Abstract
A retrospective analysis was performed using The Surveillance Network, USA, to examine the prevalence of antibiotic resistance among urine isolates from U.S. female outpatients in 2012 and assessed trends in antibiotic resistance comparing data from 2003 and 2012. The most common pathogen identified in 2012 (n = 285,325) was Escherichia coli (64.9% of isolates). In 2012, E. coli resistance to nitrofurantoin was low (<3%) across all age groups. E. coli resistance to ciprofloxacin was high among adults (11.8%) and elderly outpatients (29.1%). When comparing the 2003 and 2012 data from isolates from adults, E. coli resistance to nitrofurantoin changed only slightly (from 0.7% to 0.9%), whereas increases in resistance to ciprofloxacin (3.6% to 11.8%) and trimethoprim-sulfamethoxazole (17.2% to 22.2%) changed substantially. In the United States, E. coli has become increasingly resistant to ciprofloxacin and trimethoprim-sulfamethoxazole (TMP-SMX) in adult female outpatients. Nitrofurantoin retains high levels of antibiotic activity against urinary E. coli.
INTRODUCTION
Antibiotic use is the single most important modifiable risk factor for antibiotic resistance (1). Urinary tract infection (UTI), accounting for >8 million office visits and 1 million emergency department visits annually in the United States, is one of the most frequent indications for antibiotic use in ambulatory care (2). For the treatment of uncomplicated UTIs, or cystitis, clinical practice guidelines recommend the empirical use of nitrofurantoin or trimethoprim-sulfamethoxazole (TMP-SMX) as first-line therapy (3, 4). Fluoroquinolones, including ciprofloxacin and levofloxacin, are considered alternative agents for the treatment of uncomplicated UTIs (3).
An important consideration for the appropriate empirical treatment of UTIs is antibiotic susceptibility among uropathogens isolated from urine cultures (3). Urine cultures are often obtained for outpatients with UTIs, including those with recurrent infections, previous treatment failures, or complicated infections (5). Few investigations have explored national trends of outpatient antibiotic resistance among uropathogens in women. The objectives of this study were to examine the recent prevalence of in vitro antibiotic resistance among common bacteria isolated from urine cultures collected from U.S. female outpatients and to assess trends in antibiotic resistance by comparing data from 2003 and 2012.
MATERIALS AND METHODS
We performed a retrospective analysis of antibiotic susceptibility test results from urine cultures collected from female outpatients in the United States comparing 2003 with 2012 data from The Surveillance Network (TSN) (Eurofins Medinet, Chantilly, VA). More than 200 institutions in the United States collected and submitted susceptibility data and corresponding demographic information (age, sex, and site of infection) to TSN during these years. Outpatients were defined as individuals who visited any ambulatory care setting, such as an emergency department, hospital-based outpatient clinic, or physician's office, during the study period. Other data collected from participating labs included the isolated pathogen, tested antibiotic agents, susceptibility testing methods, and institution type and size. Individual laboratories performed their own susceptibility testing using FDA-approved methods and interpreted the results by use of Clinical and Laboratory Standards Institute (CLSI) breakpoint criteria. Laboratories participating in TSN were distributed in a way that was demographically representative of the United States at the level of the nine U.S. census regions.
Antibiotic resistance across age groups was assessed by comparing susceptibility test results among urine specimens collected from pediatric (age, 0 to 17 years), adult (age, 18 to 64 years), and older adult (age, ≥65 years) outpatients. The 10 most common bacterial species identified from all female outpatient cultures were included. Antibiotic agents were selected based on the availability of susceptibility data and clinical guideline-recommended treatment for UTIs (3). In the case of antibiotic classes for which no susceptibility data were available for oral antibiotic formulations, intravenous surrogate antibiotics were selected. The antibiotics included in this study were amoxicillin-clavulanate, ampicillin, cefazolin, ceftriaxone, cefuroxime, ciprofloxacin, nitrofurantoin, oxacillin, and TMP-SMX. Fosfomycin data were included but limited, as this agent was not commonly tested and was not widely available on antibiotic susceptibility test instrument panels. Duplicate isolates were not included, and intermediate results were not considered resistant. CLSI breakpoint recommendations for cefazolin were reduced from ≤8 μg/ml in 2003 to ≤2 μg/ml in 2011 (6) and likely contributed to the higher observed resistance rates. Cefazolin, cefuroxime, and ceftriaxone were selected as first-, second-, and third-generation cephalosporin surrogates, respectively; ampicillin was selected as a surrogate for oral penicillins.
A chi-squared test using SAS version 9.3 (SAS Institute, Cary, NC) was used to determine whether differences in isolate prevalence and antibiotic resistance between 2003 and 2012 were statistically significant. The α-level for statistical significance was set at 0.05.
RESULTS
Among urinary isolates from female outpatients in 2012 (n = 305,749), the most frequently identified bacteria were E. coli (64.9% of isolates), Klebsiella pneumoniae (10.1%), Proteus mirabilis (5.0%), Enterococcus faecalis (4.1%), and Pseudomonas aeruginosa (2.7%).
The prevalence of antibiotic resistance among urinary bacterial isolates from 2012 was stratified by age group (Table 1). Antibiotic resistance to TMP-SMX was elevated across all age groups for most bacterial species, but it was especially high among isolates of E. coli (pediatric, 25.1%; adult, 22.3%; older adult, 26.8%). Conversely, E. coli resistance to nitrofurantoin was very low (pediatric, 0.5%; adult, 0.9%; older adult, 2.6%). E. coli resistance to fosfomycin in 2012 (n = 212) was 4.2%. The prevalence of resistance increased by age group for several important pathogen-antibiotic combinations. For example, E. coli resistance to ciprofloxacin was relatively low among isolates from pediatric outpatients (5.1%), higher among isolates from adult outpatients (11.8%), and highest among isolates from older adult outpatients (29.1%). A similar pattern was observed for methicillin-resistant Staphylococcus aureus.
TABLE 1.
Prevalence of antibiotic resistance among the top 10 uropathogens obtained from U.S. female outpatients in 2012, according to age group
| Uropathogen, by age group | na | Prevalence (%) of isolates resistant tob: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amox-clavc | Ampicillin | Cefazolind | Ceftriaxone | Cefuroxime | Ciprofloxacin | Nitrofurantoin | Oxacillin | TMP-SMXe | ||
| Children 0–17 yr of age | ||||||||||
| Gram negative | ||||||||||
| Citrobacter freundii | 1,079 | 17.2 | Xf | 0.7 | 0.7 | 19.1 | ||||
| Enterobacter aerogenes | 692 | 13.8 | X | 0.0 | 36.0 | 3.4 | ||||
| Enterobacter cloacae | 1,002 | 37.7 | X | 0.0 | 24.8 | 17.9 | ||||
| Escherichia coli | 130,247 | 3.1 | 44.8 | 38.5 | 3.3 | 2.5 | 5.1 | 0.5 | 25.1 | |
| Klebsiella oxytoca | 615 | 9.7 | 10.0 | 7.1 | 10.0 | 1.3 | 0.0 | 11.8 | ||
| Klebsiella pneumoniae | 6,137 | 1.0 | 25.4 | 3.4 | 2.0 | 0.8 | 17.7 | 11.4 | ||
| Proteus mirabilis | 6,555 | 1.9 | 10.9 | 63.0 | 1.1 | 2.6 | 1.5 | 8.2 | ||
| Pseudomonas aeruginosag | 687 | 0.8 | ||||||||
| Gram positive | ||||||||||
| Enterococcus faecalis | 20,061 | 1.0 | 3.9 | 0.3 | ||||||
| Staphylococcus aureus | 2,088 | 17.5 | 12.5 | X | 8.6 | 0.0 | 16.5 | 0.5 | ||
| Adults 18–64 yr old | ||||||||||
| Gram negative | ||||||||||
| Citrobacter freundii | 4,899 | 18.0 | X | 6.7 | 2.4 | 16.6 | ||||
| Enterobacter aerogenes | 10,549 | 8.6 | X | 1.1 | 29.1 | 1.4 | ||||
| Enterobacter cloacae | 4,912 | 33.7 | X | 4.9 | 30.4 | 14.8 | ||||
| Escherichia coli | 599,722 | 3.9 | 41.3 | 37.5 | 4.5 | 3.9 | 11.8 | 0.9 | 22.3 | |
| Klebsiella oxytoca | 3,877 | 6.3 | 79.8 | 10.1 | 9.8 | 6.5 | 6.0 | 10.7 | ||
| Klebsiella pneumoniae | 66,261 | 3.1 | 27.2 | 5.7 | 5.6 | 4.0 | 20.5 | 10.6 | ||
| Proteus mirabilis | 30,756 | 0.8 | 12.3 | 59.5 | 1.0 | 1.4 | 7.5 | 11.8 | ||
| Pseudomonas aeruginosa | 1,731 | 18.9 | ||||||||
| Gram positive | ||||||||||
| Enterococcus faecalis | 20,061 | 0.5 | 13.0 | 0.1 | ||||||
| Staphylococcus aureus | 13,468 | 22.1 | 18.0 | X | 31.3 | 24.7 | 0.6 | 26.2 | 2.0 | |
| Older adults (65+ yr old) | ||||||||||
| Gram negative | ||||||||||
| Citrobacter freundii | 10,578 | 19.1 | X | 4.1 | 4.2 | 16.6 | ||||
| Enterobacter aerogenes | 6,267 | 20.3 | X | 2.0 | 33.3 | 3.3 | ||||
| Enterobacter cloacae | 10,425 | 34.6 | X | 6.1 | 25.4 | 14.9 | ||||
| Escherichia coli | 443,027 | 5.5 | 45.2 | 49.3 | 9.5 | 9.5 | 29.1 | 2.6 | 26.8 | |
| Klebsiella oxytoca | 9,963 | 2.7 | 83.1 | 5.8 | 5.5 | 2.3 | 4.1 | 6.4 | ||
| Klebsiella pneumoniae | 104,638 | 2.9 | 30.2 | 7.4 | 6.8 | 5.5 | 22.4 | 10.6 | ||
| Proteus mirabilis | 43,127 | 1.6 | 23.6 | 66.7 | 3.0 | 3.9 | 29.0 | 25.8 | ||
| Pseudomonas aeruginosa | 4,878 | 17.3 | ||||||||
| Gram positive | ||||||||||
| Enterococcus faecalis | 29,466 | 0.7 | 34.4 | 0.6 | ||||||
| Staphylococcus aureus | 13,468 | 50.8 | 46.8 | X | 71.6 | 0.2 | 56.7 | 2.2 | ||
n = total number of antibiotic susceptibility testing results.
Isolate/agent testing varied slightly across institutions based on established local antibiotic susceptibility testing panels and protocols.
Amox-clav, amoxicillin-clavulanate.
Modified CLSI susceptibility testing breakpoints for cefazolin were released in January 2011 and likely affected antibiotic susceptibility testing interpretation for this agent.
TMP-SMX, trimethoprim-sulfamethoxazole.
X, intrinsic resistance to this agent, which was not reported here.
P. aeruginosa was only routinely tested against oral antipseudomonal agents.
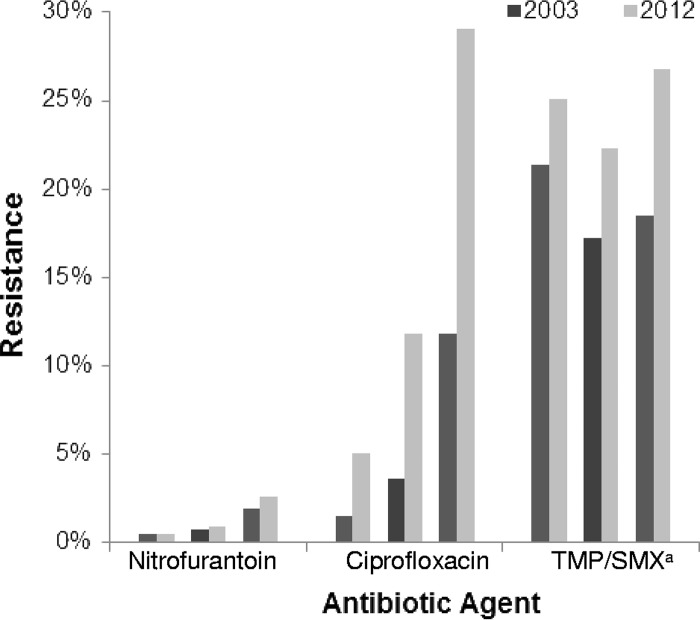
The magnitude of changes in E. coli antibiotic resistance varied when comparing 2003 with 2012 data (Fig. 1). Increases in E. coli resistance to nitrofurantoin were small (from 0.7% in 2003 to 0.9% in 2012 for adults; P < 0.001). However, ciprofloxacin resistance increased in all age groups, and increases were especially pronounced among isolates from adults (from 3.6% in 2003 to 11.8% in 2012; P < 0.001) and older adults (from 11.8% in 2003 to 29.1% in 2012; P < 0.001). Increases in E. coli resistance to TMP-SMX were observed in all age groups, including pediatric outpatients (from 21.3% to 24.9%; P < 0.001), adult outpatients (from 17.2% to 22.2%; P < 0.001), and older adult outpatients (from 18.5% to 26.7%; P < 0.001).
FIG 1.
Comparison of prevalence of E. coli antibiotic resistance among urinary isolates from female outpatients in 2003 and 2012, by age group. TMP/SMX, trimethoprim-sulfamethoxazole.
DISCUSSION
In our study of culture results from urine specimens collected from U.S. female outpatients, E. coli was the most common pathogen isolated. A comparison of 2003 and 2012 results showed that E. coli resistance to nitrofurantoin remained low, while resistance to ciprofloxacin increased substantially. Antibiotic resistance among several important pathogen-antibiotic combinations was highest among isolates from older adult outpatients.
Current guidelines emphasize the use of targeted narrow-spectrum therapies, such as nitrofurantoin, for the treatment of uncomplicated UTIs among adult women (3). However, antibiotic selection for UTI treatment and adherence to treatment guidelines are suboptimal (7). Kallen et al. (8) used National Ambulatory Care Survey and National Hospital Ambulatory Care Survey data from 2000 to 2002 to explore national trends in prescribing of antibiotics for UTI visits among outpatient women and found that quinolones had surpassed sulfanilamides as the most commonly prescribed antibiotic class. This shift has been corroborated by other studies and likely contributed to the increased ciprofloxacin resistance observed among urinary pathogens in our study (9, 10).
Fluoroquinolones have been designated by the World Health Organization as critically important antibiotics for human medicine due to their widespread utility in treating infections and “high absolute number of people affected by all diseases for which [they are] the sole/one of few therapies available” (11). The overuse of fluoroquinolones for UTIs raises important concerns for both individual and community health. For example, fluoroquinolone use induces antibiotic resistance among Gram-negative bacteria, contributes to resistant infections among inpatients, and has been implicated as a frequent cause of both health care-associated and community-associated Clostridium difficile infection (12, 13). In order to preserve the efficacy of fluoroquinolones for higher-severity infections while minimizing harm to the individual and the community, prescribing of nitrofurantoin over ciprofloxacin should be encouraged for the empirical treatment of uncomplicated UTIs in adult female outpatients (3).
The high prevalence of antibiotic-resistant uropathogens in older adult female outpatients is a concern because it complicates the effective treatment of UTIs, limits therapeutic options, and contributes to excess morbidity (14). Compared to younger individuals, older adults are more likely to have polymicrobial infections, receive a course of antibiotics during a clinical visit, and be prescribed a longer duration of treatment (14).
Furthermore, declining renal function is a normal part of aging and may limit the therapeutic value of some antibiotics (e.g., nitrofurantoin) due to a decreased ability to achieve therapeutic drug concentrations in the urinary tract. Although cost-benefit analyses and clinical practice guidelines discourage treatment of asymptomatic bacteriuria in older adults, this practice frequently occurs in ambulatory care (15). Once a diagnosis is established, antibiotic selection for UTIs among older outpatients should be based on previous uropathogen susceptibility profiles, when available, and be weighed carefully against potential adverse drug events, comorbidities, and medication interactions (15).
Our study has limitations. There is likely an underrepresentation of urine cultures from patients whose initial empirical therapy was successful, which may result in overestimation of antibiotic resistance. Furthermore, a lack of clinical information corresponding to urine cultures limits our ability to exclude patients with more complicated medical histories. Finally, several antibiotic susceptibility testing methods were used, which may have contributed to variability in susceptibility test results.
We found that the most common urinary pathogen, E. coli, has become increasingly resistant to ciprofloxacin and TMP-SMX, whereas we saw only slight increases in resistance for nitrofurantoin. Future studies should examine ongoing changes in the prescribing of outpatient antibiotics for UTIs, as antibiotic resistance patterns continue to shift. Individual clinicians should strive for judicious antibiotic selection for UTIs by selecting antibiotic therapy based on clinical guidelines, local resistance data, individual patient characteristics, and potential collateral damage. Health plans, policymakers, and health care facility administrations can help minimize the spread of community antibiotic resistance by supporting antibiotic stewardship initiatives to improve the prescribing behaviors of individual providers. The Centers for Disease Control and Prevention Get Smart: Know When Antibiotics Work program provides resources to help optimize outpatient antibiotic therapy for UTIs and other common infections (www.cdc.gov/getsmart). The widespread and dynamic nature of antibiotic resistance warrants due diligence by many stakeholders, including health systems, public health authorities, health care providers, and policymakers, to prevent, control, and track antibiotic resistance among outpatients.
ACKNOWLEDGMENTS
We acknowledge Lauri Hicks for her contributions to this work.
We have no conflicts of interest to disclose.
This study was carried out as a part of our routine work.
REFERENCES
- 1.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. 2010. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Grigoryan L, Trautner BW, Gupta K. 2014. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 312:1677–1684. doi: 10.1001/jama.2014.12842. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ML, Gaido L. 2004. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 38:1150–1158. doi: 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement CLSI M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Kim M, Lloyd A, Condren M, Miller MJ. 2015. Beyond antibiotic selection: concordance with the IDSA guidelines for uncomplicated urinary tract infections. Infection 43:89–94. doi: 10.1007/s15010-014-0659-4. [DOI] [PubMed] [Google Scholar]
- 8.Kallen AJ, Welch H, Sirovich BE. 2006. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med 166:635–639. doi: 10.1001/archinte.166.6.635. [DOI] [PubMed] [Google Scholar]
- 9.Taur Y, Smith MA. 2007. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin Infect Dis 44:769–774. doi: 10.1086/511866. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. 2014. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. 2011. Critically important antimicrobials for human medicine, 3rd rev World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.Brown KA, Khanafer N, Daneman N, Fisman DN. 2013. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 57:2326–2332. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redgrave LS, Sutton SB, Webber MA, Piddock LJV. 2014. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol 22:438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Shortliffe LM, McCue JD. 2002. Urinary tract infection at the age extremes: pediatrics and geriatrics. Am J Med 113(Suppl 1A):55S–66S. [DOI] [PubMed] [Google Scholar]
- 15.Mody L, Juthani-Mehta M. 2014. Urinary tract infections in older women: a clinical review. JAMA 311:844–854. doi: 10.1001/jama.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]