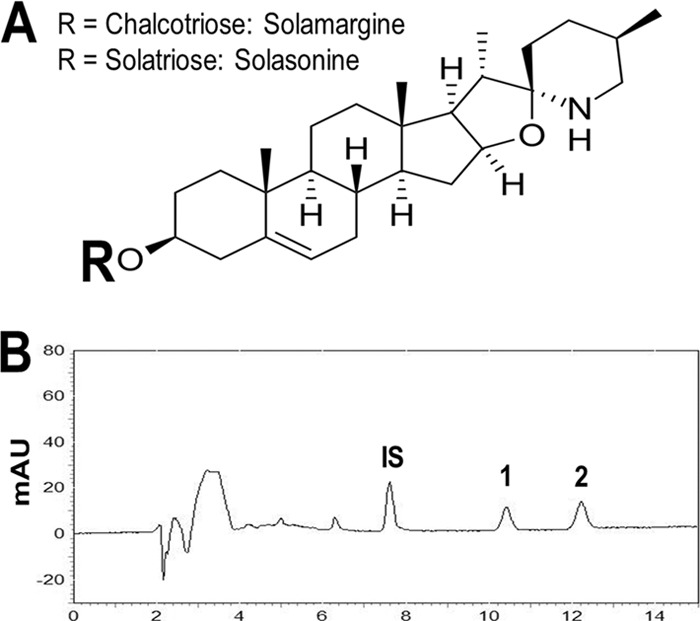
(A) Chemical structures of solamargine and solasonine. (B) Chromatographic profile of Solanum lycocarpum fruit alkaloidal extract as determined by HPLC-UV. The mobile phase was composed of acetonitrile and sodium phosphate buffer (pH 7.2; 0.01 M) in a ratio of 36.5:63.5 (vol/vol) at a flow rate of 1 ml/min with detection at 200 nm. IS, veratraldehyde; 1, solasonine; 2, solamargine. Data are expressed as milli-absorbance units (mAU) on the y axis and time (minutes) on the x axis.
Parasites.
L. mexicana parasites (MNYC/BZ/62/M379) were used in this work. For data in the supplemental material, we used an L. mexicana strain (DsRed L. mexicana) expressing firefly luciferase (LUC) and red fluorescent protein. In brief, clonal transfectants were obtained by homologous integration of a LUC-DsRed2 cassette (SwaI fragment from plasmid pIR1SAT-LUC-DsRed strain B5947) into the rRNA locus as described previously (20), and we name these parasites DsRed L. mexicana parasites. Fluorescence was verified by fluorescence microscopy in promastigotes and amastigotes (inside macrophages). All parasites (regular and transgenic) were maintained by serial passage of amastigotes into the shaven rump of 129SvE mice. Metacyclic promastigotes of L. mexicana were negatively selected by using the peanut agglutinin (PNA) negative-selection protocol: 1.5 × 108 promastigotes were treated with PNA for 30 min at room temperature and spun down at 200 × g for 5 min. Supernatants containing parasites were collected, and the parasites were washed and suspended in fresh complete M-199 culture medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio Inc., Kerrville, TX), 100 IU of penicillin, and 100 μg of streptomycin/ml (Gibco BRL, Grand Island, NY) and injected into the ear dermis of C57BL/6 mice in order to assess the in vivo effectiveness of the alkaloidal extract.
Animals.
C57BL/6 mice of both sexes were used throughout these experiments. Mice were maintained and bred at the specific-pathogen-free (SPF) animal facility at The Ohio State University animal facilities in accordance with institutional and NIH ethical guidelines. Institutional ethical approval was given to perform all animal experiments. C57BL/6 mice of both sexes were used for bone marrow-derived macrophage (BMDM) and bone marrow-derived dendritic cell (BMDDC) isolation and in vivo infection using the ear model of low-dose infection (2).
In vivo infections and treatment.
C57BL/6 mice (10 to 15 weeks old) were dosed with 1,000 PNA-negative (PNA−) parasites in the left ear dermis. Three weeks after initial infection, once palpable lesions were present, mice were treated every day for 6 weeks with the topical formulation of the standardized alkaloidal extract. Control animals were treated with only the vehicle alone (5% monoolein and 5% propylene glycol in Natrosol gel [pH 6.5]) Lesion growth was monitored every week, and parasite loads were calculated at the end of the experiment by direct counting of parasites from infected ears and by a limiting-dilution assay (2).
Parasite killing.
Promastigote killing was assessed by flow cytometry (2, 20). One million parasites suspended in 1 ml of complete M-199 culture medium were used. Solamargine or solasonine dissolved in dimethyl sulfoxide (DMSO) (10 mg/ml in DMSO from Sigma-Aldrich) was added at a known concentration (0 to 100 μM), and the cultures were incubated at 28°C for 48 h. At this point, parasites were centrifuged at 3,000 rpm for 5 min, and the pellet was resuspended in 100 μl of phosphate-buffered saline (PBS) containing 20 μg of propidium iodide (Sigma-Aldrich) and incubated for 1 h in the dark at 4°C. The parasite suspension was made up to 1 ml and analyzed by flow cytometry using a FACSCalibur instrument from Becton Dickinson, and 10,000 cells were analyzed.
In vitro infection of macrophages and dendritic cells.
Bone marrow from femurs and tibias of C57BL/6 mice were disrupted and cultured at 37°C in 5% CO2 in complete culture medium containing 20% of the supernatant from cultured L-929 cells (macrophages) or 20% granulocyte-macrophage colony-stimulating factor (GM-CSF) (dendritic cells) in separate flasks. Cells were grown in 75-cm2 cell culture flasks (Corning Inc., NY) and collected after 5 days of culture. More than 90% of differentiated macrophages were positive for CD11b, a monocyte/macrophage marker, and >90% of differentiated dendritic cells were positive for CD11c, a dendritic cell marker, as tested by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA). Half a million BMDMs or BMDDCs were seeded on top of circular glass coverslips placed at the bottom (one coverslip/well) of 24-well tissue culture plates (Corning Inc.). BMDMs were infected overnight with 5 × 106 stationary-phase L. mexicana metacyclic promastigotes (ratio of 10:1). Next, BMDMs and BMDDCs were extensively washed with Hanks' balanced salt solution (HBSS) to eliminate noninternalized parasites. At this point, infected macrophages or dendritic cells were treated with different concentrations (0 to 100 μM) of solamargine or solasonine for a period of 48 h. After this time, cells were stained with Giemsa stain and mounted for microscopic counting using a 100× objective.
Macrophage and dendritic cell viability test.
Viability of Raw cells, BMDMs, and BMDDCs was tested by using the alamarBlue protocol (2). Cells (105 cells/0.2 ml) were grown in 96 wells in a CO2 incubator (5% CO2 and 95% air) at 37°C and were cocultured with or without 10 μM solamargine or solasonine. After 48 h, 100 μl of the cell supernatant was mixed with 10 μl of the alamarBlue reagent, and the plates were incubated at 37°C for 3 h to allow cells to convert resazurin to resorufin. Next, sample results were read at 570 nm, using 600 nm as a reference wavelength (normalized to the 600-nm value), and the reduction of resazurin was calculated according to the manufacturer's instructions.
Raw NF-κB/AP-1 reporter cell assay.
The cells were transfected to allow them to stably express a secreted embryonic alkaline phosphatase (SEAP) gene inducible by the NF-κB and AP-1 transcription factors in Raw-Blue cells (InvivoGen Inc.). The enzymatic activity of SEAP was quantitated as follows. One hundred thousand cells were seeded into 96-well flat-bottom culture plates in 200 μl containing 10 μM sodium stibogluconate, 10 μM solamargine, solasonine, or sham control cells. After incubation for 48 h at 37°C in a CO2 incubator, 50 μl of each cell supernatant was transferred into separate plates, and 50 μl of Quantiblue reagent was added according to the manufacturer's instructions. Next, this mixture was incubated for 3 h at 37°C, and the absorbance was then measured at 650 nm. Results are expressed as the absorbance read at 650 nm in a plate reader.
Raw and Jaw cell infection with DsRed L. mexicana parasites.
Raw (macrophage) and Jaw (dendritic) cell lines, maintained as described above, were infected overnight with DsRed L. mexicana parasites and then treated for 48 h with 10 μM sodium stibogluconate, solamargine, or solasonine. A control culture included only cells infected with transgenic parasites. Next, cells were washed with PBS, mounted with 4′,6-diamidino-2-phenylindole (DAPI) on glass slides, and observed under a fluorescence microscope. Parasite intracellular killing can be assessed by fluorescence loss (20).
Determination of nitric oxide and cytokine levels.
The concentration of nitric oxide (NO) was determined in supernatants from BMDMs or BMDDCs stimulated overnight with 100 IU of interferon gamma (IFN-γ) and 1 μg of lipopolysaccharide (LPS), followed by coculture with solamargine or solasonine (10 μM) during 48 h. Next, Griess reagent was added to supernatants, and results were read at 570 nm in an enzyme-linked immunosorbent assay (ELISA) reader. Unknown samples were extrapolated from a standard curve (0 to 400 μM). Interleukin-6 (IL-6), IL-12p70, and tumor necrosis factor alpha (TNF-α) concentrations were determined by a capture ELISA using recombinant cytokines as standards and antibody clones from commercial sources. Results were extrapolated from a standard curve after reading in an ELISA reader at 405 nm (2).
Image-based cytometry (FlowSight imaging flow cytometry).
In the supplemental material, apoptosis of uninfected BMDMs and BMDDCs was recorded with an ImageStreamX instrument (Amnis Corporation, Seattle, WA) (image-based cytometry by measuring nucleus sizes of uninfected phagocytes). Uninfected cells were treated with 10 μM tamoxifen (positive control for apoptosis), sodium stibogluconate, solamargine, or solasonine for 48 h and then stained with DAPI. Images were acquired by utilizing a 405-nm laser at a ×20 magnification; cell images are represented as bright-field and blue fluorescence. Side scatter (SSC) was collected in channel 7 at 405 nm (430- to 505-nm filter). Doublets and larger cell clusters were eliminated from the analysis according to the manufacturer's instructions. Data analysis was performed by using IDEAS software.
Statistical analysis.
Mann-Whitney and Dunnett tests were used for statistical analysis, and 50% inhibitory concentrations (IC50s) were calculated by using an LdP Line and Prism 5 software.
RESULTS
In vitro leishmanicidal activity.
Solamargine and solasonine (Fig. 1A) are heterosidic steroids isolated from Solanum lycocarpum fruits, as described previously (9, 10). Figure 1B shows a typical HPLC chromatogram of the standardized plant extract containing solamargine and solasonine and an internal standard (veratraldehyde). The IC50s for parasites treated with solamargine and solasonine were 36.5 ± 0.26 μM and 35 ± 5.4 μM, respectively, which are significantly lower than that of the reference drug (251.3 ± 15.6 μM) (Table 1). The therapeutic index in dendritic cells is considerably higher for solamargine and solasonine than for the reference drug. However, in macrophages, only solasonine's and not solamargine's therapeutic index was higher than that of the reference drug (Table 1). Macrophages and dendritic cells were infected with L. mexicana promastigotes, and after internalization took place, infected cells were treated with solamargine, solasonine, or the reference drug (Table 1). Both alkaloids showed greater activity (lower IC50s) than that of sodium stibogluconate (Table 1). The infection rate (percentage of infected phagocytes) and infection level (average number of parasites per phagocyte) of macrophages and dendritic cells treated with alkaloids (10 μM) are shown in Fig. S1 in the supplemental material. Dendritic cells are more efficient than macrophages in internalizing and killing L. mexicana when stimulated with either alkaloid (see Fig. S1 in the supplemental material). Internalized transgenic parasites (DsRed L. mexicana) were also killed more efficiently by treatment with 10 μM the individual alkaloids than by the reference drug (see Fig. S2 in the supplemental material). This intracellular killing is shown by a loss of fluorescence of internalized parasites.
TABLE 1.
IC50s of promastigote and amastigote killing
| Compound | Mean IC50 (μM) for promastigotes ± SDa | Mean IC50 (μM) for amastigotes inside BMDMs ± SDa | Mean IC50 (μM) for amastigotes inside BMDDCs ± SDa | Therapeutic index for BMDMsb | Therapeutic index for BMDDCsb |
|---|---|---|---|---|---|
| Sodium stibogluconate | 251.3 + 15.6 | 14.32 + 1.01 | 47.91 + 6.46 | 10.6 | 4.6 |
| Solamargine | 35.06 + 5.4c | 13.36 + 3.06 | 6.03 + 2.27c | 9.3 | 43.3 |
| Solasonine | 36.5 + 0.26c | 9.30 + 0.59c | 5.93 + 0.96c | 20.2 | 38.3 |
The IC50 is the concentration of drug (micromolar) to achieve 50% killing of parasites per 106 axenic promastigotes or 2.5 × 106 amastigotes inside 0.5 × 106 BMDMs or BMDDCs after 48 h of culture.
The therapeutic index is the mean 50% cellular cytotoxicity (CC50) of uninfected BMDMs (or BMDDCs)/mean IC50 of amastigotes inside BMDMs (or BMDDCs).
Significantly different (P < 0.05) compared to sodium stibogluconate.
In vivo healing of cutaneous leishmaniasis.
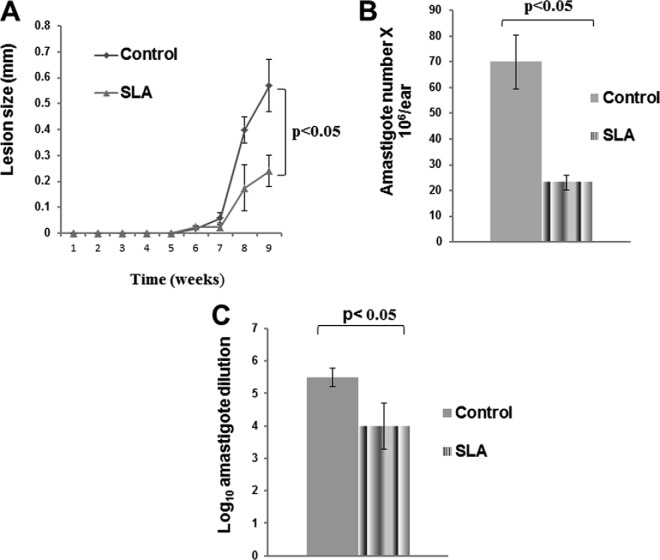
Next, we performed in vivo experiments, as shown in Fig. 2. Figure 2A shows lesion size development in mice infected with 1,000 PNA− metacyclic promastigotes and treated every day for 6 weeks with the topical preparation (SLA) containing 10 μM solamargine and solasonine per mouse per day. As can be seen in Fig. 2A, lesion growth was significantly delayed (P < 0.05) in animals treated with the topical preparation (SLA) compared to untreated animals. Figure 2B shows results obtained after counting parasites in animal ear tissue. Direct counting performed immediately after animals were sacrificed showed a significant reduction in parasite numbers (P < 0.05) (Fig. 2B), and the limiting-dilution assay of parasite suspensions from animal tissue also displayed a significant reduction (P < 0.05) (Fig. 2C) in parasite counts.
FIG 2.
Lesion growth (A) and amastigote numbers recovered from individually infected ears (B and C) of mice treated with the topical formulation. Animals were untreated (control) or treated daily during 6 weeks with a topical preparation of S. lycocarpum (SLA) (A). Parasites were recovered from individual ears, counted (B), and tested by limiting dilution after 4 days of culture (C). Data are expressed as means ± standard errors of the means.
Activation of transcription factors NF-κB and AP-1 and production of NO and cytokines.
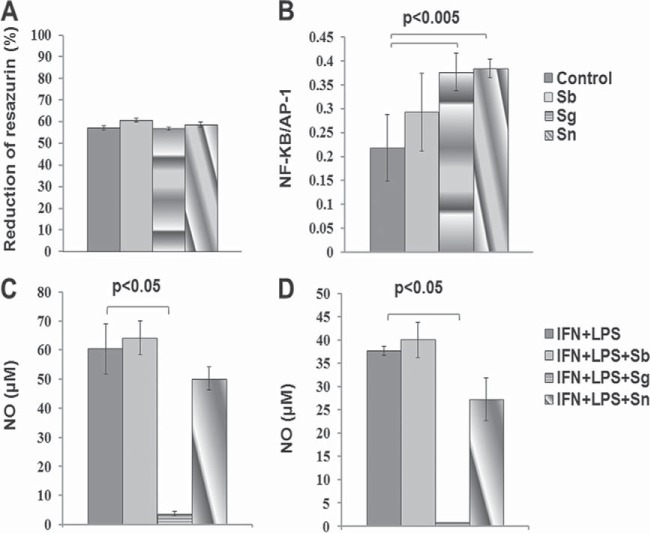
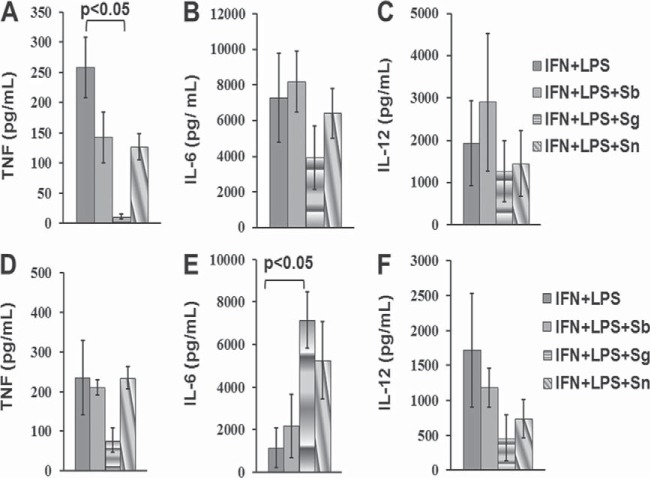
We analyzed the effects of these alkaloids on the activation of NF-κB/AP-1. These two alkaloids tested separately did not affect the viability of regular Raw cells (Fig. 3A), and they induced the expression of the NF-κB/AP-1 transcription factors in reporter transgenic Raw cells (Fig. 3B). Solamargine and solasonine but not sodium stibogluconate induced the expression of these transcription factors at 10 μM. We also measured the content of NO in supernatants of macrophages or dendritic cells treated with alkaloids or the reference drug. Macrophages (Fig. 3C) or dendritic cells (Fig. 3D) cocultured with solamargine but not solasonine presented a robust and significant reduction (P < 0.05) in NO production in cells previously treated with LPS and IFN-γ to induce NO production. These findings suggest that L. mexicana-infected macrophages or dendritic cells treated with solamargine but not solasonine would follow a NO-independent parasite-killing pathway, since this alkaloid significantly suppresses NO production (Fig. 3). The production of cytokines such as IL-6, IL-12, and TNF-α was analyzed in macrophages or dendritic cells induced by treatment with 100 IU of IFN-γ and 1 μg of LPS, followed by treatment with 10 μM the individual alkaloids. TNF-α production was significantly reduced in macrophages stimulated with IFN-γ plus LPS and treated with solamargine but not solasonine (Fig. 4A). Dendritic cells stimulated with IFN-γ plus LPS, followed by treatment with solamargine, showed a significant increase in IL-6 production, which was not observed when cells were treated with solasonine (Fig. 4E). Programmed cell death or apoptosis was also studied, and we found that it is induced by solamargine treatment (10 μM for 48 h) in both macrophages and dendritic cells. Solasonine treatment (10 μM for 48 h) induced apoptosis only in dendritic cells (see Fig. S3 in the supplemental material). In summary, alkaloids inhibit parasite proliferation, kill and reduce the number of parasites in axenic cultures, and reduce parasite counts, and solamargine reduces NO levels in macrophages and dendritic cells previously treated with LPS and IFN-γ. Alkaloids also reduce TNF-α production in both types of cells, enhance IL-6 in dendritic cells previously treated with LPS and IFN-γ, and increase the expression of NF-κB/AP-1 transcription factors in transfected Raw cells. Finally, in vivo treatment with a standardized extract rich in both alkaloids suppressed lesion growth and parasite survival and promoted healing.
FIG 3.
(A) Proliferation of Raw macrophages stimulated with 10 μM sodium stibogluconate (Sb), solamargine (Sg), or solasonine (Sn). (B) Expression of NF-κB/AP-1 transcription factors in reporter cells treated with 10 μM sodium stibogluconate, solamargine, or solasonine or left untreated (control cells). (C and D) Nitric oxide levels in supernatants from LPS/IFN-γ-stimulated macrophages (C) or dendritic cells (D). Cells were cocultured with 10 μM stibogluconate, solamargine, or solasonine. Sham control cells stimulated with LPS/IFN-γ grew in culture medium with 2% DMSO (C and D). Data are expressed as means ± standard errors of the means.
FIG 4.
Determination of cytokine levels (IL-6, IL-12, and TNF-α) in macrophages (A to C) or dendritic cells (D to F) stimulated with LPS/IFN-γ and then treated with 10 μM sodium stibogluconate (Sb), solamargine (Sg), or solasonine (Sn). Data are expressed as means ± standard errors of the means.
DISCUSSION
Development of new drugs to treat leishmaniasis is an urgent need according to WHO reports due to the emergence of drug resistance in parasites and the toxicity of drugs commonly used in areas of endemicity (1–3). Drug resistance is a major problem associated with the failure of treatment of human infections caused by this type of parasite and underscores the importance of the development of novel drugs (21–23). Plant products have long been used in traditional medicine to treat different types of illnesses (2, 6–18) and are now considered a good alternative for drug discovery. There are promising compounds originally isolated from plants with leishmanicidal properties, which could be antileishmanial drugs with commercial potential. Our group recently reported potent antileishmanial activity of one 8-amino-substituted aminoquinoline that can be orally administered (24). There are some other compounds with interesting possibilities for further development as antileishmanial drugs (24–27). Compounds isolated from S. lycocarpum have already demonstrated great potential as antimicrobials and cytotoxic products (10–18). In particular, the glycoalkaloids from this plant present potent antimicrobial activity (11, 13, 16–18). We recently demonstrated that the more abundant S. lycocarpum alkaloids present strong toxicity to extracellular L. amazonensis in vitro (9). In this work, our in vitro experiments showed that L. mexicana promastigotes are equally susceptible to solasonine and solamargine. Parasites treated with these alkaloids inhibit the proliferation of promastigotes. Solamargine and solasonine could interfere with the sterol biosynthesis and membrane stability of L. mexicana. However, more mechanistic studies are needed to prove that these alkaloids affect parasite membrane biosynthesis. Amastigotes inside macrophages and dendritic cells proved to be highly susceptible to both alkaloids. Exposure of uninfected phagocytes to these alkaloids showed that only solamargine-treated macrophages displayed a therapeutic index similar to that of the reference drug. Of particular interest is that a mixture of both alkaloids was very effective in vivo in the treatment of experimentally induced cutaneous lesions in C57BL/6 mice. The rationale for the study this combination of steroids is that we performed a simple and inexpensive extraction protocol that yielded these two alkaloids in a reproducible and pure way from a natural product that can be easily obtained. Purification of individual molecules will go through a more elaborate and expensive preparation procedure, and quantities obtained in this way would yield very small amounts. Both glycoalkaloids mixed in a topical preparation induced an effective clearance of parasites at the site of the lesion, as could be demonstrated by the significant reduction in the number of parasites recovered from lesions of treated mice compared to untreated controls (sham controls). It is important to state that antimonials, the first-line drugs currently used to treat L. mexicana infections in humans (28), do not work in mouse models of ear infection with this parasite (2). Therefore, data from positive controls during in vivo experiments in this and other works are not included (29). When we stimulated macrophages or dendritic cells with IFN-γ and LPS and then treated them with these alkaloids, only solamargine and not solasonine showed a dramatic reduction in NO production. This supports the idea that these alkaloids induce the killing of internalized parasites by using different biochemical pathways in macrophages or dendritic cells, increasing the importance of having a topical formulation containing both alkaloids. In addition, our results show that both alkaloids can increase the expression of NF-κB/AP-1 transcription factors. These factors translocate into the cell nucleus and induce the expression of a number of different genes involved in the regulation of several cellular functions such as cell growth, cytokine production, cell differentiation, apoptosis, and inflammation. Synthesis regulation of these factors by solamargine and solasonine would normalize those biological functions dysregulated by parasite infection, including NO production and inflammation at the lesion site. The significant (P < 0.05) reduction of the synthesis of the proinflammatory cytokine TNF-α in macrophages and the enhancement of the synthesis of the anti-inflammatory cytokine IL-6 in dendritic cells previously stimulated with IFN-γ plus LPS and then treated with solamargine in vitro support this conclusion. Interestingly, our previous work with humans naturally infected with this parasite showed a considerable reduction in the serum content of IL-6 that correlated with the enhancement of the serum content of TNF-α before treatment with the antimonial drug (28). We also found that solamargine induces apoptosis in both macrophages and dendritic cells, while solasonine induces apoptosis only in dendritic cells. We speculate that treatment of cutaneous lesions with an S. lycocarpum extract would lead to healing of these lesions by killing of extracellular parasites. Once alkaloids penetrate infected cells, intracellular parasites would also be killed by alkaloids or by the phagocyte-killing machinery. Bystander phagocytes in the lesion site in contact with alkaloids would speed up the process of apoptosis. Interestingly, in the Yucatan peninsula of Mexico, ear infections with this parasite in humans (chiclero's ulcers) are very common, and in this work, we used the ear model of infection in mice. Unfortunately, there are no available data in the literature regarding the pharmacokinetics of these steroids, but they constitute a new potential phytomedicine to treat cutaneous leishmaniasis. Finally, we have demonstrated that this topical preparation containing solamargine and solasonine is effective in suppression of cutaneous leishmaniasis in C57BL/6 mice.
Supplementary Material
ACKNOWLEDGMENTS
We declare that we do not have any conflict of interest.
This research was financially supported through a CONACYT (Fondo Sectorial Salud 140091) grant to A.P.I.-M. and C.M.L.-D. We are thankful to the São Paulo Research Foundation (FAPESP) for financial support.
We are grateful to A. Satoskar and S. Beverly for assistance in developing some experiments reported in this work.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02804-15.
REFERENCES
- 1.WHO. 2015. Leishmaniasis fact sheet no. 375. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs375/en/. [Google Scholar]
- 2.Lezama-Dávila CM, Pan L, Isaac-Márquez AP, Terrazas C, Oghumu S, Isaac-Márquez R, Pech-Dzib MY, Barbi J, Calomeni E, Parinandi N, Kinghorn AD, Satoskar AR. 2014. Pentalinon andrieuxii root extract is effective in the topical treatment of cutaneous leishmaniasis caused by Leishmania mexicana. Phytother Res 28:909–916. doi: 10.1002/ptr.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kevric I, Cappel MA, Keeling JH. 2015. New World and Old World Leishmania infections: a practical review. Dermatol Clin 33:579–593. doi: 10.1016/j.det.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Prasad N, Ghiya BC, Bumb RA, Kaushal H, Saboskar AA, Lezama-Davila CM, Salotra P, Satoskar AR. 2011. Heat, Oriental sore, and HIV. Lancet 377:610. doi: 10.1016/S0140-6736(10)61495-X. [DOI] [PubMed] [Google Scholar]
- 5.Lucero E, Collin SM, Gomes S, Akter F, Asad A, Kumar Das A, Ritmeijer K. 2015. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl Trop Dis 9:e0003699. doi: 10.1371/journal.pntd.0003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lezama-Dávila CM, Isaac-Márquez AP, Zamora-Crescencio P, Uc-Encalada MDR, Jusstiniano-Apolinar SY, del Angel-Robles L, Satoskar A, Hernández-Rivero L. 2007. Leishmanicidal activity of Pentalinon andrieuxii. Fitoterapia 78:255–257. doi: 10.1016/j.fitote.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Pan L, Lezama-Dávila CM, Isaac-Márquez AP, Calomeni EP, Fuchs JR, Satoskar AR, Kinghorn AD. 2012. Sterols with antileishmanial activity isolated from the roots of Pentalinon andrieuxii. Phytochemistry 82:128–135. doi: 10.1016/j.phytochem.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournet A, Angelo A, Muñoz V, Roblot F, Hocquemiller R, Cavé A. 1992. Biological and chemical studies of Pera benensis, a Bolivian plant used in folk medicine as a treatment of cutaneous leishmaniasis. J Ethnopharmacol 37:159–164. doi: 10.1016/0378-8741(92)90074-2. [DOI] [PubMed] [Google Scholar]
- 9.Abreu Miranda M, Tiossi RF, da Silva MR, Rodrigues KC, Kuehn CC, Rodrigues Oliveira LG, Albuquerque S, McChesney JD, Lezama-Dávila CM, Isaac-Márquez AP, Kenupp Bastos J. 2013. In vitro leishmanicidal and cytotoxic activities of the glycoalkaloids from Solanum lycocarpum (Solanaceae) fruits. Chem Biodivers 10:642–648. doi: 10.1002/cbdv.201200063. [DOI] [PubMed] [Google Scholar]
- 10.Tiossi RFJ, Miranda MA, Praça FSG, Sousa JPB, Bentley MVB, McChesney JD, Bastos JK. 2012. A validated reverse phase HPLC analytical method for quantitation of glycoalkaloids in Solanum lycocarpum and its extracts. J Anal Methods Chem 2012:947836. doi: 10.1155/2012/947836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda MA, Kuehn CC, Cardoso JF, Oliveira LG, Magalhães LG, Tiossi RF, Rodrigues V, Zucolloto S, Prado JC Jr, McChesney JD, Bastos JK. 2013. Immunomodulatory effect of the alkaloidic extract of Solanum lycocarpum fruits in mice infected with Schistosoma mansoni. Exp Parasitol 133:396–402. doi: 10.1016/j.exppara.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Tavares DC, Munari CC, Araújo MG, Beltrame MC, Furtado MA, Gonçalves CC, Tiossi RF, Bastos JK, Cunha WR, Veneziani RC. 2011. Antimutagenic potential of Solanum lycocarpum against induction of chromosomal aberrations in V79 cells and micronuclei in mice by doxorubicin. Planta Med 77:1489–1494. doi: 10.1055/s-0030-1270886. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Li S, Sun F, Han H, Zhang X, Fan Y, Tai G, Zhou Y. 2010. In vivo antimalarial activities of glycoalkaloids isolated from Solanaceae plants. Pharm Biol 48:1018–1024. doi: 10.3109/13880200903440211. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Sharma B, Bakshi N. 2009. Biological activity of alkaloids from Solanum dulcamara L. Nat Prod Res 23:719–723. doi: 10.1080/14786410802267692. [DOI] [PubMed] [Google Scholar]
- 15.Blankemeyer JT, McWilliams ML, Rayburn JR, Weissenberg M, Friedman M. 1998. Developmental toxicology of solamargine and solasonine glycoalkaloids in frog embryos. Food Chem Toxicol 36:383–389. doi: 10.1016/S0278-6915(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 16.Chataing B, Concepción JL, Lobatón R, Usubillaga A. 1998. Inhibition of Trypanosoma cruzi growth in vitro by Solanum alkaloids: a comparison with ketoconazole. Planta Med 64:31–36. doi: 10.1055/s-2006-957361. [DOI] [PubMed] [Google Scholar]
- 17.Fewell AM, Roddick JG, Weissenberg M. 1994. Interactions between the glycoalkaloids solasonine and solamargine in relation to inhibition of fungal growth. Phytochemistry 37:1007–1011. doi: 10.1016/S0031-9422(00)89518-7. [DOI] [PubMed] [Google Scholar]
- 18.Thorne HV, Clarke GF, Skuce R. 1985. The inactivation of herpes simplex virus by some Solanaceae glycoalkaloids. Antiviral Res 5:335–343. doi: 10.1016/0166-3542(85)90003-8. [DOI] [PubMed] [Google Scholar]
- 19.Tiossi Renata FJ, Da Costa JC, Miranda MA, Praça FSG, McChesney JD, Bentley MVLB, Bastos JK. 2014. In vitro and in vivo evaluation of the delivery of topical formulations containing glycoalkaloids of Solanum lycocarpum fruits. Eur J Pharm Biopharm 88:28–33. doi: 10.1016/j.ejpb.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Lezama-Dávila CM, Isaac-Márquez AP, Kapadia G, Owens K, Oghumu S, Beverley S, Satoskar AR. 2012. Leishmanicidal activity of two naphthoquinones against Leishmania donovani. Biol Pharm Bull 35:1761–1764. doi: 10.1248/bpb.b12-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alizadeh R, Hooshyar H, Bandehpor M, Arbabi M, Kazemi F, Talari A, Kazemi BB. 2011. Detection of drug resistance gene in cutaneous leishmaniasis by PCR in some endemic area of Iran. Iran Red Crescent Med J 13:863–867. [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Victoria JM, Pérez-Victoria FJ, Parodi-Talice A, Jiménez IA, Ravelo AG, Castanys S, Gamarro F. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob Agents Chemother 45:2468–2474. doi: 10.1128/AAC.45.9.2468-2474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson DM, Sifri CD, Rodgers M, Wirth DF, Hendrickson N, Ullman B. 1992. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol 12:2855–2865. doi: 10.1128/MCB.12.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaac-Márquez AP, McChesney JD, Nanayakara NP, Satoskar AR, Lezama-Dávila CM. 2010. Leishmanicidal activity of racemic +/− 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline. Nat Prod Commun 5:387–390. [PubMed] [Google Scholar]
- 25.Courtney KO, Thompson PE., Hodgkinson R, Fitzsimmons JR. 1959–1960. Paromomycin as a therapeutic substance for intestinal amebiasis and bacterial enteritis. Antibiot Annu 7:304–309. [PubMed] [Google Scholar]
- 26.Frankenburg S, Gross A, Jonas F, Klaus S. 1993. Effect of topical paromomycin on cell-mediated immunity during cutaneous leishmaniasis. Int J Dermatol 32:68–70. doi: 10.1111/j.1365-4362.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 27.Monge-Maillo B, López-Vélez R. 2015. Miltefosine for visceral and cutaneous leishmaniasis: drug characteristics and evidence-based treatment recommendations. Clin Infect Dis 60:1398–1404. doi: 10.1093/cid/civ004. [DOI] [PubMed] [Google Scholar]
- 28.Lezama-Dávila CM, Isaac-Márquez AP. 2006. Systemic cytokine response in humans with chiclero's ulcers. Parasitol Res 99:546–553. doi: 10.1007/s00436-006-0203-2. [DOI] [PubMed] [Google Scholar]
- 29.Cummings HE, Barbi J, Reville P, Oghumu S, Zorko N, Sarkar A, Keiser TL, Lu B, Rückle T, Varikuti S, Lezama-Davila C, Wewers MD, Whitacre C, Radzioch D, Rommel C, Seveau S, Satoskar AR. 2012. Critical role for phosphoinositide 3-kinase gamma in parasite invasion and disease progression of cutaneous leishmaniasis. Proc Natl Acad Sci U S A 109:1251–1256. doi: 10.1073/pnas.1110339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.