Abstract
Voriconazole (VCZ) exhibits great inter- and intrapatient variability. The latter variation cannot exclusively be explained by concomitant medications, liver disease or dysfunction, and genetic polymorphisms in cytochrome P450 2C19 (CYP2C19). We hypothesized that inflammatory response in patients under VCZ medication might also influence this fluctuation in concentrations. In this study, we explored the association between inflammation, reflected by the C-reactive protein (CRP) concentration, and VCZ trough concentrations over time. A retrospective analysis of data was performed for patients with more than one steady-state VCZ trough concentration and a CRP concentration measured on the same day. A longitudinal analysis was used for series of observations obtained from many study participants over time. The approach involved inclusion of random effects and autocorrelation in linear models to reflect within-person cross-time correlation. A total of 50 patients were eligible for the study, resulting in 139 observations (paired VCZ and CRP concentrations) for the analysis, ranging from 2 to 6 observations per study participant. Inflammation, marked by the CRP concentration, had a significant association with VCZ trough concentrations (P < 0.001). Covariates such as age and interacting comedication ([es]omeprazole), also showed a significant correlation between VCZ and CRP concentrations (P < 0.05). The intrapatient variation of trough concentrations of VCZ was 1.401 (confidence interval [CI], 0.881 to 2.567), and the interpatient variation was 1.756 (CI, 0.934 to 4.440). The autocorrelation between VCZ trough concentrations at two sequential time points was calculated at 0.71 (CI, 0.51 to 0.92). The inflammatory response appears to play a significant role in the largely unpredictable pharmacokinetics of VCZ, especially in patients with high inflammatory response, as reflected by high CRP concentrations.
INTRODUCTION
Voriconazole (VCZ) is the first-line treatment for invasive aspergillosis (1–3). It undergoes extensive hepatic metabolism, principally by cytochrome P450 2C19 (CYP2C19) and to a lesser extent by CYP3A4 and CYP2C9 (4, 5). It is catalyzed into inactive metabolites, with VCZ N-oxide being the principal circulating metabolite (6). The trough concentration of VCZ under steady-state conditions in relation to the MIC is used for practical reasons as the predictive pharmacokinetic/pharmacodynamic parameter (7).
VCZ exhibits nonlinear pharmacokinetics in adults with a large inter- and intrapatient variability of drug exposure. These variations might be caused by many factors, including the use of concomitant medications, liver disease or liver dysfunction (1, 3, 5, 8), and, especially, genetic polymorphisms of CYP2C19 (8). However, none of these factors provides a complete explanation of the variability in VCZ concentrations observed in previous studies (9, 10).
Data are now emerging that identify a decrease in the capacity of metabolic pathways to handle drugs during infections and diseases that involve an inflammatory response (11–14). We were the first to associate inflammation, reflected by the C-reactive protein (CRP) concentration, with the VCZ trough concentration (15), as well as finding an association between the CRP concentration and the VCZ N-oxide/VCZ ratio (16), identifying a possible explanation for some of the variability in VCZ concentrations.
Previously, the relationship between CRP and the VCZ trough concentration was evaluated at a single point in time (15). However, the inflammatory response represents a coordinated set of physiological events, while the metabolic and inflammatory status of each patient is different and may vary over time during VCZ treatment. Therefore, to detect changes in VCZ trough concentrations of the patients at both the group and the individual levels, the studies should be extended beyond a single moment in time in order to explore all sequences of events.
To further explore the intrapatient variability, our aim was to evaluate the effect of inflammation on VCZ trough concentrations over time within patients.
MATERIALS AND METHODS
Patient selection.
This retrospective chart review study was performed in the University Medical Center Groningen, Groningen, the Netherlands. For patients who received VCZ treatment, laboratory and diagnostic test reports were retrospectively analyzed. All patients aged ≥18 years were considered eligible for the study if they had at least one set of more than one steady-state VCZ trough concentration and a CRP concentration measured on the same day. The steady-state concentration of VCZ was defined as the state after 6 doses when 2 loading doses were administered (17), after 10 doses when less than 2 loading doses were administered (18), and after 6 doses when the dosage had been adjusted (19). VCZ concentrations were measured by a validated liquid chromatography coupled with tandem mass spectrometry (LC–MS-MS) assay (20, 21). CRP concentrations were measured by a turbidimetric assay (Roche Modular; Roche, Mannheim, Germany).
In accordance with Dutch law (Medical Research Involving Human Subjects Act) and because of the retrospective nature of this study with anonymous data, a waiver was obtained from the local ethical committee (IRB 2013-491).
Data collection.
For each eligible patient, all data were collected from the medical chart, including demographic data, medical history, and laboratory parameters, i.e., CRP concentrations, alkaline phosphatase, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), γ-glutamyl transferase (γGT), and total bilirubin. VCZ dosing information, frequency, route of administration, treatment duration, comedication, and dose adjustments were also extracted from the hospital files.
Data analyses. (i) Descriptive analysis.
A descriptive analysis of the patients' characteristics was conducted with frequencies and percentages for discrete variables, as well as medians with interquartile ranges for quantitative (continuous) variables.
(ii) Longitudinal analysis.
A linear mixed model was used to analyze the repeated VCZ trough concentrations in relation to CRP concentrations. Each patient may have a typical or unique average level, or pattern, of VCZ trough concentrations, and this was addressed by including a random effect for patients. “Time after first dose” entered the model as a continuous variable to obtain an average time profile for the patients. To address the correlations among repeated VCZ trough concentrations within a patient over time, a first-order autoregressive correlation structure was selected. This structure assumes that the correlation between observations closer together in time is higher than that between observations further apart. To investigate an association of inflammation, reflected by CRP concentrations, with VCZ trough concentrations over time, the Wald type III test was conducted after correcting for the covariates: gender, age, VCZ dose and route of administration, alkaline phosphatase, ALAT, ASAT, γGT, total bilirubin, and the use of interacting comedication. The analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Characteristics of the patient population.
A total of 50 patients were eligible for inclusion during the period January 2006 to December 2010. Hematologic malignancy was the most common underlying condition (22/50; 44%). Demographic information, underlying disease, VCZ dosage, laboratory parameters, and comedication are described in Table 1. All solid-organ transplant recipients were on a combination of tacrolimus and prednisolone as immunosuppressants. Half of the patients (11/22; 50%) with a hematological disease received prednisolone, and some received additional treatment with cyclosporine (6/11; 55%). Most of the solid-organ transplant recipients (16/17; 94%) and about 40% (9/22) of the patients with a hematological disease received (es)omeprazole.
TABLE 1.
Patient characteristics
| Characteristic | Valuea |
|---|---|
| Demographics (n = 50) | |
| Female | 20 (40) |
| Age (yr) | 57 (44–63) |
| Weight (kg) | 78 (64–87) |
| Height (m) | 1.76 (1.69–1.84) |
| BMIb (kg/m2) | 24.3 (21.1–26.6) |
| Underlying disease | |
| Hematologic malignancy | 22 (44) |
| Leukemia | 9 (18) |
| Lymphoma | 5 (10) |
| Other | 8 (16) |
| Solid-organ transplant | 17 (34) |
| Liver | 7 (14) |
| Lung | 10 (20) |
| Otherc | 11 (22) |
| Voriconazole treatment | |
| Dose (mg/kg of body wt) | 3.5 (2.5–4.2) |
| Intravenous administration | 18 (36) |
| Laboratory parameters | |
| Voriconazole concn (mg/liter) | 2.8 (1.8–3.7) |
| CRP (mg/liter) | 67 (14–153) |
| Alkaline phosphatase (U/liter) | 114 (71–183) |
| ALAT (U/liter) | 30 (18–61) |
| ASAT (U/liter) | 33 (20–48) |
| γ-Glutamyl transferase (U/liter) | 119 (49–240) |
| Total bilirubin (μmol/liter) | 8 (6–14) |
Data are presented as n (%) or median (interquartile range).
BMI, body mass index.
Other, patients diagnosed with various disorders, including chronic pulmonary obstructive disease, cystic fibrosis, and granulomatosis and polyangiitis.
In total, 139 observations were available for analysis, ranging from 2 to 6 observations per patient. VCZ was administered orally to 64% of the patients (32/50); as VCZ treatment is routinely switched from intravenous to oral administration when possible, this high percentage was expected. The mean concentration of CRP was 67 mg/liter (range, 14 to 153 mg/liter), and that of VCZ was 2.8 mg/liter (range, 1.8 to 3.7 mg/liter). The median values of the laboratory parameters were inside the normal range, except for CRP and γGT values that were above the upper limit of normal. Only (es)omeprazole was used simultaneously with VCZ as a concomitant medication that potentially might influence VCZ concentrations.
Longitudinal analysis.
The intrapatient variation of trough concentrations of VCZ was calculated at 1.401 (confidence interval [CI], 0.881 to 2.567), and the interpatient variation was calculated at 1.756 (CI, 0.934 to 4.440). The correlation between VCZ trough concentrations between two sequential time points was calculated at 0.71 (CI, 0.51 to 0.92). The VCZ trough concentrations have a significant association with CRP: 0.014 (0.011 to 0.018; P < 0.001), as well as with age (P = 0.013) and with the use of (es)omeprazole (P = 0.036). For none of the other variables could an association be determined at a significance level where α was equal to 0.05.
DISCUSSION
Inflammation, reflected by the CRP concentration, is associated with VCZ trough concentrations. Apparently, inflammation partly explains the variability observed between patients and within patients over time. A similar association between CRP and the VCZ trough concentration was found in a previous cross-sectional analysis (15). These findings suggest that an inflammatory state affects the pharmacokinetics/trough concentration of VCZ; however, the clinical impact of inflammation on VCZ trough concentrations has not yet been fully clarified.
In this study, we implemented a linear mixed model to analyze repeated observations over time. This method is particularly useful to evaluate the changes in the inflammatory response while observing the same individuals over the study period, which enables us to expand on our previous findings.
Researchers have claimed that the observed variability of VCZ in blood may be caused by multiple factors, such as changes in absorption, patient protein status, liver function, and disease (9, 10). Thus, we have suggested that inflammation is one of the other factors that might influence the variability of the VCZ concentration. Unfortunately, there is meager clinical information on the effects of inflammation and infection on CYP isoenzymes to aid in understanding how drug metabolism is regulated during an inflammatory stimulus (22, 23). Therefore, we retrospectively analyzed data on our patients at more than one time point, especially since inflammation is a complex biochemical and cellular process in which host cells, blood vessels, proteins, and other mediators are involved and interact in a complex fashion. Moreover, the inflammatory response differs among individuals, and it may also fluctuate over time during VCZ treatment within a particular patient. Unfortunately, the sample size was not sufficient to perform a subgroup analysis.
In previous studies, we have observed that an inflammatory state raised the concentration of VCZ in terms of its metabolic reduction (16). It has been determined that for every 1-mg/liter increase in the CRP concentration, the VCZ trough concentration was 0.015 mg/liter higher (15), and as the longitudinal estimate (0.014) is very close to this value, this suggests that it is stable over time.
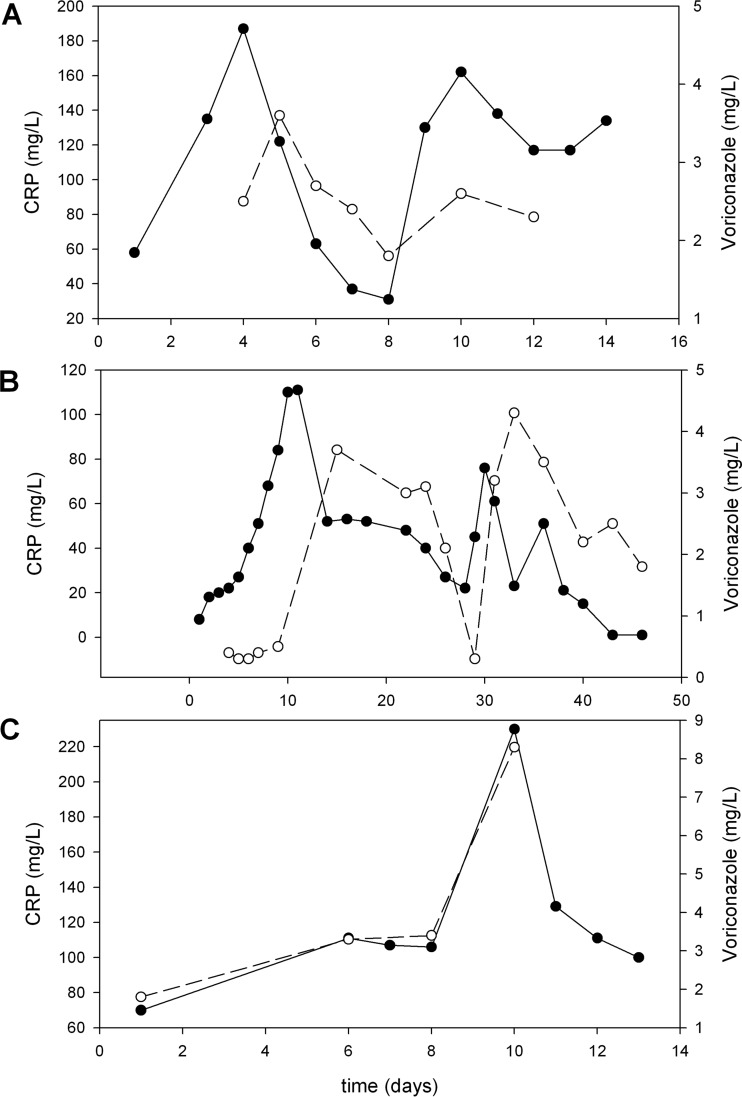
Additionally, the metabolic ratio, defined as VCZ N-oxide/VCZ, decreased by 0.010 for every unit increase in the CRP concentration (16). We therefore hypothesized that inflammation induces downregulation of CYP2C19 in patients treated with VCZ (15, 16), suggesting that elevated amounts of inflammatory cytokines influence the CYP isoenzymes and thus change the rate of metabolism of VCZ, resulting in increased drug concentrations (15, 16). For example, an increase in the CRP concentration from 11 mg/liter to 196 mg/liter is likely to result in an increase in the VCZ concentration from 2.7 mg/liter to approximately 5.3 mg/liter. If the dose of VCZ is subsequently reduced to attain a concentration of 2.5 mg/liter, the concentration will likely drop to subtherapeutic levels if the infection has subsided. To show the typical pattern, we provide a CRP and VCZ concentration-time graph (Fig. 1).
FIG 1.
Typical graphs of voriconazole-CRP concentrations over time. The time courses of both voriconazole and CRP are shown. Clearly, the voriconazole concentration-time curve mirrors the CRP concentration-time curve. We selected three patients representative of the cohort: a 44-year-old male patient after lung transplantation receiving voriconazole as empirical treatment under intensive care (A), a 53-year-old male liver transplant recipient receiving voriconazole after a bronchoalveolar lavage specimen was positive for Aspergillus (B), and a 44-year-old male patient after allogeneic hematopoietic stem cell transplantation suffering from neutropenic fever and receiving piperacillin-tazobactam and voriconazole (C). Solid circles, CRP values; open circles, voriconazole concentrations.
Morgan and collaborators (22, 24) have provided an explanation for this phenomenon. Inflammation stimulates the release of cytokines, resulting in the modulation of transcription factor activities in the liver. These changes ultimately lead to downregulation of most CYP genes, affecting the production of the metabolizing proteins and consequently reducing the clearance of certain drugs (22). In vitro studies have provided compelling evidence that proinflammatory cytokines, particularly interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α), downregulate the biosynthesis of some hepatic CYP isoenzymes, such as CYP2C9, CYP2C19, and CYP3A4, that are involved in VCZ metabolism (22–24). Nevertheless, more data are needed for a better understanding of this molecular mechanism. Clinical studies on transcriptional regulation under inflammatory conditions may yield further information, i.e., transcriptional control through transcription factors may provide cellular signaling pathways by which inflammatory mediators suppress expression of the enzymes.
Recently, it has been hypothesized that inflammation induces phenoconversion of polymorphic drug-metabolizing enzymes (DME) (25). Phenoconversion is an event whereby a genotypic extensive metabolizer of a DME is converted into a transient phenotypic poor metabolizer of that DME. This hypothesis has been based on direct clinical evidence of genotype-phenotype mismatch and on nonclinical evidence of downregulation of mRNA and the corresponding DME activity, as well as unexplained changes in concentrations of a drug within a patient (25). In particular, phenoconversion of CYP2C19 has been documented under inflammatory conditions associated with elevated cytokines (25). However, clinical evidence was not always consistent (25).
Furthermore, it has been suggested that long-term exposure to VCZ causes autoinduction of cytochrome P450 isoenzymes, resulting in decreasing VCZ concentrations (26). Although autoinduction has not been observed in studies of pharmacokinetics with healthy volunteers (27), there has been some, although scarce, clinical evidence of the phenomenon reported in single cases of children and adults receiving higher than usual doses of VCZ and/or after prolonged courses (longer than 2 months) (28). More research is clearly needed to investigate further the occurrence of autoinduction in patients. In our study, autoinduction was not considered to play a role as a source of variability. Conceptually, the observations that are explained by autoinduction could very well be the result of reduced inflammation as a response to the treatment. Therefore, the activities of CYP isoenzymes would become normal, resulting in lower concentrations of VCZ. It is clear that more studies are necessary to better characterize the profiles of the DMEs involved in VCZ metabolism.
Here, we must add that the present study has some limitations, although sufficient information regarding the demographic characteristics and the clinical features of the population studied were available. First, its retrospective nature could have led to a possible selection bias. Nevertheless, no impact on the results due to possible selection bias was expected because the measurement of VCZ has been processed routinely in most patients and not only when efficacy or safety issues arose. Second, in this study no evaluation of genetic variations for the hepatic CYP isoenzymes was available. As genetic variants of CYP2C19 alter the concentration of VCZ (29, 30), further studies including CYP2C19 genotypes would contribute to clarifying our results. Nonetheless, it is unlikely that CYP2C19 polymorphisms completely explain the variability of VCZ pharmacokinetics due to a low incidence among Caucasians, who made up the vast majority of our study population (31). Third, CRP concentrations in patients receiving VCZ may not completely reflect the degree of inflammation. Although the onset and offset of CRP are not specific for an acute inflammatory response (32), CRP measurement has some favorable properties and valuable clinical uses (i.e., as a marker of inflammation or as a participant in the immune system and cardiovascular diseases) in critically ill patients (32). CRP has a relatively long half-life of 19 h, and it is principally regulated at the transcriptional level by the cytokine IL-6 and, to a lesser degree, by IL-1β and TNF-α (32, 33). Further studies that focus on IL-6 and proinflammatory mediators might shed more light on the molecular regulation of CYP isoenzyme genes. Finally, therapeutic drug monitoring of VCZ needs more standardization to control the timing of samples.
In conclusion, based on the results of our longitudinal analysis and as corroborated by our previous studies (15, 16), these findings may provide estimates of the inflammatory response as a source of variability of VCZ concentrations. The inflammatory response plays a role in the largely unpredictable pharmacokinetics of VCZ, especially in patients with a higher and/or variable inflammatory status. Patients are at risk for adverse events during high inflammatory response and for subtherapeutic concentrations when the inflammatory response subsides.
REFERENCES
- 1.Leveque D, Nivoix Y, Jehl F, Herbrecht R. 2006. Clinical pharmacokinetics of voriconazole. Int J Antimicrob Agents 27:274–284. doi: 10.1016/j.ijantimicag.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, Burger DM. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 3.Chu HY, Jain R, Xie H, Pottinger P, Fredricks DN. 2013. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis 13:105. doi: 10.1186/1471-2334-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamics profile of voriconazole. Clin Pharmacokinet 45:649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 5.Eiden C, Cociglio M, Hillaire-Buys D, Eymard-Duvernay S, Ceballos P, Fegueux N, Peyriere H. 2010. Pharmacokinetic variability of voriconazole and N-oxide voriconazole measured as therapeutic drug monitoring. Xenobiotica 40:701–706. doi: 10.3109/00498254.2010.503814. [DOI] [PubMed] [Google Scholar]
- 6.Hyland R, Jones B, Smith D. 2003. Identification of the cytochrome p450 enzymes involved in the n-oxidation of voriconazole. Drug Metab Dispos 31:540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 7.Karthaus M, Lehrnbecher T, Lipp H, Kluge S, Buchheidt D. 2015. Therapeutic drug monitoring in the treatment of invasive aspergillosis with voriconazole in cancer patients: an evidence-based approach. Ann Hematol 94:547–556. doi: 10.1007/s00277-015-2333-z. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J, ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. 2009. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol 49:196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 9.Trifilio SM, Yarnold PR, Scheetz MH, Pi J, Pennick G, Mehta J. 2009. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob Agents Chemother 53:1793–1796. doi: 10.1128/AAC.01316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brüggemann RJ, Blijlevens NM, Burger DM, Franke B, Troke PF, Donnelly JP. 2010. Pharmacokinetics and safety of 14 days intravenous voriconazole in allogeneic haematopoietic stem cell transplant recipients. J Antimicrob Chemother 65:107–113. doi: 10.1093/jac/dkp416. [DOI] [PubMed] [Google Scholar]
- 11.Renton KW. 2001. Alteration of drug biotransformation and elimination during infection and inflammation. Pharmacol Ther 92:147–163. doi: 10.1016/S0163-7258(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 12.Renton KW. 2005. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol 1:629–640. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
- 13.Slaviero KA, Clarke SJ, Rivory LP. 2003. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol 4:224–232. doi: 10.1016/S1470-2045(03)01034-9. [DOI] [PubMed] [Google Scholar]
- 14.Kulmatycki KM, Jamali F. 2005. Drug disease interactions: role of inflammatory mediators in disease and variability in drug response. J Pharm Pharm Sci 8:602–625. [PubMed] [Google Scholar]
- 15.van Wanrooy MJP, Span LFR, Rodgers MGG, van den Heuvel ER, Uges DRA, van der Werf TS, Kosterink JGW, Alffenaar JWC. 2014. Inflammation is associated with voriconazole trough concentrations. Antimicrob Agents Chemother 58:7098–7101. doi: 10.1128/AAC.03820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Encalada Ventura MA, Span LFR, van den Heuvel ER, Groothuis GMM, Alffenaar JWC. 2015. Influence of inflammation on voriconazole metabolism. Antimicrob Agents Chemother 59:2942–2943. doi: 10.1128/AAC.04789-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purkins L, Wood N, Greenhalgh K, Eve MD, Oliver SD, Nichols D. 2003. The pharmacokinetics and safety of intravenous voriconazole—a novel wide-spectrum antifungal agent. Br J Clin Pharmacol 56(Suppl 1):S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purkins L, Wood N, Greenhalgh K, Allen MJ, Oliver SD. 2003. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br J Clin Pharmacol 56(Suppl 1):S10–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother 46:2546–2553. doi: 10.1128/AAC.46.8.2546-2553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alffenaar JWC, Wessels AMA, van Hateren K, Greijdanus B, Kosterink JGW, Uges DRA. 2010. Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 878:39–44. doi: 10.1016/j.jchromb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Lempers VJ, Alffenaar JW, Touw DJ, Burger DM, Uges DR, Aarnoutse RE, Brüggemann RJ. 2014. Five year results of an international proficiency testing programme for measurement of antifungal drug concentrations. J Antimicrob Chemother 69:2988–2994. doi: 10.1093/jac/dku242. [DOI] [PubMed] [Google Scholar]
- 22.Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, Richardson TA, Sharma R, Sinal CJ. 2008. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36:205–216. doi: 10.1124/dmd.107.018747. [DOI] [PubMed] [Google Scholar]
- 23.Aitken AE, Richardson TA, Morgan ET. 2006. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 24.Morgan ET. 2001. Regulation of cytochrome P450 by inflammatory mediators: why and how? Drug Metab Dispos 29:207–212. [PubMed] [Google Scholar]
- 25.Shah RR, Smith RL. 2015. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos 43:400–410. doi: 10.1124/dmd.114.061093. [DOI] [PubMed] [Google Scholar]
- 26.Moriyama B, Elinoff J, Danner RL, Gea-Banacloche J, Pennick G, Rinaldi MG, Walsh TJ. 2009. Accelerated metabolism of voriconazole and its partial reversal by cimetidine. Antimicrob Agents Chemother 53:1712–1714. doi: 10.1128/AAC.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 31:731–741. doi: 10.1124/dmd.31.6.731. [DOI] [PubMed] [Google Scholar]
- 28.Hsu AJ, Dabb A, Arav-Boger R. 2015. Autoinduction of voriconazole metabolism in a child with invasive pulmonary aspergillosis. Pharmacotherapy 35:e20–e26. doi: 10.1002/phar.1566. [DOI] [PubMed] [Google Scholar]
- 29.Scholz I, Oberwittler H, Riedel K, Burhenne J, Weiss J, Haefeli WE, Mikus G. 2009. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol 68:906–915. doi: 10.1111/j.1365-2125.2009.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. 2010. Voriconazole therapeutic drug monitoring: focus on safety. Expert Opin Drug Saf 9:125–137. doi: 10.1517/14740330903485637. [DOI] [PubMed] [Google Scholar]
- 31.Desta Z, Zhao X, Shin JG, Flockhart DA. 2002. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ho KM, Lipman J. 2009. An update on C-reactive protein for intensivists. Anaesth Intensive Care 37:234–241. [DOI] [PubMed] [Google Scholar]
- 33.Black S, Kushner I, Samols D. 2004. C-reactive protein. J Biol Chem 279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]