Abstract
Biofilm growth is a universal survival strategy for bacteria, providing an effective and resilient approach for survival in an otherwise hostile environment. In the context of an infection, a biofilm provides resistance and tolerance to host immune defenses and antibiotics, allowing the biofilm population to survive and thrive under conditions that would destroy their planktonic counterparts. Therefore, the disruption of the biofilm is a key step in eradicating persistent bacterial infections, as seen in many types of chronic disease. In these studies, we used both in vitro minimum biofilm eradication concentration (MBEC) assays and an in vivo model of chronic biofilm infection to demonstrate the biofilm-disrupting effects of an alginate oligomer, OligoG CF-5/20. Biofilm infections were established in mice by tracheal instillation of a mucoid clinical isolate of Pseudomonas aeruginosa embedded in alginate polymer beads. The disruption of the biofilm by OligoG CF-5/20 was observed in a dose-dependent manner over 24 h, with up to a 2.5-log reduction in CFU in the infected mouse lungs. Furthermore, in vitro assays showed that 5% OligoG CF-5/20 significantly reduced the MBEC for colistin from 512 μg/ml to 4 μg/ml after 8 h. These findings support the potential for OligoG CF-5/20 as a biofilm disruption agent which may have clinical value in reducing the microbial burden in chronic biofilm infections.
INTRODUCTION
It has been suggested that biofilms are present in more than 65% of all bacterial infections (1). Biofilm-associated bacteria show an innate resistance to antibiotics, disinfectants, and host defense mechanisms (2, 3) and are thought to contribute to survival in chronic lung infections. Several in vitro studies have demonstrated that bacteria growing in a biofilm can become 10 to 1,000 times more resistant to the effects of antimicrobial agents than planktonic-growing bacteria of the same strain (4, 5). Patients with cystic fibrosis (CF) are highly susceptible to lung infections. As the disease progresses their lungs become chronically infected by P. aeruginosa as a biofilm infection (6), contributing to the destructive cycle of inflammation and fibrosis (7). Clinically, the presence of mucoid variants is associated with poor prognosis, deterioration of lung function, and increased tissue damage (2, 8). Therefore, the disruption of the biofilm is a key step in eradicating persistent bacterial infections, as is seen in many types of chronic disease.
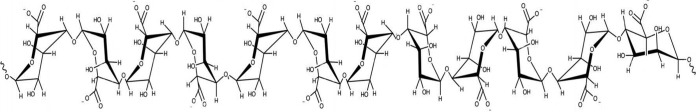
The novel alginate oligomer OligoG CF-5/20 is a low-molecular-weight oligosaccharide enriched from sodium alginate polysaccharides. It is an oligomer composed of α-l-guluronic acid (>85%) and β-d-mannuronic acid (<15%) (Fig. 1). OligoG CF-5/20 is currently in phase 2b clinical trials in CF patients. Previous in vitro studies have shown the ability of OligoG CF-5/20 to perturb biofilm formation, thereby reducing tolerance to antibiotic treatment (9–12).
FIG 1.
Structure of 1→4-α-l-guluronic acid (G) and 1→4-β-d-mannuronic acid (M); OligoG CF-5/20 has at least 85% of the monomer as G residues.
High-molecular-weight polymeric alginate is one of the components produced by P. aeruginosa in the formation of biofilms in the airways of CF patients (13, 14). This characteristic has been exploited in mouse models addressing the role of P. aeruginosa in lung infections, wherein the inoculum is artificially embedded in an ultrapure polymeric alginate microbead to mimic the presence of a biofilm infection. We used this approach in the biofilm lung infection model in this study to investigate the biofilm-disrupting capabilities of the novel alginate oligomer OligoG CF-5/20.
MATERIALS AND METHODS
Preparation of biofilm-embedded beads.
Wild-type P. aeruginosa PAO1 (nonmucoid) (15) and clinical strain P. aeruginosa NH57388A (mucoid), isolated from the lungs of a CF patient (16), were used in this study. A high-molecular-weight alginate polymer bead preparation was used to embed P. aeruginosa and simulate biofilm infection. P. aeruginosa was cultured on Blue agar plates (a selective medium for Gram-negative rods, containing 10 g/liter peptone, 5 g/liter yeast extract, 5 g/liter NaCl, 1 ml/liter 5% Maranil, 2 ml/liter 50% Na2S2O3, 10 ml/liter 1% bromothymol blue, 27 ml/liter 33% lactose, 1.2 ml/liter 33% glucose, 10 g/liter Bacto agar in ion-exchanged water, pH 7.7 to 7.8) (17) (SSI Diagnostica) from frozen stocks. Liquid inocula for the growth experiment were prepared by inoculating P. aeruginosa from a plate in filtered ox broth (Statens Serum Institut, Copenhagen, Denmark) overnight at 37°C and 170 rpm. The bacteria were centrifuged, resuspended, and diluted in 0.9% saline. Planktonic PAO1 or NH57388A was harvested from overnight cultures at 37°C grown in filtered ox broth (Statens Serum Institut, Copenhagen, Denmark). The bacteria were resuspended and diluted in 0.9% saline (1 × 107 CFU/ml of suspension for in vitro assays and 1 × 109 CFU/ml of suspension for in vivo administration), and 1 ml of this suspension then was mixed with 9 ml of 1% (mass/vol) Pronova alginate. The mixture was passed through a needle into a solution of 0.1 M CaCl2 and 0.1 M Tris–HCl buffer (pH 7.0) (Sigma-Aldrich) to create the alginate beads. The bead size (100 ± 15 μm) and number of alginate beads was confirmed by microscopic examination. The procedure was performed aseptically. The concentration of bacteria in the alginate beads was checked before use in the bacterial challenge studies. The concentration of bacteria inside the beads was checked by serial dilution. The inoculum of alginate beads was adjusted with sterile 0.9% saline to 1 × 106 CFU/ml for in vitro assay and 1 × 108 CFU/ml for in vivo study. The procedure was performed aseptically (18). Alginate beads were diluted in saline and stored at 4°C 2 days prior to use.
MIC and MBEC biofilm susceptibility assay.
Ciprofloxacin and colistin were pharmaceutical grade. The MIC was determined using the Etest (AB Biodisk, Solna, Sweden). For P. aeruginosa NH57388A, the MIC of colistin is 0.094 μg/ml and that of ciprofloxacin is 2 μg/ml; for P. aeruginosa PAO1, the MIC of colistin is 2 μg/ml and that of ciprofloxacin is 0.125 μg/ml. OligoG CF-5/20 was provided by AlgiPharma AS (Norway). The alginate beads containing the bacteria (106 CFU/ml) were transferred into flat-bottom microtiter plates containing different concentrations of ciprofloxacin or colistin with or without the presence of 5% OligoG CF-5/20 in 0.2 ml of saline per well (antibiotic challenge plate) and incubated for 0 h, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h at 37°C. The wells with only 5% (mass/vol) OligoG CF-5/20 treatment on alginate beads were used as a control. The pilot studies were performed by the kill-curve method to check the killing effect of different concentrations of OligoG CF-5/20. After incubation with antibiotic, the samples (3 samples/time point/concentration) were harvested and serially diluted. After overnight culture on plates, the CFU were determined. The lower limit of detection on plates was 10 CFU/ml. The minimal biofilm eradication concentration (MBEC) of antibiotics for biofilm alginate beads was determined as outlined previously (4, 19). MBEC was defined as the concentration of antimicrobials at which no bacterial growth on the recovery plate was observed.
Mouse lung infection model and OligoG CF-5/20.
Eight-week-old female BALB/cJ mice (Taconic, Denmark) weighing 20 to 26 g were used. The mice were maintained on standard mouse chow and water ad libitum for a week and randomly divided into treatment groups (12 mice/group) before bacterial challenge. All animal experiments were performed under authorization from the National Animal Ethics Committee of Denmark.
The BALB/cJ mice were lightly anesthetized by subcutaneous injection of a 1:1 mixture of fentanyl-fluanisone (Hypnorm) and midazolam at a dose of 5 ml/kg of body weight. The trachea was exposed to allow the instillation of drugs to the lower left lung with 0.04 ml of P. aeruginosa NH57388A embedded biofilm alginate beads (1 × 108 CFU/ml) (biofilm model) or planktonic culture (5 × 109 CFU/ml) (planktonic model) (18). At each time point mice were sacrificed and lung samples were removed for bacteriology and histopathology.
To determine the effect of different concentrations of OligoG CF-5/20 on biofilms, 0.04 ml alginate beads (1 × 108 CFU/ml) of P. aeruginosa NH57388A alone or combined with saline or 0.2%, 1%, and 5% OligoG was delivered into the lower left lung. OligoG was administered by tracheal instillation. Lungs were removed 24 h after bacterial challenge for bacteriology. Additional time points at 12 h, 24 h, 72 h, and 7 days after challenge were used to determine the effects over time. To demonstrate the tolerance of biofilm growth to conventional antibiotic treatment, three different antibiotics (aztreonam, colistin, and tobramycin) were tested at 16×MIC in both the planktonic and biofilm growth experiments.
Bacteriology.
Eight randomly selected mice from each group were prepared for quantitative bacteriology (20). The whole lung of each mouse was excised aseptically and homogenized in 5 ml of sterile 0.9% saline, and 100 μl of serially diluted lung homogenates was plated on Blue agar plates and incubated at 37°C. CFU counting for P. aeruginosa colonies was performed after 35 to 40 h. The lower limit of detection was 10 CFU/ml.
Tissue preparation and histopathology.
The remaining mice from each group were used for lung histopathology (19). Prior to fixation in formalin buffer (4% formaldehyde [pH 7.0]; Bie & Berntsen, Copenhagen, Denmark), pictures of the lungs were taken. The formalin fixation for at least 1 week was followed by embedding in paraffin wax, and the wax then was cut into 4- to 5-μm sections. Mounted sections were stained with hematoxylin and eosin (H&E) combined with alcian blue (Sigma-Aldrich, Denmark) and nuclear fast red (Sigma-Aldrich, Denmark).
Measurement of IL-1α.
Interleukin-1α (IL-1α) is a proinflammatory cytokine and plays a central role in the regulation of immune responses (21). To observe the effects of OligoG CF-5/20 treatment on the systemic inflammatory response in the biofilm infection model, the level of IL-1α expression in the blood of mice was tested. IL-1α levels were determined in normal and infected mice after treatment with 0%, 0.2%, 1%, and 5% OligoG CF-5/20 using the enzyme-linked immunosorbent assay (ELISA) kit for mouse IL-1α (BioLegend, San Diego, CA) by following the instructions of the manufacturers. The blood was collected from four randomly selected mice of each group 24 h after infection, with measurements performed in duplicate, and the mean values for each group were calculated.
Data analysis and statistical methods.
The pharmacodynamic analysis was performed using the inhibitory sigmoid dose-effect model (Origin, version 8.0; OriginLab, Northampton, MA). The formula is E = E0 − Emax · Xy/(Xy+ EC50), where E is the measure of effect (log10 numbers of CFU/lung) and X is the concentration of OligoG CF-5/20. E0 is the effect in the absence of drug, Emax is the maximal drug effect, EC50 is the value of the target of OligoG CF-5/20 required to achieve 50% of Emax, and y is the Hill coefficient of the OligoG CF-5/20 effect curve. The correlation between the efficacy and the concentrations of OligoG CF-5/20 was determined by nonweighted, nonlinear least-squares regression. One-way analysis of variance (ANOVA) was used to determine whether there were any significant differences in the bacteriology or cytokine values from the different treatment groups.
RESULTS
MBEC assay for 5% OligoG CF-5/20 combined with ciprofloxacin or colistin.
In Table 1 and Table 2, the minimum biofilm eradication concentrations (MBEC) of ciprofloxacin and colistin are shown. The MBEC of ciprofloxacin on alginate bead biofilms in saline at 24 h of treatment was 1 and >32 μg/ml for PAO1 and NH57388A, respectively, and the MBEC of colistin was 4 and 128 μg/ml for PAO1 and NH57388A, respectively. The combination of colistin and 5% OligoG CF-5/20 showed synergistic effects, especially in the alginate-overproducing clinical strain NH57388A. The maximum combined killing of colistin and OligoG CF-5/20 was seen after 8 h of treatment. The synergistic effect of colistin and OligoG CF-5/20 was observed as early as 1 h. There also was synergism between ciprofloxacin and OligoG for the PAO1 strain but not for the NH58388A strain. A significant synergistic effect of colistin and OligoG was found in vitro on biofilms of the mucoid P. aeruginosa NH57388A clinical isolate (128-fold reduction). A 4-fold reduction was observed for the PAO1 biofilms treated with the same drug combination. The synergistic effect also was found in mucoid strains P. aeruginosa PDO300 and PAO579 (clinical strain) in vitro (data not shown).
TABLE 1.
MBEC in vitro of OligoG CF-5/20 with ciprofloxacin and colistin on P. aeruginosa PAO1 alginate bead biofilm in saline
| Treatment time (h) | MBECa (μg/ml) |
|||
|---|---|---|---|---|
| Ciprofloxacin |
Colistin |
|||
| 0% OligoG | 5% OligoG | 0% OligoG | 5% OligoG | |
| 1 | 8 | 4 | >256 | 256 |
| 2 | 8 | 4 | >256 | 128 |
| 4 | 8 | 4 | 64 | 16 |
| 8 | 4 | 1 | 16 | 4 |
| 12 | 2 | 0.5 | 8 | 4 |
| 24 | 1 | 0.5 | 4 | 4 |
Etest for P. aeruginosa PAO1 found a colistin MIC of 2 μg/ml and a ciprofloxacin MIC of 0.125 μg/ml.
TABLE 2.
MBEC in vitro of OligoG CF-5/20 with ciprofloxacin and colistin on P. aeruginosa NH57388A alginate bead biofilm in saline
| Treatment time (h) | MBECa (μg/ml) |
|||
|---|---|---|---|---|
| Ciprofloxacin |
Colistin |
|||
| 0% OligoG | 5% OligoG | 0% OligoG | 5% OligoG | |
| 1 h | >32 | >32 | >512 | 512 |
| 2 h | >32 | >32 | >512 | 256 |
| 4 h | >32 | >32 | >512 | 16 |
| 8 h | >32 | >32 | 512 | 4 |
| 12 h | >32 | >32 | 256 | 2 |
| 24 h | >32 | 32 | 128 | <2 |
Etest for P. aeruginosa NH57388A found a colistin MIC of 0.094 μg/ml and a ciprofloxacin MIC of 2 μg/ml.
Mouse lung infection model and OligoG CF-5/20.
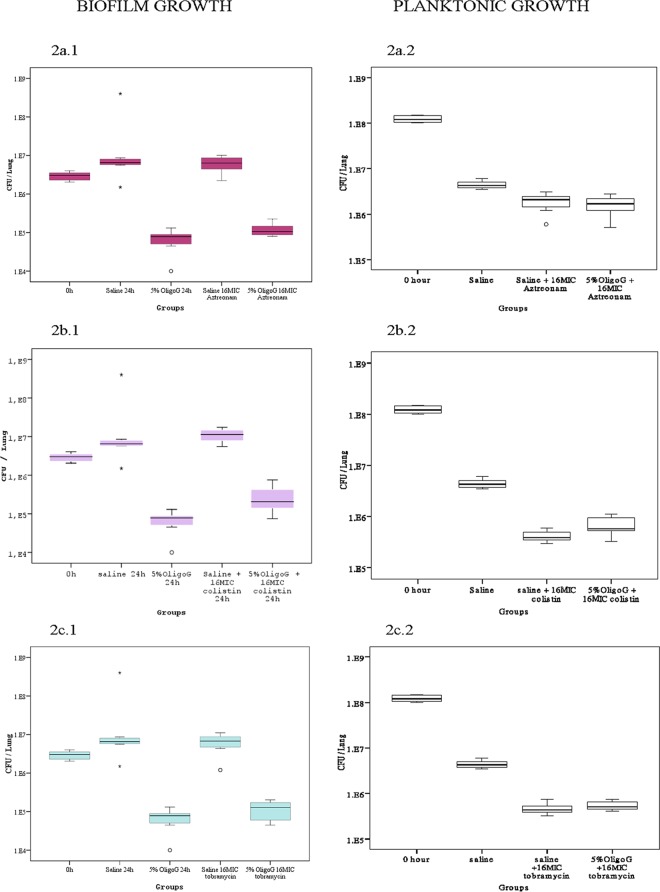
The in vivo effects of antibiotics in the biofilm and planktonic models are shown in Fig. 2a, b, and c. These data clearly showed a reduced antibiotic efficacy on biofilms compared to that of planktonic infections. In the biofilm model the 16×MIC local antibiotic treatments showed no significant reduction in CFU, illustrating the tolerance of biofilm growth to conventional antibiotic treatment. This was in marked contrast to the effects of antibiotics in the planktonic growth model, where a 2-log reduction from the zero time point in microbial burden was observed. As expected, in the absence of biofilm, OligoG CF-5/20 had no apparent effect on the planktonic growth model. However, a significant effect was shown in the biofilm groups receiving OligoG CF-5/20. A statistically significant reduction in biofilm microbial burden (up to 2.5-log reduction in CFU) was observed in those mice that received 5% OligoG CF-5/20 (P < 0.01) (Fig. 2). Additionally, this reduction also was seen in a dose-dependent manner with a clear reduction in microbial burden, which also was observed at concentrations as low as 1% OligoG CF-5/20 (Fig. 3 and 4).
FIG 2.
(a) Killing effect of 5% OligoG CF-5/20 combined with local 16×MIC aztreonam treatment on alginate bead biofilm and planktonic P. aeruginosa NH57388A in vivo. (b) Killing effect of 5% OligoG CF-5/20 combined with local 16×MIC colistin treatment on alginate bead biofilm and planktonic P. aeruginosa NH57388A in vivo. (c) Killing effect of 5% OligoG CF-5/20 combined with local 16×MIC tobramycin treatment on alginate bead biofilm and planktonic P. aeruginosa NH57388A in vivo.
FIG 3.
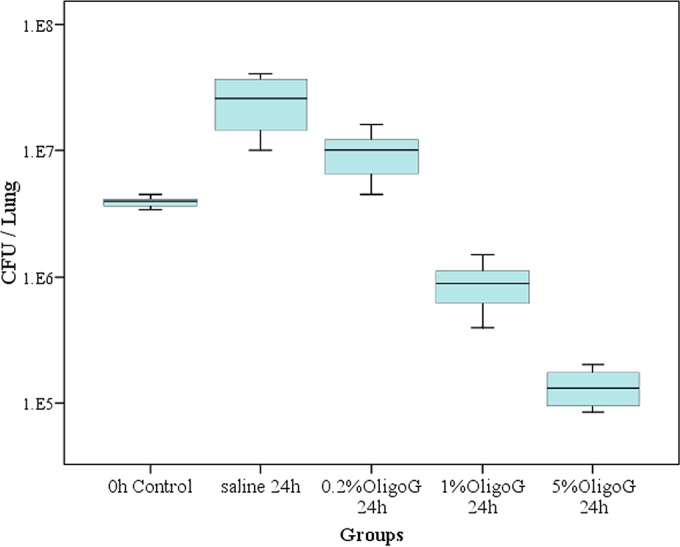
Effect of 0%, 0.2%, 1%, and 5% OligoG CF-5/20 on alginate bead biofilm of P. aeruginosa NH57388A in vivo.
FIG 4.
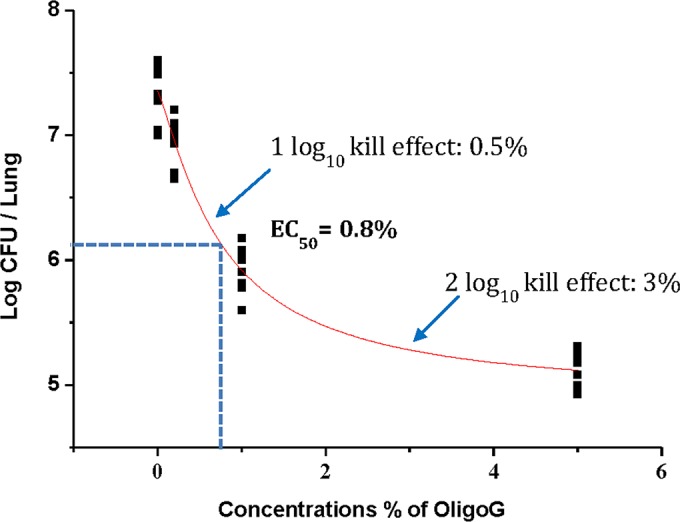
Sigmoidal Emax modeling for the effect of OligoG CF-5/20 on biofilms of P. aeruginosa NH57388A in vivo.
Pharmacodynamics of OligoG CF-5/20 and sigmoidal Emax modeling.
The EC50 for OligoG CF-5/20 on the in vivo biofilm infection model was 0.8%, with the majority of biofilm-embedded cells killed within 24 h after a single treatment of 3% OligoG CF-5/20 (Fig. 3 and 4).
Histopathology and macroscopic pathology of lung.
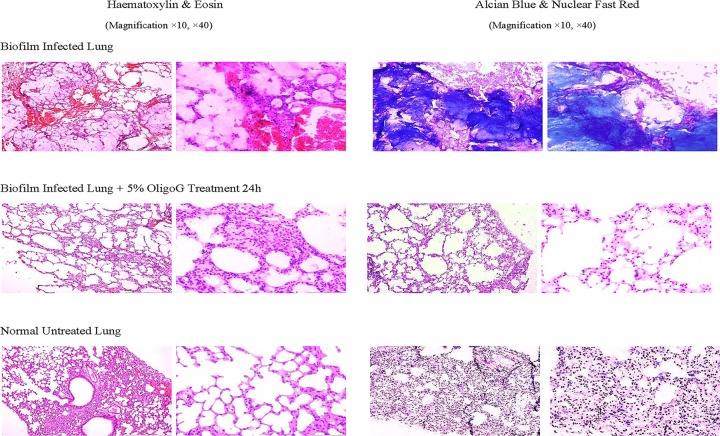
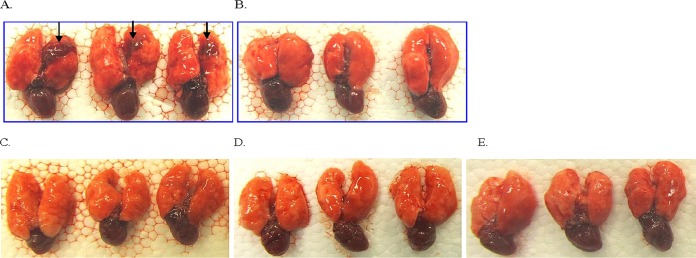
In Fig. 5, H&E staining of lung sections from infected mice clearly showed signs of infection. Staining with alcian blue showed clear differences in biofilm burden between treated and untreated groups, with biofilms surrounded by polymorphonuclear neutrophils (PMNs). Animals treated with 5% OligoG CF-5/20 exhibited a marked reduction in alcian blue staining of alginate, reflecting a significant disruption of biofilm in the infected lungs. The macroscopic change of infected lung is shown in Fig. 6.
FIG 5.
Representative histology of H&E- and alcian blue-stained sections of lung tissues from controls and OligoG CF-5/20-treated mice.
FIG 6.
Macroscopic lung pathology of mouse. The biofilms of alginate beads of NH57388A were administered by the intratracheal route to the lower left lung either left untreated (A) or with 5% OligoG (50 mg/ml) treatment only (B), with local 5% OligoG treatment and 16×MIC aztreonam treatment (C), with local 5% OligoG and 16×MIC colistin treatment (D), and with local 5% OligoG and 16×MIC tobramycin treatment (E). Lungs were removed at 24 h after bacterial challenge.
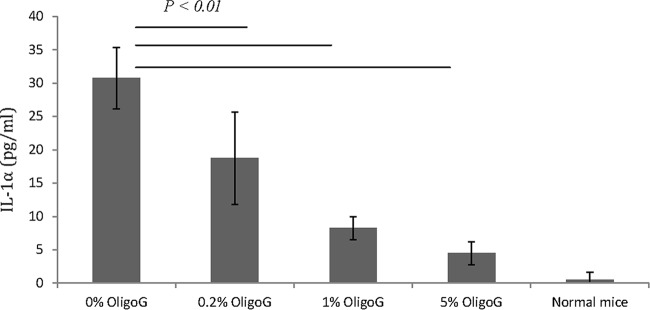
IL-1α expression after OligoG CF-5/20 treatment in biofilm infection.
The concentration of IL-1α in the blood of mice exposed to the biofilm infection with P. aeruginosa NH57388A is shown in Fig. 7. The data clearly showed a statistically significant (P < 0.01) reduction in IL-1α concentrations after treatment with different concentrations of OligoG CF-5/20, with mean values of 30.8 pg/ml (untreated mice), 18.8 pg/ml (0.2% OligoG), 8.3 pg/ml (1% OligoG), and 4.5 pg/ml (5% OligoG).
FIG 7.
IL-1α with 0%, 0.2%, 1%, and 5% OligoG CF-5/20 on biofilm infection of P. aeruginosa NH57388A in vivo.
DISCUSSION
The data presented in these studies support the role of the novel alginate oligomer OligoG CF-5/20 as a biofilm-disrupting agent. In the in vivo lung infection model, a single treatment of OligoG CF-5/20 by tracheal instillation significantly reduced the mucoid P. aeruginosa biofilm from the lungs of immunocompetent mice compared to that for saline controls. These findings support previous in vitro studies demonstrating the biofilm disruption effects (10, 11, 12).
The 2.5-log reduction in CFU burden observed in the in vivo biofilm lung infection model after OligoG treatment is of a magnitude similar to that of the CFU reduction attributed to the host immunity in a previous planktonic P. aeruginosa infection model (22). Given the existing knowledge of the mechanism of action from in vitro studies (9–12), it is likely that the dramatic reduction in microbial burden is due to the innate host immune defenses gaining access to the infection through the disruption of the biofilm by OligoG CF-5/20.
Pseudomonal biofilms in the lungs of CF patients represent a significant barrier to eradication by many antibiotics. The biofilm provides such a degree of tolerance to antibiotics that the concentrations of antibiotic required to eradicate the infection often are physiologically unattainable. The concentration of nebulized colistin that is achievable in CF patients is about 40 μg/ml (23), while the corresponding MBEC of colistin for P. aeruginosa is at least 64 μg/ml (4). In the absence of a biofilm-disrupting agent, this disparity makes the eradication of biofilm infection in CF patients extremely difficult.
In the present study, the MBEC of colistin combined with OligoG is 4 μg/ml (Tables 1 and 2), and it represents a promising combination approach to eradicate biofilm infections in CF patients at far lower concentrations of antibiotic, with the additional benefit of reducing the risk of toxicity (19). The data in the present study show that coadministration treatment of in vitro biofilms with the novel alginate oligomer OligoG CF-5/20 and colistin results in a 128-fold reduction in the MBEC value. The burden of biofilm cells was significantly decreased in vivo after the administration of 5% OligoG, while the combined effect of 5% OligoG and antibiotics at 16×MIC was not superior to that of 5% OligoG alone in this study in immunocompetent mice. However, the effect of antibiotic combination in biofilm infections might be more appropriate using a neutropenic mouse model (24).
The data presented support the idea that the biofilm is disrupted by OligoG CF-5/20, which makes P. aeruginosa NH57388A more susceptible to eradication by the host innate immune response (i.e., PMNs). The higher efficacy of antibiotics on planktonic cells compared to that of biofilm also is shown by our results. Moreover, by removing the protective nature of the biofilm, any quiescent cells also would become more vulnerable/sensitive to antibiotics and/or host immune defenses (24).
From the sigmoidal Emax modeling, in this mouse infection model, a single dose of 3% OligoG CF-5/20 would result in the eradication of 99% of the biofilm infection. While the complex environment of the human CF lung likely will present a greater challenge, these data represent a promising approach of clinical value.
In addition to the obvious reduction in microbial burden afforded by OligoG CF-5/20 treatment, the marked decrease of IL-1α in blood reflects a general reduction in inflammatory response that further confirms the disruption of the biofilm (Fig. 5). The reduced inflammatory response as measured by IL-1α concentrations showed a dose-dependent decrease with increasing concentrations of OligoG CF-5/20 (Fig. 7).
These findings, combined with previous in vitro data, support the use of OligoG CF-5/20 as a promising new strategy to prevent and disrupt P. aeruginosa biofilm infections in vivo.
ACKNOWLEDGMENTS
This study was sponsored by AlgiPharma AS, Sandvika, Norway. E.O. and P.D.R. are directors at AlgiPharma AS. W.H. was supported by a grant from the University of Copenhagen Research Center for Control of Antibiotic Resistance (UC-CARE).
REFERENCES
- 1.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Ciofu O, Fussing V, Bagge N, Koch C, Høiby N. 2001. Characterization of paired mucoid/non-mucoid Pseudomonas aeruginosa isolates from Danish cystic fibrosis patients: antibiotic resistance, β-lactamase activity and RiboPrinting. J Antimicrob Chemother 48:391–396. doi: 10.1093/jac/48.3.391. [DOI] [PubMed] [Google Scholar]
- 3.Høiby N, Bjarnsholt T, Moser C, Bassi G, Coenye T, Donelli G, Hall-Stoodley L, Holá V, Imbert C, Kirketerp-Møller K. 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21:S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2011. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 55:4469–4474. doi: 10.1128/AAC.00126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceri H, Olson M, Stremick C, Read R, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Høiby N, Olling S. 1977. Pseudomonas aeruginosa infection in cystic fibrosis. Acta Pathol Microbiol Scand C Immunol 85:107–114. [PubMed] [Google Scholar]
- 7.Høiby N. 1982. Microbiology of lung infections in cystic fibrosis patients. Acta Paediatr 71:33–54. [Google Scholar]
- 8.Pedersen SS. 1992. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS Suppl 28:1–79. [PubMed] [Google Scholar]
- 9.Powell LC, Pritchard MF, Emanuel C, Onsoyen E, Rye PD, Wright CJ, Hill KE, Thomas DW. 2014. A nanoscale characterization of the interaction of a novel alginate oligomer with the cell surface and motility of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 50:483–492. doi: 10.1165/rcmb.2013-0287OC. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JL, Khan S, Emanuel C, Powell LC, Pritchard MF, Onsoyen E, Myrvold R, Thomas DW, Hill KE. 2013. An in vitro study of alginate oligomer therapies on oral biofilms. J Dentistry 41:892–899. doi: 10.1016/j.jdent.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Powell LC, Sowedan A, Khan S, Wright CJ, Hawkins K, Onsøyen E, Myrvold R, Hill KE, Thomas DW. 2013. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling 29:413–421. doi: 10.1080/08927014.2013.777954. [DOI] [PubMed] [Google Scholar]
- 12.Khan S, Tøndervik A, Sletta H, Klinkenberg G, Emanuel C, Onsøyen E, Myrvold R, Howe RA, Walsh TR, Hill KE. 2012. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob Agents Chemother 56:5134–5141. doi: 10.1128/AAC.00525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Z, Wu H, Ciofu O, Kong K-F, Høiby N, Rygaard J, Kharazmi A, Mathee K. 2003. Pseudomonas aeruginosa alginate is refractory to Th1 immune response and impedes host immune clearance in a mouse model of acute lung infection. J Med Microbiol 52:731–740. doi: 10.1099/jmm.0.05122-0. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob Agents Chemother 51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Høiby N. 1974. Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol Microbiol Scand B Microbiol Immunol 82:541–550. [PubMed] [Google Scholar]
- 18.Pedersen SS, Shand GH, Hansen BL, Hansen GN. 1990. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS 98:203–211. doi: 10.1111/j.1699-0463.1990.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 19.Hengzhuang W, Høiby N, Ciofu O. 2014. Pharmacokinetics and pharmacodynamics of antibiotics in biofilm infections of pseudomonas aeruginosa in vitro and in vivo, p 239–254. In Donelli G. (ed), Microbial biofilms: methods and protocols. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann N, Rasmussen TB, Jensen P, Stub C, Hentzer M, Molin S, Ciofu O, Givskov M, Johansen HK, Høiby N. 2005. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun 73:2504–2514. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, Kanneganti T-D. 2013. RIP1-driven autoinflammation targets IL-1 [agr] independently of inflammasomes and RIP3. Nature 498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange K, Hougen H, Høiby N, Fomsgaard A, Rygaard J, Johansen HK. 1995. Experimental chronic Pseudomonas aeruginosa lung infection in rats. APMIS 103:367–374. doi: 10.1111/j.1699-0463.1995.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 23.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, Van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 24.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2012. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother 56:2683–2690. doi: 10.1128/AAC.06486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]