Abstract
Stenotrophomonas maltophilia harbors six lytic transglycosylases (LTs): mltA, mltB1, mltB2, mltD1, mltD2, and slt. LT deletion increased susceptibility of S. maltophilia to aminoglycosides (AGs) and macrolides, and the underlying mechanisms were investigated. The expression of AG-modifying enzymes and efflux pumps was evaluated by quantitative reverse transcription-PCR (qRT-PCR). Susceptibility to 1-N-phenylnaphthylamine, vancomycin, SDS, and bile salts was measured to assess outer membrane permeability. In conclusion, increased outer membrane permeability contributes to LT deletion-mediated increase in aminoglycoside and macrolide susceptibility.
TEXT
Lytic transglycosylases (LTs) are an important class of bacterial enzymes recognized by their role in creating space within the peptidoglycan (PG) sacculus for its biosynthesis and recycling, cell division, and the insertion of macromolecular complexes spanning the cell wall, such as flagella, pili, and secretion systems (1). Many bacteria encode an array of different LTs that may be functionally redundant. With respect to their involvement in the biosynthesis and recycling of the PG sacculus, LTs are also linked to the expression of chromosomal β-lactamase genes in some ampR-β-lactamase-bearing Gram-negative bacteria (2). Therefore, studies concerning LT-mediated antibiotic resistance primarily focus on β-lactams over other antibiotics.
Stenotrophomonas maltophilia, a nonfermentative, Gram negative, aerobic bacillus, is an opportunistic pathogen responsible for many nosocomial infections (3). It exhibits resistance to a broad array of antibiotics. According to the sequenced genomes, S. maltophilia harbors six putative LT genes: mltA, mltB1, mltB2, mltD1, mltD2, and slt (4). However, very little research has been published concerning the S. maltophilia LTs, except our recent report, in which we demonstrated that inactivation of mltD1 confers a partial basal-level β-lactamase derepression phenotype (5). In this article, we assessed the relationship between LTs and susceptibility to antibiotics besides β-lactams in S. maltophilia.
S. maltophilia LT single deletion mutant (ΔmltA, ΔmltB1, ΔmltB2, ΔmltD1, ΔmltD2, and Δslt) strains and double mutant (ΔmltD1 ΔmltB1 and ΔmltD1 ΔmltD2) strains and a triple mutant (ΔmltD1 ΔmltB1 ΔmltD2) strain were prepared in our previous study (5). To rule out growth rate-mediated changes in susceptibility, first we assessed whether LT inactivation affected bacterial growth by monitoring the optical density at 450 nm (OD450) in 24 h with an interval of 3 h. No growth differences were observed between the wild-type KJ strain and its LT mutants (data not shown). The drug susceptibilities of wild-type KJ and its derived individual LT deletion mutants were assessed following Clinical and Laboratory Standards Institute (CLSI) guidelines (6). Inactivation of each LT did not significantly affect the susceptibility of S. maltophilia to chloramphenicol, nalidixic acid, or tetracycline, as the margin of error for the susceptibility test is in the 2-fold MIC value range (Table 1). It is worth mentioning that each LT mutant was more susceptible to aminoglycosides (AGs); the ΔmltB1 and Δslt mutants were the most susceptible (Table 1). Furthermore, the ΔmltB1, ΔmltD1, ΔmltD2, and Δslt mutants exhibited a prominent decrease in macrolide resistance. Since LT inactivation had never before been associated with an increased susceptibility to AGs and macrolides, we focused on elucidating the underlying mechanisms responsible for this phenotype.
TABLE 1.
Antimicrobial susceptibilities of S. maltophilia KJ and its derived LT deletion mutants
| Strain | MIC (μg/ml)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CHL | NAL | TET | AMK | GEN | KAN | ERY | LM | VAN | |
| KJ wild type | 8 | 8 | 16 | 1,024 | 1,024 | 256 | 64 | 256 | 512 |
| ΔmltA mutant | 8 | 4 | 16 | 256 | 256 | 128 | 32 | 128 | 256 |
| ΔmltB1 mutant | 8 | 4 | 16 | 64 | 128 | 64 | 8 | 16 | 128 |
| ΔmltB2 mutant | 8 | 4 | 16 | 256 | 128 | 128 | 32 | 256 | 256 |
| ΔmltD1 mutant | 8 | 4 | 16 | 128 | 512 | 128 | 32 | 32 | 64 |
| ΔmltD2 mutant | 8 | 4 | 16 | 128 | 256 | 128 | 32 | 32 | 128 |
| Δslt mutant | 8 | 8 | 16 | 64 | 128 | 64 | 32 | 64 | 128 |
| ΔmltD1 ΔmltB1 mutant | 8 | 4 | 16 | 256 | 512 | 64 | 32 | 64 | 128 |
| ΔmltD1 ΔmltD2 mutant | 8 | 8 | 16 | 128 | 512 | 128 | 32 | 64 | 128 |
| ΔmltD1 ΔmltB1 ΔmltD2 mutant | 8 | 8 | 16 | 256 | 512 | 128 | 32 | 128 | 128 |
CHL, chloramphenicol; NAL, nalidixic acid; TET, tetracycline; AMK, amikacin; GEN, gentamicin; KAN, kanamycin; ERY, erythromycin; LM, leucomycin; VAN, vancomycin.
Bacterial resistance to AGs can be mediated by target modification through mutation or methylation of 16S rRNA, drug modification by AG-modifying enzymes (AMEs), drug extrusion by efflux pumps, and changes in membrane permeability (7). The known mechanisms responsible for macrolide resistance include 23S rRNA modification, macrolide hydrolysis or modification, efflux pump extrusion, and changes in outer membrane permeability (8). In this article, knocking out LT genes by homologous recombination did not affect bacterial rRNA modification. Furthermore, no homologs of ere, mph, and lin, which encode proteins involved in macrolide hydrolysis and modification, were found in S. maltophilia by whole-genome searching (8). Therefore, we ruled out the possibility that rRNA and macrolide modification contributed to the LT deletion-mediated decrease in AG and macrolide resistance.
Five putative AMEs, aminoglycoside phosphotransferase (Smlt0191), AAC(2′)-Ic (Smlt1669), APH(3′)-IIc (Smlt2120), streptomycin 3′-phosphotransferase (Smlt2336), and AAC(6′)-Iz (Smlt3615), and an array of efflux pumps were found in the sequenced S. maltophilia K279a genome (4). Of them, expression of aph(3′)-IIc, aac(6′)-Iz, smeDEF, smeIJK, smeOP, smeVWX, smeYZ, and macABCsm has been proven to be related to AG and/or macrolide susceptibility in S. maltophilia (9–16). To assess whether the LT inactivation-mediated decrease in AG and macrolide resistance was linked to AMEs and efflux pumps, the transcripts of aph(3′)-IIc, aac(6′)-Iz, smeE, smeJ, smeP, smeW, smeX, smeZ, and macBsm from wild-type KJ and the LT mutants were quantified by quantitative reverse transcription-PCR (qRT-PCR). Total RNAs from wild-type KJ and the LT mutants were prepared from logarithmic-phase bacterial cultures and then reverse transcribed into cDNA as previously described (14). The primers used for qRT-PCR are listed in Table S1 in the supplemental material. The 16S rRNA gene was chosen as the normalizing gene. The relative quantities of mRNA from each gene of interest were determined by the comparative cycle threshold method. The results revealed that the transcripts of AME and efflux pump-encoding genes in the LT mutants were comparable to that of the wild-type KJ, except smeJ (see Fig. S1 in the supplemental material). The smeJ transcript had a moderate increase in the ΔmltD1 and Δslt mutants compared to wild-type KJ (see Fig. S1); however, it cannot account for the AG resistance decrease of both mutants, since increased expression of the SmeIJK pump should enhance the AG resistance (12). The possible reasons for the smeJ increase in expression in ΔmltD1 and Δslt mutants will be discussed later. Therefore, at the moment, we cannot attribute the AG and macrolide resistance decrease of LT mutants to the decreased expression of the AME and efflux pump genes assayed.
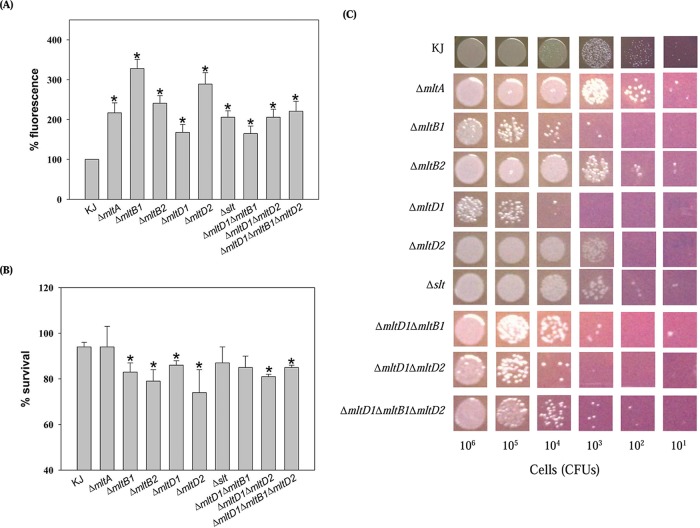
AGs and macrolides have been reported to enter the Gram-negative outer membrane by a self-promoted uptake mechanism (17), which relies on specific properties of the outer membrane. LTs are a family of enzymes that participate in peptidoglycan homeostasis and further affect the envelope integrity of bacteria. The loss of LTs has been reported to compromise membrane integrity (18). Therefore, we considered the possibility that LT deletion would have an impact on some aspect of the outer membrane. The hydrophobic fluorescent probe 1-N-phenylnaphthylamine (NPN) was used to study the permeabilizing effects of self-promoted uptake molecules on bacterial cells (19). Overnight cultures were subcultured into fresh LB broth and grown to mid-logarithmic phase. The cells were harvested by centrifugation, washed with 5 mM HEPES buffer (pH 7.2), and the suspension's optical density was adjusted to an OD450 of 0.5 using the same buffer. Then, 100-μl aliquots of cell suspension were pipetted into 96-well microtiter plates, and NPN was added to a final concentration of 15 μM. Fluorescence was monitored after 5 min of incubation from three parallel wells per sample using a fluorescence spectrophotometer at excitation and emission wavelengths of 355 nm and 402 nm, respectively. The outer membranes of Gram-negative bacteria exclude the hydrophobic probe NPN. Fluorescence is emitted by NPN only after it partitions into the membrane; therefore, greater emission of fluorescence represents greater outer membrane permeability to NPN. LT inactivation caused higher uptake of NPN (Fig. 1A), suggesting there is a membrane defect and therefore AG may be gotten into cells better.
FIG 1.
Outer membrane permeability and integrity of S. maltophilia KJ and its derived LT mutants. Values are averages from three replicates. P values were calculated using Student's t test comparing LT mutants to the wild-type KJ strain. *, P < 0.01. (A) 1-N-Phenylnaphthylamine (NPN) uptake assay. The bacterial cultures assayed were harvested, washed, and then adjusted to an OD450 of 0.5 with 5 mM HEPES buffer (pH 7.2). Then 100 μl of bacterial cells was pipetted into 96-well microtiter plates, and NPN was added to a final concentration of 15 μM. Fluorescence was monitored by fluorescence spectrophotometer at excitation and emission wavelengths of 355 nm and 402 nm, respectively. (B) SDS survival assay. The survival of wild-type KJ and its derived LT mutants in LB broth without or with 0.01% SDS was determined by CFU counting. The percentage of survival was defined as the CFU ratio of the SDS-additive group to the SDS-free counterpart. (C) Bile acid susceptibility. The logarithmic-phase bacterial cells were collected, adjusted to a concentration of 5 × 105 CFU/μl, and 10-fold serially diluted. Then 2 μl of bacterial cells was spotted onto the bile salts-containing MacConkey agars. After 18 h of incubation at 37°C, the growth of bacterial cells was observed.
The outer membrane of Gram-negative bacilli is a general barrier for high-molecular-weight antibiotics such as vancomycin or macrolides. The vancomycin susceptibility can be used as an indicator for evaluating the outer membrane permeability of Gram-negative bacteria for high-molecular-weight substances. Next, we investigated whether LT inactivation altered the outer membrane permeability for high-molecular-weight substances. The susceptibility of LT mutants to vancomycin was evaluated. Compared to that of wild-type KJ, the MICs of the ΔmltB1, ΔmltD1, ΔmltD2, and Δslt mutants to vancomycin decreased (Table 1). The elevated outer membrane permeability for high-molecular-weight substances in the ΔmltB1, ΔmltD1, ΔmltD2, and Δslt mutants can provide an explanation for the increased macrolide susceptibility observed in the ΔmltB1, ΔmltD1, ΔmltD2, and Δslt mutants.
In view of the outer membrane permeability alteration in LT mutants, we hypothesize that LT inactivation causes the membrane defect. To further support this hypothesis, the susceptibility of LT mutants to sodium dodecyl sulfate (SDS) and bile salts was assessed. The logarithmic-phase wild-type KJ and LT mutant cells were treated with 0.01% SDS or left untreated. CFU was determined after 10 min of incubation without shaking. The percentage of survival was defined as the CFU ratio of the SDS-additive group to the SDS-free counterpart. The SDS susceptibility increased in the ΔmltB1, ΔmltB2, ΔmltD1, and ΔmltD2 mutants compared to that in wild-type KJ (Fig. 1B). For the bile salts susceptibility assay, the logarithmic-phase bacterial cells of 5 × 105 CFU/μl were 10-fold serially diluted. Then, 2 μl of bacterial cells was spotted onto the bile salts-containing MacConkey agars. The growth of bacterial cells was observed after 18 h of incubation at 37°C. The susceptibility of ΔmltB1, ΔmltD1, and ΔmltD2 mutants to bile salts obviously increased (Fig. 1C). Taken together, these results further support that LT inactivations cause a membrane defect.
Since individual LT mutants display increased membrane susceptibility to NPN, SDS, and bile salts (Fig. 1), it would be interesting to determine whether inactivation of more than one LT simultaneously would show an additive change in the membrane permeability. The ΔmltD1 ΔmltB1, ΔmltD1 ΔmltD2, and ΔmltD1 ΔmltB1 ΔmltD2 mutants were thus assessed. Simultaneous inactivation of multiple LTs did not further increase the membrane susceptibility to aminoglycoside, macrolides, NPN, SDS, and bile salts (Table 1, Fig. 1).
Cavallari et al. have demonstrated that LT mutations in Pseudomonas aeruginosa do not affect membrane permeability and that these LT mutants exhibit no changes in susceptibility to tobramycin (2). In contrast to the observations in P. aeruginosa, LT inactivation in S. maltophilia increases the outer membrane permeability for self-promoted uptake compounds and high-molecular-weight substances, thus resulting in increased susceptibility to AGs and macrolides. The alteration in the outer membrane integrity of LT mutants can be an envelope stress to S. maltophilia. This may be the reason why smeJ transcripts of the ΔmltD1 and Δslt mutants are increased (see Fig. S1 in the supplemental material), since smeIJK is a member of σE-mediated envelope stress response regulon in S. maltophilia (12). To our knowledge, this is the first report linking LT inactivation to a decrease in AG and macrolide resistance.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant MOST 104-2320-B-010-023-MY3 from Ministry of Science and Technology of Taiwan and grant 30219003 from the Professor Tsuei-Chu Mong Merit Scholarship.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03026-15.
REFERENCES
- 1.Koraimann G. 2003. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell Mol Life Sci 60:2371–2388. doi: 10.1007/s00018-003-3056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavallari JF, Lamers RP, Scheurwater EM, Matos AL, Burrows LL. 2013. Changes to its peptidoglycan-remodeling enzyme repertoire modulate β-lactam resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:3078–3084. doi: 10.1128/AAC.00268-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang YT, Lin CY, Chen YH, Hsueh PR. 2015. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 6:893. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N, Adlem E, Kerhornou A, Lord A, Murphy L, Seeger K, Squares R, Rutter S, Quail MA, Rajandream MA, Harris D, Churcher C, Bentley SD, Parkhill J, Thomson NR, Avison MB. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YW, Wu CJ, Hu RM, Lin YT, Yang TC. 2015. Interplay among membrane-bound lytic transglycosylases D1, the CreBC two-component regulatory system, the AmpNG-AmpDI-NagZ-AmpR regulatory circuit, and L1/L2 β-lactamase expression in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 59:6866–6872. doi: 10.1128/AAC.05179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing, 20th informational supplement. M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Poole K. 2005. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:479–487. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of resistance elements and their clinical implications. Clin Infect Dis 34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 9.Lambert T, Ploy M-C, Denis F, Courvalin P. 1999. Characterization of the chromosomal aac(6′)-Iz gene of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 43:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okazaki A, Avison MB. 2007. Aph(3′)-IIc, an aminoglycoside resistance determinant from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 51:359–360. doi: 10.1128/AAC.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Martinez JL. 2000. Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 44:3079–3086. doi: 10.1128/AAC.44.11.3079-3086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YW, Liou RS, Lin YT, Huang HH, Yang TC. 2014. A linkage between SmeIJK efflux pump, cell envelope integrity, and σE-mediated envelope stress response in Stenotrophomonas maltophilia. PLoS One 9:e111784. doi: 10.1371/journal.pone.0111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CW, Huang YW, Hu RM, Yang TC. 2014. SmeOP-TolCSm efflux pump contributes to the multidrug resistance of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 58:2405–2408. doi: 10.1128/AAC.01974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CH, Huang CC, Chung TC, Hu RM, Huang YW, Yang TC. 2011. Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 55:5826–5833. doi: 10.1128/AAC.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YT, Huang YW, Chen SJ, Chang CW, Yang TC. 2015. The SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics and virulence in mice. Antimicrob Agents Chemother 59:4067–4073. doi: 10.1128/AAC.00372-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YT, Huang YW, Liou RS, Chang YC, Yang TC. 2014. MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J Antimicrob Chemother 69:3221–3226. doi: 10.1093/jac/dku317. [DOI] [PubMed] [Google Scholar]
- 17.Hancock RE, Farmer SW, Li ZS, Poole K. 1991. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother 35:1309–1314. doi: 10.1128/AAC.35.7.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro C, Fang X, Ahmad I, Gomelsky M, Romling U. 2011. Regulation of biofilm components in Salmonella enteric serovar Typhimurium by lytic transglycosylase involved in cell wall turnover. J Bacteriol 193:6443–6451. doi: 10.1128/JB.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh B, Grant C, Hancock RE. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551. doi: 10.1128/AAC.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.