Abstract
Aminoglycoside treatment of carbapenem-resistant (CR) Klebsiella pneumoniae bacteremia was associated with a 70% rate (23/33) of 30-day survival. Successful treatment was associated with sources of bacteremia amenable to reliable aminoglycoside pharmacokinetics (P = 0.037), acute physiology and chronic health evaluation II (APACHE II) scores of <20 (P = 0.16), and nonfatal underlying diseases (P = 0.015). Success rates were 78% and 100% if ≥2 and all 3 factors were present, respectively. Clinicians may consider the use of aminoglycosides against CR K. pneumoniae bacteremia if strains are susceptible and the sources of infection are amenable to reliable pharmacokinetics.
TEXT
Treatment options for carbapenem-resistant (CR) Klebsiella pneumoniae infections are limited. Aminoglycosides are active against ≥50% of CR K. pneumoniae isolates in vitro (1–3) and exhibit rapid bactericidal activity against susceptible strains during time-kill assays (2). Aminoglycosides have been shown to be more effective than polymyxin B or tigecycline in eradicating CR K. pneumoniae bacteriuria (4). Treatment with a regimen that included gentamicin was associated with reduced mortality among patients with sepsis, due to an outbreak strain of colistin-resistant, CR K. pneumoniae (5). The effectiveness of aminoglycosides against CR K. pneumoniae bacteremia in a nonoutbreak setting is unknown. Prior to the 1980s, aminoglycosides were reported to successfully treat ∼70% of Gram-negative bacteremia cases (6). More recently, the availability of well-tolerated, broad-spectrum β-lactam antibiotics has relegated aminoglycosides to second-line status. Aminoglycoside therapy is limited by nephrotoxicity, a need for therapeutic drug monitoring, and poor penetration into abdominal and pulmonary sites of infection (7–10). The objective of this study was to review our clinical experience with aminoglycosides as primary therapy for CR K. pneumoniae bacteremia.
We conducted a retrospective study of patients at our center with CR K. pneumoniae bacteremia between February 2010 and September 2014. CR K. pneumoniae was defined by nonsusceptibility to a carbapenem and all third-generation cephalosporins (11). Patients with bacteremia who were initially treated with an aminoglycoside for ≥3 days were included. For patients with normal renal function, standard extended-interval aminoglycoside doses were recommended and adjusted according to the Hartford nomogram (7). Among patients with renal impairment, adjustments were made according to current recommendations (12). Bacteremia was classified as primary or secondary by the independent review of two investigators (13). Sources of bacteremia were considered amenable or unamenable to the achievement of reliable aminoglycoside pharmacokinetics at the site. Amenable sites were primary bacteremia, urine, and soft tissues (14, 15). Unamenable sites included the abdominal cavity, respiratory tract, and bone (8–10). Underlying diseases were classified as fatal or nonfatal, according to the criteria of McCabe and Jackson (16). Clinical success was defined as survival at 30 days following the onset of CR K. pneumoniae bacteremia, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation, completion of planned antimicrobial therapy, and the absence of recurrent CR K. pneumoniae infections within 30 days. MICs were determined using reference Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods (17). Strains were tested for multilocus sequence type (ST) and K. pneumoniae carbapenemase (KPC) variants, as described previously (18). Comparisons between groups were made by Fisher's exact test for categorical variables and Mann-Whitney U test for continuous variables. Significance was defined as a P value of ≤0.05 (two-tailed).
Thirty-six consecutive patients with CR K. pneumoniae bacteremia were evaluated; 3 patients died after 1 day of aminoglycoside therapy and were excluded. The data for the remaining 33 patients are summarized in Table 1. The median acute physiology and chronic health evaluation II (APACHE II) score was 17 (range, 3 to 35). Primary bacteremia was diagnosed in 39% (13/33) of patients; secondary bacteremia resulted from sites in the abdomen (42% [14/33]), respiratory tract (6% [2/33]), urinary tract (6% [2/33]), soft tissue (3% [1/33]), and bone (3% [1/33]). Forty-eight percent (16/33) of infections were amenable to reliable aminoglycoside pharmacokinetics. Thirty-one CR K. pneumoniae isolates were available for strain typing. Ninety percent (28/31) were sequence type 258 (ST-258); 81% (25/31) and 13% (4/31) expressed KPC-2 and KPC-3 carbapenemases, respectively.
TABLE 1.
Patient demographics, clinical characteristics, and outcomes of CR K. pneumoniae bloodstream infections
| Patient | Age (yr) (sex)a | Underlying disease(s)b | McCabe and Jackson scorec | APACHE II scored | Time to BSI (days)e | Type of bacteremia | Source control (day)f | Duration of BSI (days)g | Time to initiation of therapy (h)h | Antimicrobial regimen (days of therapy) | MIC (μg/ml) fori: |
Patient outcomej | Time to death in days | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycoside | Carbapenem | Other | |||||||||||||
| 22 | 58 (F) | Lung transplant | 3 | 13 | 17 | Primary | Catheter removal (2) | 1 | 87 | Gentamicin (9) | 1 | NA | NA | Clinical success | Alive |
| 29 | 28 (M) | Pancreatitis, DM | 3 | 3 | 25 | Primary | Catheter removal (1) | 1 | 110 | Gentamicin (14) | 2 | NA | NA | Clinical success | Alive |
| 103 | 64 (M) | Parkinson's disease, cholangitis | 3 | 19 | 16 | Secondary abdominal | IR-guided drainage of pancreatic abscess (2) | 1 | 65 | Gentamicin (13) | 0.5 | NA | NA | Clinical success | Alive |
| 140 | 45 (F) | Multivisceral transplant | 3 | 17 | 0 | Primary | Port and catheter removal (4) | 7 | 114 | Gentamicin + doripenem (21) | 1 | 8 | NA | Clinical success | Alive |
| 88 | 85 (F) | Pancreatitis | 3 | 8 | 4 | Secondary abdominal | None | 1 | 52 | Gentamicin + ertapenem (14) | 0.5 | 8 | NA | Clinical success | Alive |
| 116 | 56 (F) | Liver transplant | 3 | 20 | 1 | Secondary abdominal | Biliary stent removal (11) | 1 | 64 | Gentamicin + doripenem (14) | 1 | 32 | NA | Clinical success | Alive |
| 117 | 55 (M) | Liver transplant | 3 | 10 | 1 | Secondary abdominal | None | 1 | 67 | Gentamicin + doripenem (10) | 0.5 | 8 | NA | Clinical success | Alive |
| 79 | 53 (M) | ESRD | 2 | 14 | 25 | Secondary wound | Bedside debridement | 1 | 22 | Gentamicin + doripenem (14) | 0.5 | 8 | NA | Clinical success | Alive |
| 138 | 63 (M) | AML, febrile neutropenia | 3 | 14 | 48 | Primary | Catheter removal (4) | 2 | 36 | Gentamicin + ciprofloxacin + meropenem (14) | 0.5 | 2 | Cipro, 0.06 | Clinical success | Alive |
| 144 | 33 (M) | Paraplegia | 3 | 9 | 8 | Primary | Catheter removal (2 and 7) | 6 | 63 | Gentamicin (3) + meropenem-RPX7009 (8) | 0.5 | 8 | NA | Clinical success | Alive |
| 142 | 70 (M) | DM s/p cardiac arrest | 2 | 29 | 1 | Secondary respiratory tract | None | 1 | 75 | Gentamicin (7) + inhaled gent (7) meropenm (14) | 0.5 | 2 | NA | Clinical success | Alive |
| 119 | 57 (M) | Kidney transplant | 3 | 15 | 48 | Secondary urinary tract | Foley catheter removed (1) | 5 | 31 | Gentamicin + doripenem (18) | 1 | 4 | NA | Clinical success | Alive |
| 120 | 58 (M) | ESLD, CCA | 2 | 16 | 3 | Secondary abdominal | PTC catheter exchange (6) | 2 | 60 | Gentamicin + doripenem (15) | 1 | 256 | NA | Clinical success | 33 (due to underlying disease) |
| 69 | 69 (M) | HIV, Burkitt's lymphoma | 2 | 26 | 12 | Primary | None | 1 | 59 | Gentamicin (10) + colistin (8) + meropenem (12) | 0.5 | 256 | Colistin, 0.25 | Clinical success | 81 (due to Pseudomonas BSI) |
| 66 | 74 (M) | CHF, DM | 2 | 12 | 7 | Primary | Catheter removal (3) | 2 | 94 | Gentamicin (6) | 1 | NA | NA | Clinical success | 84 (due to underlying disease) |
| 49 | 65 (M) | Liver transplant and ESRD | 2 | 19 | 5 | Primary | Catheter removal (2) | 5 | 86 | Gentamicin (14) | 2 | NA | NA | Clinical success | 73 (due to underlying disease) |
| 30 | 30 (M) | Lung transplant | 3 | 21 | 72 | Primary | Catheter removal (4) | 3 | 95 | Gentamicin (7) | 0.25 | NA | NA | Clinical success | 564 (due to underlying disease) |
| 121 | 32 (M) | Multivisceral transplant | 3 | 11 | 91 | Primary | Catheter exchange over wire (1), and then removed (3) | 3 | 55 | Gentamicin + doripenem (15) | 0.5 | 256 | NA | Clinical success | 126 (due to relapse CR-K. pneumoniae BSI) |
| 61 | 84 (F) | ESRD, chronic respiratory failure, CAD | 2 | 35 | 1 | Primary | Catheter removed (1) | 1 | 4 | Gentamicin + meropenem (5) | 1 | 8 | NA | Failure due to death | 6 |
| 999 | 64 (M) | Liver transplant | 3 | 15 | 3 | Secondary abdominal | Biliary balloon dilation (2) | 7 | 72 | Gentamicin + doripenem (4) | 4 | 8 | NA | Failure due to death | 7 |
| 127 | 54 (M) | Liver transplant and AML | 1 | 17 | 8 | Secondary urinary tract | None | 3 | 70 | Gentamicin + colistin + meropenem (5) | 0.5 | 4 | Colistin, 16 | Failure due to death | 8 |
| 89 | 67 (F) | Rheumatoid arthritis | 1 | 16 | 10 | Secondary abdominal | None | 1 | 66 | Gentamicin (6) | 0.25 | NA | NA | Failure due to death | 9 |
| 37 | 85 (F) | Chronic respiratory failure and CAD | 2 | 20 | 2 | Primary | Catheter removal (1) | 1 | 26 | Gentamicin (5) | 1 | NA | NA | Failure due to death | 10 |
| 78 | 57 (M) | Alcoholic cirrhosis | 2 | 25 | 14 | Secondary respiratory tract | None | 1 | 55 | Gentamicin + colistin + doripenem (7) | 0.25 | 128 | Colistin, 4 | Failure due to death | 10 |
| 64 | 62 (F) | Pancreatic CA | 1 | 8 | 69 | Secondary abdominal | None | 1 | 97 | Gentamicin (6) | 1 | NA | NA | Failure due to death | 14 |
| 128 | 66 (M) | ESLD, ESRD | 2 | 26 | 19 | Secondary abdominal | Chronic cholecyctostomy tube | 1 | 28 | Gentamicin + doripenem (14) | 0.5 | 128 | NA | Failure due to death | 19 |
| 74 | 61 (F) | ESRD | 2 | 15 | 52 | Secondary wound/bone | Multiple bedside debridements and wound vacuum | 1 | 46 | Gentamicin + meropenem (14) | 0.5 | 128 | NA | Failure due to death | 22 |
| 131 | 73 (F) | Cholangiocarcinoma | 2 | 26 | 3 | Secondary abdominal | None | 4 | 83 | Amikacin + doripenem (23) | 16 | 2 | NA | Failure due to death | 30 |
| 114 | 56 (M) | Liver transplant | 3 | 25 | 1 | Secondary abdominal | Biliary stent removal (2), biliary drainage and PTC catheter placement (12) | 4 | 43 | Gentamicin + doripenem (18) | 1 | 16 | NA | Failure due to persistent IAI | 35 |
| 136 | 52 (M) | Pancreatic cancer | 2 | 13 | 4 | Primary | Port removed (2) | 1 | 33 | Gentamicin + meropenem (10) | 1 | 8 | NA | Failure due to recurrent BSI (day 20) | NAk |
| 39 | 65 (M) | Liver transplant | 2 | 18 | 1 | Secondary abdominal | Balloon dilation of choledocojejunostomy to remove stone (6) | 4 | 37 | Gentamicin (10) | 2 | NA | NA | Failure due to recurrent BSI (day 10) and new IAI (day 11) | 1,044 |
| 48 | 60 (M) | Liver transplant | 2 | 22 | 61 | Secondary abdominal | Abdominal washout (9) | 11 | 127 | Gentamicin + colistin + doripenem (8) | 0.5 | 64 | Colistin, 0.25 | Failure due to persistent BSI requiring change of therapy | Alive |
| 91 | 60 (M) | Pancreatitis | 3 | 18 | 7 | Secondary abdominal | Percutaneous drainage (3) | 1 | 23 | Gentamicin + doripenem (14) | 0.5 | 16 | NA | Failure due to persistent IAI requiring change of therapy | Alive |
F, female; M, male.
DM, diabetes mellitus; ESRD, end-stage renal disease; AML, acute myeloid leukemia; s/p, status-post; ESLD, end-stage liver disease; CCA, cholangiocarcinoma; CHF, congestive heart failure; CAD, coronary artery disease; CA, cancer.
As defined by the McCabe and Jackson classification of underlying diseases (16), where 1 = rapidly fatal, 2 = ultimately fatal, and 3 = nonfatal.
At the onset of bloodstream infection.
Time from hospital admission to positive blood culture. BSI, bloodstream infection (bacteremia).
IR, infrared; PTC, percutaneous transhepatic cholangiography.
Days of positive blood cultures.
Time from collection of blood culture to first dose of combination therapy.
NA, not available.
IAI, intra-abdominal infection.
Discharged to hospice, so date of death was not available.
Thirty percent (10/33) of patients received gentamicin monotherapy. Ninety-seven percent (32/33) of CR K. pneumoniae strains were susceptible to gentamicin, and all were susceptible to amikacin. All patients infected with a gentamicin-susceptible strain were treated with a regimen that included gentamicin; the patient infected with the gentamicin-nonsusceptible (MIC, 8 μg/ml) and amikacin-susceptible (MIC, 16 μg/ml) strain was treated with amikacin plus doripenem (Table 1, patient 131). Aminoglycoside therapy was initiated at a median of 63 h (range, 4 to 127 h) after the first positive blood culture was collected.
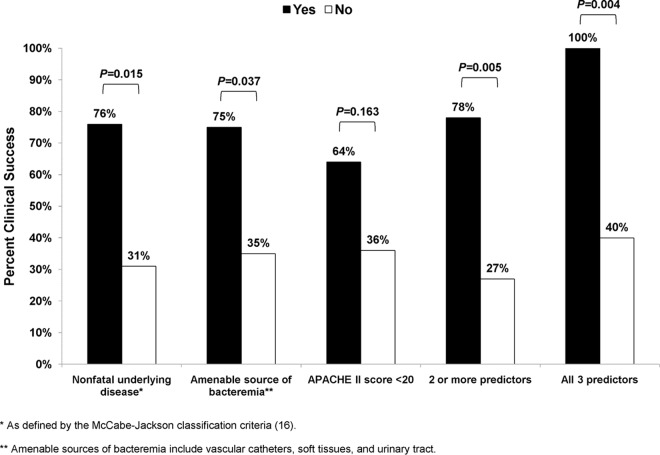
The 14- and 30-day survival rates were 78% (26/33) and 70% (23/33), respectively. Clinical success was achieved in 54% (18/33) of the patients. Clinical success was more likely for patients with primary rather than secondary bacteremia (77% [10/13] versus 40% [8/20], respectively; P = 0.07), amenable rather than unamenable sources of bacteremia (75% [12/16] versus 35% [6/17], respectively; P = 0.037), and APACHE II scores of <20 rather than ≥20 at the onset of bacteremia (64% [14/22] versus 36% [4/11], respectively; P = 0.16). Success rates were higher among patients with underlying diseases that were classified as nonfatal rather than fatal (76% [13/17] versus 31% [5/16], respectively; P = 0.015). Overall, the clinical success rate was 78% (14/18) for patients in whom ≥2 factors linked to favorable outcomes were present (amenable source, APACHE II score <20, and/or nonfatal underlying disease), compared to 27% (4/15) for other patients (P = 0.005). For patients in whom all 3 favorable factors were present, the clinical success rate was 100% (8/8) compared to 40% (10/25) for others (Fig. 1; P = 0.004). Other variables, including time to initiation of treatment and aminoglycoside combination therapy, were not associated with clinical responses. For combination therapy, success rates were comparable against infections caused by strains that exhibited carbapenem MICs of ≤8 μg/ml and >8 μg/ml (62% [8/13] versus 40% [4/10], respectively; P = 0.41). Outcomes did not differ by strain ST or KPC subtype.
FIG 1.
Factors associated with clinical success following aminoglycoside therapy for CR K. pneumoniae (CR-Kp) bacteremia.
Thirty-six percent (9/25) developed acute kidney injury (AKI) at some point during aminoglycoside therapy (defined as a 1.5-fold increase in serum creatinine level from baseline [19]), with one patient requiring renal replacement therapy. The median time to AKI was 10 days (range, 2 to 18 days). One patient receiving gentamicin monotherapy had a recurrent bloodstream infection (11 days from initial bacteremia) due to an aminoglycoside-resistant, CR K. pneumoniae strain; otherwise, the emergence of aminoglycoside resistance was not identified.
Taken together, our data demonstrate that aminoglycosides are effective in treatment against CR K. pneumoniae bacteremia, provided the causative strain is aminoglycoside susceptible and the infection originates from a site amenable to targeted aminoglycoside concentrations. In these settings, our clinical success rate of 75% is comparable to pooled response rates reported for patients with CR K. pneumoniae infections who received two or more in vitro active agents (20–22). This success rate is also similar to the 80% rate we previously reported at our center for patients with CR K. pneumoniae bacteremia treated with a carbapenem and colistin, if both agents were active (carbapenem MIC, ≤8 μg/ml) (23).
Our findings support and extend those of a recent study that showed a survival advantage among patients with colistin-resistant, CR K. pneumoniae sepsis who were treated with gentamicin-based regimens (5). The earlier study focused exclusively on sepsis caused by an outbreak-associated ST-512, KPC-3-producing strain over a 9-month period. In contrast, our data were collected over a 4-year period and were not outbreak associated. Moreover, most of our patients were infected with KPC-2- or KPC-3-producing CR K. pneumoniae strains of the predominant international clonal group ST-258. Therefore, the utility of aminoglycosides against CR K. pneumoniae infections is not limited by strain ST but rather by drug susceptibility and pharmacokinetic considerations.
In both studies, there were no differences in outcomes for patients who received aminoglycoside monotherapy or those who received combination regimens. Tigecycline was the agent used in combination in the earlier study, as opposed to a carbapenem in this study. The different regimens and small sample sizes preclude us from drawing definitive conclusions about the usefulness of aminoglycoside mono- or combination therapy. Nevertheless, our data indicate that aminoglycoside activity is a major driver of clinical outcomes, since the success rates for combination therapy were comparable for strains with carbapenem MICs of ≤8 μg/ml or >8 μg/ml.
It is notable that our previously reported success rate in treating CR K. pneumoniae bacteremia with carbapenem-colistin combination therapy was only 30% if colistin was the sole active agent (23). Indeed, the presence of either a major ompK36 mutation or a doripenem MIC of ≤8 μg/ml predicted a lack of CR K. pneumoniae responsiveness to doripenem and colistin in vitro (24). Polymyxins alone or in combination have been linked to suboptimal treatment responses among patients with CR K. pneumoniae infections in another study (25). The emergence of colistin resistance during treatment is a well-recognized limitation of this agent (26–28). In contrast, aminoglycosides are rapidly and durably bactericidal in vitro (1, 2), and the emergence of resistance was uncommon in our clinical experience. Aminoglycosides and colistin are each limited by nephrotoxicity, which we observed for a minority of patients in this study. Given these data, we generally recommend an aminoglycoside-containing rather than colistin-containing regimen at our center if the causative strain is susceptible to both agents and the infected sites are amenable to aminoglycoside pharmacokinetics. On the other hand, larger cohort studies have failed to show superiority of aminoglycoside- versus colistin-based regimens (21). Thus, we encourage centers to internally audit patient outcomes prior to selecting preferred therapeutic approaches against CR K. pneumoniae bacteremia. Such approaches should be prospectively tailored to include new antimicrobial agents as they become available.
Our findings are plausible based on aminoglycoside pharmacokinetics. Aminoglycoside peak concentrations of 10× the MIC against the infecting pathogen are associated with optimal bactericidal killing (29) and suppression of aminoglycoside resistance (30). Peak serum concentrations range from 14 to 24 μg/ml among patients receiving extended-interval dosing regimens (7). This concentration range approximates 10× the MIC against 94% (31/33) of CR K. pneumoniae isolates in our study, including all isolates from patients with primary bacteremia. Aminoglycoside concentrations in urine are even higher (15). On the other hand, aminoglycoside concentrations in bile are 25 to 50% of those of serum and are virtually undetectable in the presence of biliary obstruction or hepatic damage (8, 10). Likewise, less than one-third of gentamicin serum concentrations are detectable in the alveolar lining fluid and respiratory secretions of critically ill patients (9, 31). In keeping with our experience, aminoglycosides are less efficacious than comparator agents for the treatment of intra-abdominal infections (32).
Optimal antimicrobial therapy is not the sole determinant of outcomes among patients with CR K. pneumoniae bacteremia (18). Underlying diseases and severity of illness at the onset of infection are also major predictors of mortality (21, 22, 33). Indeed, we found that clinical response rates were only 31% and 36% among patients with fatal underlying diseases and APACHE II scores of ≥20, respectively. When these factors were combined with unamenable sources of bacteremia, clinical response rates dropped to 22% and 29%, respectively. An important avenue of future investigation will be whether outcomes can be improved with newer agents, such as ceftazidime-avibactam, which is active against Enterobacteriaceae that produce KPC and other β-lactamases (34).
Primary bacteremia and bacteremia that was secondary to intra-abdominal sources predominated in our study. All patients with bacteremia stemming from intra-abdominal sources underwent an interventional procedure, but it is possible that the higher aminoglycoside failure rate in the population was due to suboptimal source control. Our results cannot necessarily be extrapolated to other types of infection. Based on pharmacokinetic and clinical data, it is reasonable to anticipate that aminoglycosides will be useful against bacteremia due to urinary sources (4, 8, 15). We also cannot exclude that clinician biases in choosing aminoglycoside-based regimens may have influenced our findings. Last, 70% of patients received an aminoglycoside in combination with another antimicrobial agent; thus, the value of aminoglycoside monotherapy for CR K. pneumoniae bacteremia is not clear.
In conclusion, despite a small sample size, our study provides much-needed insight into the potential roles for aminoglycoside treatment against CR K. pneumoniae bacteremia. As new agents against CR K. pneumoniae and other highly drug-resistant Gram-negative bacteria enter the clinic, it will be imperative to employ them judiciously to preserve their long-term utility. In this regard, it is critical to understand where older agents, like aminoglycosides, have useful roles. Moving forward, it will be important to determine if aminoglycosides can be used in combination with newer agents to improve efficacy and limit the emergence of resistance.
ACKNOWLEDGMENTS
This project was supported by funding provided to the XDR Pathogen Laboratory by the University of Pittsburgh Medical Center and by the National Institutes of Health (NIH) under award no. K08AI114883 awarded to R.K.S. and award no. R21AI111037 awarded to C.J.C.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside modifying enzymes, which exert varying effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy CJ, Hao B, Shields RK, Chen L, Perlin DS, Kreiswirth BN, Nguyen MH. 2014. Doripenem, gentamicin, and colistin, alone and in combinations, against gentamicin-susceptible, KPC-producing Klebsiella pneumoniae strains with various ompK36 genotypes. Antimicrob Agents Chemother 58:3521–3525. doi: 10.1128/AAC.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 4.Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ, Jenkins SG, Calfee DP. 2011. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 55:5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, Natera C, Rodríguez M, Salcedo I, Rodríguez-López F, Rivero A, Rodríguez-Baño J. 2015. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 6.McHenry MC, Gavan TL, VanOmmen RA, Hawk WA. 1971. Therapy with gentamicin for bacteremic infections: results with 53 patients. J Infect Dis 124(Suppl):S164–S173. [DOI] [PubMed] [Google Scholar]
- 7.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riff LJ, Jackson GG. 1971. Pharmacology of gentamicin in man. J Infect Dis 124(Suppl):S98–S105. [DOI] [PubMed] [Google Scholar]
- 9.Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. 2005. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 128:545–552. doi: 10.1378/chest.128.2.545. [DOI] [PubMed] [Google Scholar]
- 10.Pitt HA, Roberts RB, Johnson WD Jr. 1973. Gentamicin levels in the human biliary tract. J Infect Dis 127:299–302. doi: 10.1093/infdis/127.3.299. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS. 2014. The Sanford guide to antimicrobial therapy, 44th ed. Antimicrobial Therapy, Inc. Sperryville, VA. [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Lorentzen H, Kallehave F, Kolmos HJ, Knigge U, Bülow J, Gottrup F. 1996. Gentamicin concentrations in human subcutaneous tissue. Antimicrob Agents Chemother 40:1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labovitz E, Levison ME, Kaye D. 1974. Single-dose daily gentamicin therapy in urinary tract infection. Antimicrob Agents Chemother 6:465–470. doi: 10.1128/AAC.6.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCabe WR, Jackson GG. 1962. Gram-negative bacteremia: I. Etiology and ecology. Arch Intern Med 110:847–855. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, Silveira FP, Abdel-Massih R, Bogdanovich T, Humar A, Perlin DS, Kreiswirth BN, Hong Nguyen M. 2013. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD. 2013. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 20.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 21.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. [DOI] [PubMed] [Google Scholar]
- 22.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields RK, Nguyen MH, Potoski BA, Press EG, Chen L, Kreiswirth BN, Clarke LG, Eschenauer GA, Clancy CJ. 2015. Doripenem MICs and ompK36 porin genotypes of sequence type 258, KPC-producing Klebsiella pneumoniae may predict responses to carbapenem-colistin combination therapy among patients with bacteremia. Antimicrob Agents Chemother 59:1797–1801. doi: 10.1128/AAC.03894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clancy CJ, Chen L, Hong JH, Cheng S, Hao B, Shields RK, Farrell AN, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Mutations of the ompK36 porin gene and promoter impact responses of sequence type 258, KPC-2-producing Klebsiella pneumoniae strains to doripenem and doripenem-colistin. Antimicrob Agents Chemother 57:5258–5265. doi: 10.1128/AAC.01069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira MS, de Assis DB, Freire MP, Boas do Prado GV, Machado AS, Abdala E, Pierrotti LC, Mangini C, Campos L, Caiaffa Filho HH, Levin AS. 2015. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 21:179e1–179e7. [DOI] [PubMed] [Google Scholar]
- 26.Shields RK, Clancy CJ, Gillis LM, Kwak EJ, Silveira FP, Massih RC, Eschenauer GA, Potoski BA, Nguyen MH. 2012. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One 7:e52349. doi: 10.1371/journal.pone.0052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) . 2015. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21:1106.e1–1106.e8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 30.Blaser J, Stone BB, Groner MC, Zinner SH. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother 31:1054–1060. doi: 10.1128/AAC.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valcke Y, Pauwels R, Van der Straeten M. 1990. Pharmacokinetics of antibiotics in the lungs. Eur Respir J 3:715–722. [PubMed] [Google Scholar]
- 32.Bailey JA, Virgo KS, DiPiro JT, Nathens AB, Sawyer RG, Mazuski JE. 2002. Aminoglycosides for intra-abdominal infection: equal to the challenge? Surg Infect (Larchmt) 3:315–335. doi: 10.1089/109629602762539544. [DOI] [PubMed] [Google Scholar]
- 33.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 34.Nicolau DP. 2015. Focus on ceftazidime-avibactam for optimizing outcomes in complicated intra-abdominal and urinary tract infections. Expert Opin Invest Drugs 24:1261–1273. doi: 10.1517/13543784.2015.1062873. [DOI] [PubMed] [Google Scholar]