Abstract
The three direct-acting antiviral agent (3D) regimen is a novel combination of direct-acting antiviral agents (DAAs) that has proven effective for the treatment of hepatitis C virus (HCV) infection. Given the potential for coadministration in patients with human immunodeficiency virus infection, possible drug interactions with antiretroviral drugs must be carefully considered. Four phase 1, multiple-dose pharmacokinetic studies were conducted in healthy volunteers (n = 66). The 3D regimen of 150/100 mg daily paritaprevir/ritonavir, 25 mg daily ombitasvir, and 400 mg twice-daily dasabuvir was administered alone or in combination with 200 mg daily of emtricitabine and 300 mg daily of tenofovir disoproxil fumarate (tenofovir DF), 25 mg daily of rilpivirine, or 400 mg of raltegravir twice daily. A 2-DAA regimen of 150/100 mg daily paritaprevir/ritonavir and 400 mg of dasabuvir twice daily was also studied in combination with efavirenz/emtricitabine/tenofovir DF at 600/200/300 mg daily, respectively (Atripla; Bristol-Myers Squibb). Pharmacokinetic parameters were determined from plasma drug concentrations. No clinically significant drug interactions were observed (≤32% change in exposure) between the 3D regimen and that of emtricitabine plus tenofovir DF. Raltegravir exposure was increased up to 134% when the drug was coadministered with the 3D regimen. Although coadministration with rilpivirine was well tolerated in healthy volunteers, observed elevations in rilpivirine exposures may increase the potential for adverse drug reactions. Concomitant use of the 2-DAA regimen and efavirenz/emtricitabine/tenofovir DF was discontinued owing to poor tolerability and adverse events. No dose adjustment is required during coadministration of raltegravir, tenofovir DF, or emtricitabine with the 3D regimen. Rilpivirine is not recommended and efavirenz is contraindicated for coadministration with the 3D regimen.
INTRODUCTION
Coinfection with the hepatitis C virus (HCV) is common among patients diagnosed with the human immunodeficiency virus (HIV). Approximately 25% of HIV-infected individuals in the United States are also infected with HCV (1). Although advances in antiretroviral (ARV) therapy have had positive effects on the disease course and life expectancy of HIV-infected individuals, coinfection with HCV substantially increases the risk for liver disease and its associated morbidity and mortality (2, 3).
Treatment of HCV is complicated by the risk for drug-drug interactions (DDIs) with ARV therapy in patients with comorbid HIV. Treatment regimens for both conditions often involve the coadministration of multiple drugs, which may have overlapping metabolic enzyme, transporter, and/or elimination pathways. In clinical practice, use of medications with DDI potential is commonplace among patients with HCV/HIV coinfection who are initiating treatment with a direct-acting antiviral agent (DAA), a drug class that has emerged as a mainstay of HCV treatment (4). For certain DAAs, caution or avoidance of use in combination with several ARV regimens, such as tenofovir disoproxil fumarate (tenofovir DF)-based treatments, is advised owing to alterations in drug exposures (5).
One of the newest additions to the HCV treatment armamentarium is the all-oral, interferon-free, three direct-acting antiviral agent (3D) regimen. This combination therapy consists of paritaprevir (coadministered with ritonavir to enhance drug exposure [designated paritaprevir/r]), ombitasvir, and dasabuvir. The 3D regimen with or without ribavirin has proven to be efficacious and well tolerated in phase 3 clinical trials, with sustained virologic response rates 12 weeks after treatment (SVR12) of 92% to 100% in patients with genotype 1 HCV infection (6–9). Ombitasvir/paritaprevir/ritonavir and dasabuvir with and without ribavirin were approved in the United States and the European Union for the treatment of patients with chronic genotype 1 HCV infection, including those with compensated cirrhosis and those with HCV/HIV-1 coinfection.
Based on in vitro data, paritaprevir and ritonavir are primarily metabolized by cytochrome P450 3A (CYP3A), dasabuvir is primarily metabolized by CYP2C8, and ombitasvir is predominantly metabolized by amide hydrolysis followed by oxidative metabolism (D. A. J. Bow, J. Liu, O. Kavetskaia, R. Menon, S. M. de Morais, M. Nijsen, J. Sydor, V. Fischer, and M. Shebley, presented at the Special Conference on Hepatitis C of the American Association for the Study of Liver Disease-European Association for the Study of the Liver, New York, NY, 12 to 13 September, 2014). The DAAs and ritonavir are in vitro substrates of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), and paritaprevir is also a substrate of organic anion transporting polypeptide (OATP) 1B1/1B3 (10; Bow et al., conference presentation). In addition, the DAAs are inhibitors of UDP-glucuronosyltransferase 1A1 (UGT1A1), and ritonavir is an inhibitor of CYP3A4 (11). At clinically relevant concentrations, paritaprevir is an inhibitor of OATP 1B1/1B3 and paritaprevir, ritonavir, and dasabuvir are potential inhibitors of P-gp and BCRP (10; Bow et al., conference presentation).
Among the HIV antiretroviral agents, tenofovir DF and emtricitabine are primarily renally eliminated as unchanged drugs by a combination of glomerular filtration and active tubular secretion; tenofovir DF is a substrate of OAT1 (12–14). These data suggest that tenofovir DF and emtricitabine are the least likely to have a potential for pharmacokinetic interaction with the 3D regimen. On the other hand, raltegravir is eliminated mainly by metabolism via a UGT1A1-mediated glucuronidation pathway, rilpivirine is primarily metabolized by CYP3A, and efavirenz is a CYP3A inducer (15–18); hence, these drugs have the potential to have a pharmacokinetic interaction with the 3D regimen.
This series of DDI studies evaluated the pharmacokinetic effects and tolerability of coadministering the 3D regimen (or a modified version thereof) and commonly prescribed ARVs representing different agent classes. These studies were designed to test the feasibility of treating HCV infection with the 3D regimen in HCV/HIV-1-coinfected patients receiving ARV therapy.
MATERIALS AND METHODS
Study participants.
Healthy adult male and female participants aged 18 to 55 years with a body mass index (BMI) of ≥18 and <30 kg/m2 were eligible to participate in these studies. Volunteers with positive test results for hepatitis A, hepatitis B, HCV, or HIV or those who regularly used prescription or over-the-counter medications, vitamins, or supplements were excluded. Participants were prohibited from receiving any drug by injection within 30 days prior to study drug administration; ingesting a known inhibitor or inducer of cytochrome P450 3A (CYP3A), CYP2C8, or OATP 1B1 within 1 month prior to study drug administration; using tobacco or nicotine-containing products within 6 months of study initiation; or consuming grapefruit juice, star fruit, Seville oranges, or products containing these ingredients within 72 h prior to study drug administration. All study participants provided written informed consent as approved by the RCRC and Vista Health System institutional review boards.
Study design.
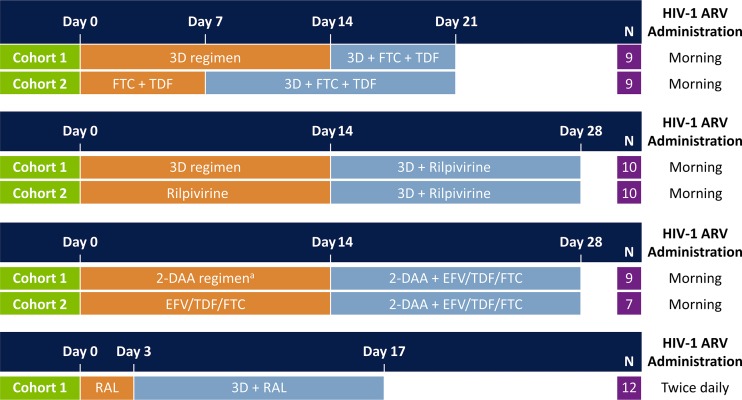
Data were extracted from four phase 1, single-center, multiple-dose, open-label studies (Fig. 1). Three of the four studies involved two participant cohorts: cohort 1 received a DAA regimen for the first 14 days before addition of the selected ARV regimen for another 7 to 14 days; cohort 2 received the ARV regimen for 7 to 14 days before addition of the DAA regimen for another 14 days. In the fourth study, all participants received the ARV regimen for 3 days, followed by the addition of the DAA regimen for another 14 days.
FIG 1.
Study design of drug-drug interaction studies. All study drugs were administered under nonfasting conditions. Paritaprevir/ritonavir and ombitasvir once-daily doses were administered in the morning, and dasabuvir was administered in the morning and evening. The drug-drug interaction study of efavirenz (EFV)/tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) (i.e., Atripla) with direct-acting antiviral (DAA) agents was conducted with the 2-DAA regimen of paritaprevir/ritonavir and dasabuvir. 3D, paritaprevir/ritonavir, ombitasvir, and dasabuvir; ARV, antiretroviral; RAL, raltegravir.
Dosing of ARV drugs was based on label-recommended doses. In study 1, the 3D regimen (paritaprevir/r at 150/100 mg once daily [QD], ombitasvir at 25 mg QD, and dasabuvir at 400 mg twice daily [BID]) was combined with emtricitabine at 200 mg QD (Emtriva; Gilead Sciences, Inc., Foster City, CA, USA) and tenofovir DF at 300 mg QD (Viread; Gilead Sciences, Inc., Foster City, CA, USA). In study 2, the 3D regimen was combined with 25 mg of rilpivirine (Edurant; Tibotec, Inc., Raritan, NJ, USA) administered QD in the morning. In study 3 efavirenz/emtricitabine/tenofovir DF at 600/200/300 mg daily (Atripla; Bristol-Myers Squibb, Princeton, NJ, USA) was combined with a 2-DAA regimen, which was one of the initial DAA combination regimens evaluated in clinical studies and consisted of paritaprevir/r at 150/100 mg QD and dasabuvir at 400 mg BID. In study 4, the 3D regimen was combined with raltegravir at 400 mg BID (Isentress; Merck & Co., Inc., Whitehouse Station, NJ, USA). The 400-mg dose of dasabuvir used in these studies provides equivalent exposures to that of the 250-mg marketed dose of dasabuvir.
The studies were conducted in accordance with the International Conference of Harmonisation guidelines, applicable regulations, and guidelines governing clinical study conduct and ethical principles that have their origin in the Declaration of Helsinki.
Pharmacokinetic and safety assessments.
Pharmacokinetic assessments were performed at steady state. Blood samples were collected prior to dosing and at regular intervals after dosing for up to 96 h to quantify plasma concentrations of paritaprevir, ombitasvir, ritonavir, dasabuvir, and ARV drugs. Intensive blood sampling for the determination of maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve during a dosing interval (AUCτ; AUC24 for drugs administered QD and AUC12 for drugs administered BID) was performed in the last dosing interval of each treatment phase. Blood draws for the measurement of trough drug concentration (Ctrough) levels were performed periodically during the treatment phase in each study. The Ctrough levels were measured at 24 h postdose (C24) for drugs administered QD and at 12 h after the morning dose (C12) for drugs administered BID.
Plasma concentrations of paritaprevir, ritonavir, ombitasvir, and dasabuvir were determined using a validated protein precipitation method and online solid-phase extraction method with liquid chromatography and tandem mass spectrometric detection (LC-MS/MS) (19). Plasma concentrations of emtricitabine/tenofovir, rilpivirine, efavirenz/emtricitabine/tenofovir, and raltegravir were determined by validated LC-MS/MS methods at a commercial laboratory (PPD, Middleton, WI). The lower limits of quantitation were 20.0/5.00 ng/ml, 0.500 ng/ml, 100/20.0/5.00 ng/ml, and 10.0 ng/ml, respectively.
Safety and tolerability were evaluated based on adverse event (AE) monitoring, vital sign measurements, physical examinations, electrocardiography, and laboratory assessments. All AEs were recorded, severity was assessed, and their relationship to the study drug was evaluated.
Pharmacokinetic and statistical analyses.
Pharmacokinetic analyses were performed via noncompartmental methods, and statistical analyses were performed using SAS, version 9.2 (Cary, NC, USA). Geometric mean ratios (GMRs) and 90% confidence intervals (CIs) comparing exposures (Cmax, AUCτ, and Ctrough) of DAA or ARV regimens alone with those of DAA/ARV coadministration were calculated through repeated-measures analysis. With the exception of the raltegravir DDI study, data from cohort 1 of each study were used to assess the effect of the ARV drugs on the pharmacokinetics of DAAs and ritonavir. Data from cohort 2 were used to determine the effect of the 3D regimen on ARV drug exposure. The effects of raltegravir on the pharmacokinetics of DAAs and ritonavir were evaluated by cross-study comparison of the study data from the 3D regimen plus raltegravir with historical data for the 3D regimen administered alone (data on file, AbbVie, Inc., USA). Raltegravir has minimal potential for a pharmacokinetic interaction with metabolizing enzymes or drug transporters that affect the disposition of DAAs and, hence, was not anticipated to alter the pharmacokinetics of DAAs. For DAAs, exposures up to 50% lower or 100% higher (GMR, 0.5 to 2.0) during coadministration with ARV drugs versus DAA administration alone were considered not to be significant. For ARV drugs, GMR and 90% CIs in the range of 0.8 to 1.25 for the comparison of exposures during coadministration with DAAs versus ARV drugs administered alone were not considered to be significant. The clinical significance of the exposures of ARV drugs outside the range of 0.8 to 1.25 was based on their dosing recommendations as per U.S. prescribing information or summaries of product characteristics.
Demographic and safety/tolerability data were evaluated using descriptive statistics.
RESULTS
Participants.
A total of 66 healthy volunteers were enrolled in the study cohorts that contributed to this analysis (Table 1). All participants in studies 1 and 4 completed their respective studies as planned. Two (10%) participants were prematurely discontinued from study 2 (3D regimen with rilpivirine): one at the discretion of the investigator and one because of an AE. The DDI study of the 2-DAA regimen with efavirenz/emtricitabine/tenofovir DF (study 3) was terminated early owing to the occurrence of AEs that led 9 of 16 participants to be discontinued from the study. Pharmacokinetic analysis for this combination was, therefore, not performed.
TABLE 1.
Study participant demographics and baseline characteristicsa
| Parameter | Study 1 (3D + FTC + TDF) | Study 2 (3D + RPV) | Study 3 (2-DAA + EFV/TDF/FTC) | Study 4 (3D + RAL) |
|---|---|---|---|---|
| No. of subjects | 18 | 20 | 16 | 12 |
| Age (yr) | 32.7 ± 9.5 | 29.9 ± 6.9 | 35.2 ± 8.6 | 42.1 ± 11.5 |
| Weight (kg) | 81.2 ± 6.3 | 75.9 ± 9.1 | 82.7 ± 8.5 | 80.9 ± 11.6 |
| Height (cm) | 177 ± 6.4 | 173 ± 8.8 | 179 ± 7.3 | 174 ± 8.5 |
| Sex (no. of subjects [%]) | ||||
| Male | 18 (100.0) | 15 (75.0) | 15 (93.8) | 8 (66.7) |
| Female | 0 | 5 (25.0) | 1 (6.3) | 4 (33.3) |
| Race (no. of subjects [%]) | ||||
| White | 7 (38.9) | 8 (40.0) | 9 (56.3) | 9 (75.0) |
| Black | 10 (55.6) | 12 (60.0) | 6 (37.5) | 3 (25.0) |
| Asian | 1 (5.6) | 0 | 0 | 0 |
| Multirace | 0 | 0 | 1 (6.3) | 0 |
Data include all participants enrolled in the relevant treatment arms of the study. Values are means ± standard deviations unless otherwise noted. 2-DAA, paritaprevir/ritonavir and dasabuvir; 3D, paritaprevir/ritonavir, ombitasvir, and dasabuvir; EFV, efavirenz; FTC, emtricitabine; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate.
Effect of the 3D regimen on HIV-1 antiretroviral pharmacokinetics.
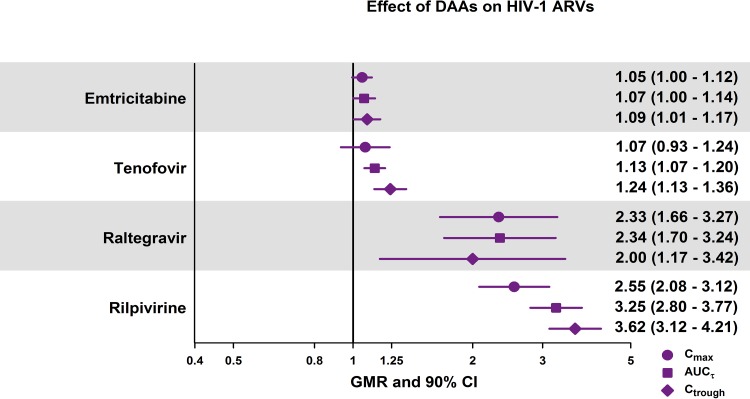
Emtricitabine exposure was not affected by coadministration of the 3D regimen; Cmax, AUCτ, and Ctrough GMRs indicated an increase of <10% during coadministration of emtricitabine and tenofovir DF with the 3D regimen (Fig. 2). Tenofovir Cmax and AUCτ values were not affected, with approximately 7% and 13% increases, respectively, but the tenofovir Ctrough was 24% higher during coadministration of emtricitabine and tenofovir DF with the 3D regimen. These minor changes were within the prespecified GMR range of 0.8 to 1.25 for Cmax and AUC and are, therefore, not expected to be clinically meaningful.
FIG 2.
Effect of the 3D regimen on the Cmax, AUCτ, and Ctrough values of HIV-1 antiretroviral drugs. AUCτ, area under the plasma concentration-time curve during a dosing interval; CI, confidence interval; Cmax, maximum observed plasma concentration; Ctrough, minimum observed plasma concentration; GMR, geometric mean ratio.
Raltegravir and rilpivirine exposures were notably elevated when these drugs were administered with the 3D regimen. The Cmax, AUCτ, and Ctrough values for raltegravir increased by 100% to 134%. GMRs for the pharmacokinetic parameters of rilpivirine were in the range of 2.55 to 3.62 for the comparison of rilpivirine plus the 3D regimen versus rilpivirine alone, indicating a 155% to 262% increase in rilpivirine exposure.
Effect of HIV-1 antiretroviral drugs on 3D regimen pharmacokinetics.
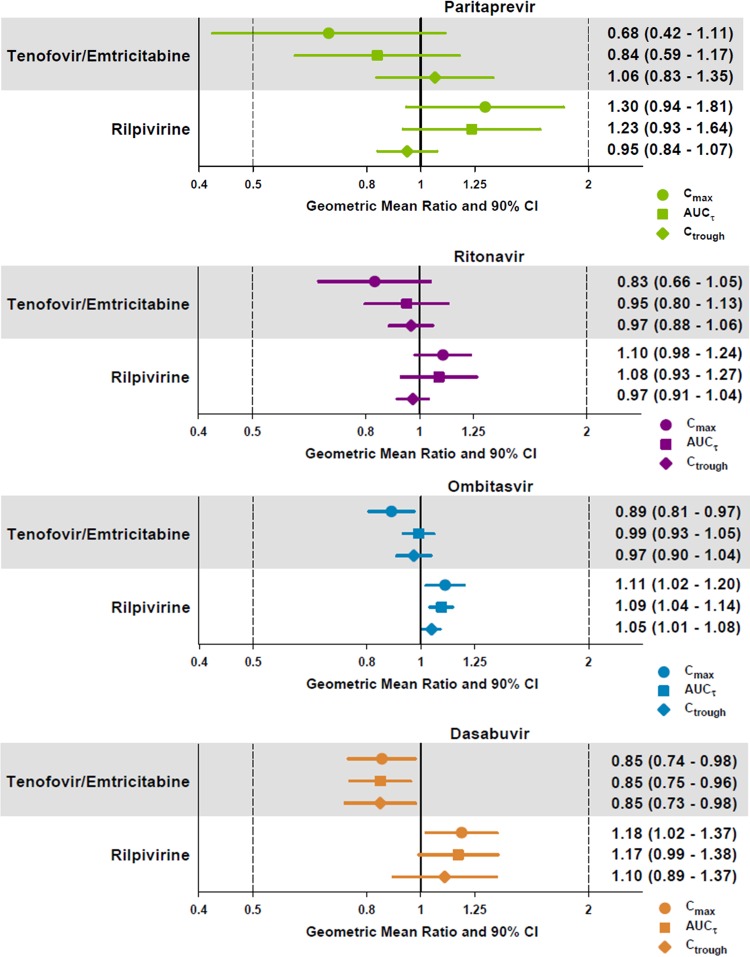
Paritaprevir AUC and Ctrough values were minimally affected, whereas the Cmax GMR decreased by 32% when the 3D regimen was coadministered with emtricitabine plus tenofovir DF (Fig. 3). When the 3D regimen was coadministered with rilpivirine, changes in paritaprevir exposures ranged from a 5% decrease to a 30% increase. Pharmacokinetic parameters for ritonavir, ombitasvir, and dasabuvir were not affected (≤20% change) when the 3D regimen was coadministered with emtricitabine plus tenofovir DF or rilpivirine (Fig. 3).
FIG 3.
Effect of HIV-1 antiretroviral drugs on the Cmax, AUCτ, and Ctrough values of paritaprevir, ritonavir, ombitasvir, and dasabuvir. AUCτ, area under the plasma concentration-time curve during a dosing interval; CI, confidence interval; Cmax, maximum observed plasma concentration; Ctrough, minimum observed plasma concentration.
Cross-study comparisons of geometric mean AUC values indicated that exposures for paritaprevir, ritonavir, ombitasvir, and dasabuvir during coadministration of the 3D regimen with raltegravir were within the range of AUC values for the 3D regimen alone in historical phase 1 studies.
Safety and tolerability.
The most common treatment-emergent AEs (TEAEs) that occurred in the study with emtricitabine plus tenofovir DF were headache and abdominal pain (n = 2 each). All TEAEs in this study were mild in severity. No clinically significant changes in vital signs or laboratory measurements were observed.
Adverse events reported during the rilpivirine study were generally mild in severity and considered to be unrelated to study drug administration. The most common TEAEs were headache and constipation. One (5%) participant was discontinued from the rilpivirine study owing to a TEAE of increased blood creatinine phosphokinase and grade 3 aspartate aminotransferase (AST) elevation. The event was deemed unrelated to study treatment and resolved during follow-up on study day 38. Two other participants experienced isolated elevations in aminotransferases (grade 2 AST [n = 1] or alanine aminotransferase [ALT; n = 1]) that were not accompanied by a concurrent elevation in bilirubin and resolved with continued combination dosing. No clinically significant changes in vital signs or other laboratory measurements were observed.
Three participants experienced a single TEAE with concomitant administration of the 3D regimen and raltegravir. These events were mild and included dyspepsia, bilirubin (indirect) increase, and ALT increase (grade 1). All TEAEs resolved during the course of the study. No serious TEAEs occurred, and no clinically significant changes in vital signs or other laboratory measurements were reported.
Of the 16 participants enrolled in the study with efavirenz/emtricitabine/tenofovir DF, nine were prematurely discontinued owing to TEAEs (including dizziness, headache, nausea, vomiting, and aminotransferase elevations) that occurred in the second study period (on or after study day 15). Discontinuations were more frequent in cohort 2, wherein efavirenz/emtricitabine/tenofovir DF was administered prior to initiation of paritaprevir/r and dasabuvir. The determination was made to terminate the study on study day 17, 3 days after initiation of the coadministration of the 2-DAA regimen and efavirenz/emtricitabine/tenofovir DF, based on the AE profile. No serious TEAEs were reported; however, one participant experienced a severe TEAE (severe nausea, vomiting, and feeling abnormal), and three participants experienced grade 3 or 4 aminotransferase elevations. All reported events resolved without sequelae after the discontinuation of study medication.
DISCUSSION
This series of DDI studies indicates that HCV therapy with the 3D regimen is compatible with several commonly prescribed ARV agents. Based on drug exposure data, combining the 3D regimen with emtricitabine plus tenofovir DF or with raltegravir BID dosing should not require dose adjustment. The 3D regimen had minimal to no effect on the pharmacokinetic profile of emtricitabine or tenofovir, and exposures to 3D regimen components were not affected or only minimally influenced by emtricitabine plus tenofovir DF or raltegravir. The greatest change in 3D component exposure in the presence of emtricitabine and tenofovir DF occurred with paritaprevir, for which the Cmax decreased by 32%. Paritaprevir, dasabuvir, and ombitasvir at doses of 100 to 250 mg administered QD, 300 to 800 mg administered BID, and 1.5 to 200 mg administered QD, respectively, provided comparable efficacies and were well tolerated in phase 2 studies (20–24; I. A. Gaultier, D. E. Cohen, E. O. Dumas, L. M. Larsen, T. J. Podsadecki, and B. Bernstein, presented at the 21st Conference of the Asian Pacific Association for the Study of the Liver, 17 to 20 February, 2011, Bangkok, Thailand). These DAA doses used in phase 2 studies, which produce exposures up to 55% lower and up to 250% higher than those used as part of the 3D regimen, along with the exposure-response relationship, suggest that exposure increases of 100% (2×) or decreases of 50% (0.5×) do not necessitate dose adjustment (10, 25).
Although raltegravir exposure increased by 100% to 134% during 3D regimen coadministration, this elevation is not expected to be clinically important owing to the wide safety margin of raltegravir. In the U.S. Food and Drug Administration summary review of raltegravir's new drug application, an increase by as much as 2-fold in raltegravir AUC and a decrease of 60% in Ctrough were not considered clinically relevant based on the results of phase 2 and 3 clinical trials (26). Moreover, data from a DDI study of raltegravir with gastric pH-altering agents demonstrated greater increases in raltegravir exposure (e.g., 315% and 212% in Cmax and AUC, respectively) than observed during 3D regimen treatment, yet coadministration of gastric pH-altering agents in phase 3 studies of raltegravir revealed no unique safety signals (27). Because the increase in raltegravir exposure during its coadministration with the 3D regimen is within the safety margin for raltegravir established in phase 2 and 3 clinical trials, no dose adjustment for raltegravir is anticipated to be necessary during coadministration with the 3D regimen.
The influence of raltegravir on DAA pharmacokinetics was not evaluated directly as raltegravir does not inhibit or induce metabolic enzymes or drug transporters known to affect the disposition of paritaprevir, ritonavir, ombitasvir, or dasabuvir. Cross-study comparisons with previous phase 1 study data affirmed that DAA pharmacokinetics were within the expected range during coadministration of raltegravir with the 3D regimen.
Coadministration of the 3D regimen with rilpivirine is not recommended. Addition of the 3D regimen to steady-state rilpivirine resulted in a greater than 2-fold increase in rilpivirine exposure. Alternate dosing regimens of rilpivirine with the 3D regimen, including administration of rilpivirine in the evening after an evening snack or at night approximately 4 h after dinner, have also been tested with comparable results. Results from previous studies of rilpivirine suggest that increases in rilpivirine exposure could result in corrected QT (QTc) prolongation; therefore, coadministration of any drugs that appreciably increase the exposure of rilpivirine is not recommended (16).
Coadministration of the 3D regimen with efavirenz-containing ARV regimens is contraindicated. When the 2-DAA regimen of paritaprevir/r and dasabuvir was coadministered with efavirenz/emtricitabine/tenofovir DF at 600/200/300 mg QD, respectively, 9 of 16 patients were discontinued from the study owing to AEs. These safety findings were attributed to efavirenz, a CYP3A inducer, as coadministration of the 3D regimen with emtricitabine and tenofovir DF was well tolerated in healthy volunteers. Similar safety findings were observed when the CYP3A inducer rifampin or efavirenz was coadministered with ritonavir-boosted protease inhibitors such as lopinavir and saquinavir in healthy volunteers (28–30). Patients treated with these therapy combinations reported AEs consistent with our observations, including abdominal symptoms and transaminase elevations.
Coadministration of the 3D regimen with emtricitabine plus tenofovir DF, raltegravir, or rilpivirine for 7 to 14 days was generally well tolerated in these phase 1 DDI studies. Multiple-dose administration did not reveal new safety findings of concern in any study.
Additional studies have evaluated and are exploring potential DDIs between the 3D regimen and other commonly prescribed HIV-1 medications, including atazanavir, darunavir, lopinavir/ritonavir, dolutegravir, and abacavir/lamivudine. Moreover, coadministration of the 3D regimen plus ribavirin with the ARV drugs tenofovir DF, emtricitabine, atazanavir, or raltegravir is being investigated in a phase 2/3 study (Turquoise-I) that enrolled patients with genotype 1 chronic HCV and HIV-1 coinfection. After 12 weeks of treatment with 3D plus ribavirin in part 1a of Turquoise-I, 94% (29/31; 95% CI, 79% to 98%) of patients achieved SVR12, no serious TEAEs were reported, and no patients prematurely discontinued study treatment owing to an AE (31). Patients on stable darunavir-containing ARV regimens are being evaluated in the ongoing part 1b of the study.
Despite the robust study designs used herein, one possible limitation was the enrollment of healthy volunteers. Greater variability is seen in the real world or clinical trial setting than in pharmacokinetic studies with healthy volunteers owing to the effects of sex, ethnicity, age, medication adherence, body weight/BMI, genetic background, comorbidities, and concomitant medications. Patients who are coinfected with HIV and HCV represent the target population for 3D/ARV combination therapy; however, the evaluation of DDIs in this patient population is not only challenging due to the unpredictable relationship between population dose response and individual concentration response but also because a clinically significant DDI could put these patients at risk for treatment failure or AEs. Nonetheless, results from the Turquoise-I study do not indicate differences in treatment responses or a notable elevation in the risk for adverse drug reactions with combination 3D/ARV therapy in the target population (31). Other factors that may affect real-world applicability of our results include the small number of subjects, limited duration of the studies, and restrictions on concomitant medications. With regard to the latter, phase 3 studies in which patients were allowed wider lenience in terms of concomitant medications have not yielded any interactions of concern (R. Menon, P. Badri, A. Khatri, T. Wang, D. Bow, A. Polepally, T. Podsadecki, W. Awni, and S. Dutta, presented at the 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy, Washington, DC, 19 to 21 May 2014).
In conclusion, the interferon-free 3D regimen may be appropriate for a broad range of patients with HCV infection, including those with HIV. The low risk for clinically significant DDIs when the 3D regimen is coadministered with the ARV agent emtricitabine, tenofovir DF, or raltegravir makes it a potentially viable treatment option for patients who are coinfected with HCV and HIV.
ACKNOWLEDGMENTS
We thank the study investigators, subjects, clinical sites, AbbVie personnel, including Lillian Lee, Natalie Hycner, Bifeng Ding, Xia Wan, and Jun Zhang, for their contribution to various aspects of the studies, and Rosemary Reinke and Crystal Murcia of the JB Ashtin Group, Inc., for medical writing support.
AbbVie contributed to the study design, research, interpretation of data, writing, reviewing, and approval for publication. All authors are AbbVie employees and may hold AbbVie stocks or options.
Funding Statement
These studies were sponsored and supported by AbbVie, Inc.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2014. HIV and viral hepatitis fact sheet. Centers for Disease Control and Prevention Atlanta, GA. [Google Scholar]
- 2.Monga HK, Rodriguez-Barradas MC, Breaux K, Khattak K, Troisi CL, Velez M, Yoffe B. 2001. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis 33:240–247. doi: 10.1086/321819. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LE, Swan T, Matthews GV. 2013. Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral-based therapy. Clin Infect Dis 57(Suppl 2):S118–S124. doi: 10.1093/cid/cit326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Colominas E, Broquetas T, Retamero A, Garcia-Retortillo M, Canete N, Coll S, Pellicer R, Gimenez MD, Cabrero B, Bory F, Knobel H, Salas E, Sola R, Carrion JA. 2015. Drug-drug interactions of telaprevir and boceprevir in HCV-monoinfected and HIV/HCV-coinfected patients can modify the adherence. Liver Int 35:1557–1565. doi: 10.1111/liv.12729. [DOI] [PubMed] [Google Scholar]
- 5.Gilead Sciences, Inc. 2015. Harvoni prescribing information. Gilead Sciences, Inc., Forest City, CA. https://www.gilead.com/∼/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. [Google Scholar]
- 6.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Mullhaupt B, Horsmans Y, Weiland O, Reesink HW, Rodrigues L Jr, Hu YB, Podsadecki T, Bernstein B. 2014. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 147:359–365.e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 7.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR. 2014. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 8.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, Forns X, Lovell SS, Da Silva-Tillmann B, Collins CA, Campbell AL, Podsadecki T, Bernstein B. 2014. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 9.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. 2014. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 10.Menon R, Badri P, Wang T, Polepally A, Zha J, Khatri A, Wang H, Hu B, Coakley E, Podsadecki T, Awni W, Dutta S. 2015. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir and dasabuvir. J Hepatol 63:20–29. doi: 10.1016/j.jhep.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 11.AbbVie, Inc. 2015. Viekira Pak prescribing information. AbbVie, Inc., North Chicago, IL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206619lbl.pdf. [Google Scholar]
- 12.Gilead Sciences, Inc. 2013. Truvada prescribing information. Gilead Sciences, Inc., Foster City, CA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021752s030lbl.pdf. [Google Scholar]
- 13.Cihlar T, Ho ES, Lin DC, Mulato AS. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641–648. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 14.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merck Sharp and Dohme Corp. 2014. Isentress prescribing information. Merck Sharp and Dohme Corp., Whitehouse Station, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022145s018lbl.pdf. [Google Scholar]
- 16.Janssen Therapeutics. 2014. Edurant prescribing information. Janssen Therapeutics, Titusville, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202022s000lbl.pdf. [Google Scholar]
- 17.Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. 2002. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther 72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 18.Bristol-Myers Squibb Pharmaceutical, Ltd. 2015. Sustiva prescribing information. Bristol-Myers Squibb Pharmaceutical, Ltd., Uxbridge, United Kingdom. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202022s000lbl.pdf.
- 19.Polepally AR, Dutta S, Hu B, Podsadecki TJ, Awni WM, Menon RM. 24 January 2016. Drug-drug interaction of omeprazole with the HCV direct-acting antiviral agents paritaprevir/ritonavir and ombitasvir with and without dasabuvir. Clin Pharmacol Drug Develop doi: 10.1002/cpdd.246. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Torres M, Lawitz E, Cohen D, Larsen LM, Menon R, Collins C, Marsh T, Gibbs S, Bernstein B. 2009. Treatment-naïve, HCV genotype 1-infected subjects show significantly greater HCV RNA decreases when treated with 28 days of ABT-333 plus peginterferon and ribavirin compared to peginterferon and ribavirin alone. Hepatology 50(Suppl 4):91A. [Google Scholar]
- 21.Sullivan GJ, Rodrigues-Torres M, Lawitz E, Poordad F, Kapoor M, Campbell A, Setze C, Xie W, Khatri A, Dumas E, Krishnan P, Pilot-Matias T, Williams L, Bernstein B. 2012. ABT-267 combined with pegylated interferon alpha-2a/ribavirin in genotype 1 (GT1) HCV-infected treatment-naive subjects: 12 week antiviral and safety analysis. J Hepatol 56:S480. doi: 10.1016/S0168-8278(12)61222-7. [DOI] [Google Scholar]
- 22.Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Heckaman M, Larsen L, Menon R, Koev G, Tripathi R, Pilot-Matias T, Bernstein B. 2013. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 23.Epstein M, Felizarta F, Marbury T, Badri P, Mullally V, Pilot-Matias T, Liu X, Setze CM, Campbell AL, Bernstein B. 2013. Study of ABT-267 2-day monotherapy followed by 12-week combination therapy in treatment-naive patients with chronic HCV genotype 1 infection. J Hepatol 58:S484. doi: 10.1016/S0168-8278(13)61191-5. [DOI] [Google Scholar]
- 24.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski MS, Xie W, Pilot-Matias T, Liossis G, Larsen L, Khatri A, Podsadecki T, Bernstein B. 2014. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med 370:222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 25.Center for Drug Evaluation and Research. 2014. Summary review for regulatory action, NDA 206619. Food and Drug Administration, Rockville, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206619Orig1s000SumR.pdf. [Google Scholar]
- 26.Center for Drug Evaluation and Research. 2007. Decisional review for NDA 22-128. Food and Drug Administration, Rockville, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022145_SumR.pdf. [Google Scholar]
- 27.Iwamoto M, Wenning LA, Nguyen BY, Teppler H, Moreau AR, Rhodes RR, Hanley WD, Jin B, Harvey CM, Breidinger SA, Azrolan N, Farmer HF Jr, Isaacs RD, Chodakewitz JA, Stone JA, Wagner JA. 2009. Effects of omeprazole on plasma levels of raltegravir. Clin Infect Dis 48:489–492. doi: 10.1086/596503. [DOI] [PubMed] [Google Scholar]
- 28.Nijland HM, L'homme RF, Rongen GA, van Uden P, van Crevel R, Boeree MJ, Aarnoutse RE, Koopmans PP, Burger DM. 2008. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS 22:931–935. doi: 10.1097/QAD.0b013e3282faa71e. [DOI] [PubMed] [Google Scholar]
- 29.Jamois C, Riek M, Schmitt C. 2009. Potential Hepatotoxicity of efavirenz and saquinavir/ritonavir coadministration in healthy volunteers. Arch Drug Inf 2:1–7. doi: 10.1111/j.1753-5174.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt C, Riek M, Winters K, Schutz M, Grange S. 2009. Unexpected hepatotoxicity of rifampin and saquinavir/ritonavir in healthy male volunteers. Arch Drug Inf 2:8–16. doi: 10.1111/j.1753-5174.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyles DL, Sulkowski MS, Eron JJ, Trinh R, Lalezari J, Slim J, Gathe JC, Wang CC, Elion R, Bredeek F, Brennan RO, Blick G, Khatri A, Gibbons K, Hu Y, Fredrick L, Pilot-Matias T, Da Silva-Tillmann B, McGovern B, Campbell AL, Podsadecki T. 2014. TURQUOISE-I: 94% SVR12 in HCV/HIV-1 coinfected patients treated with ABT-450/r/ombitasvir, dasabuvir and ribavirin. Hepatology 60:1136A.24668800 [Google Scholar]