Abstract
We tested the effects of various putative efflux pump inhibitors on colistin resistance in multidrug-resistant Gram-negative bacteria. Addition of 10 mg/liter cyanide 3-chlorophenylhydrazone (CCCP) to the test medium could significantly decrease the MICs of colistin-resistant strains. Time-kill assays showed CCCP could reverse colistin resistance and inhibit the regrowth of the resistant subpopulation, especially in Acinetobacter baumannii and Stenotrophomonas maltophilia. These results suggest colistin resistance in Gram-negative bacteria can be suppressed and reversed by CCCP.
TEXT
Colistin is regarded as the last resort antibiotic for infections caused by multidrug-resistant (MDR) and extensively drug-resistant (XDR) Gram-negative bacteria. However due to colistin's widespread use, resistant strains are increasingly being isolated in the clinic (1). Resistance to colistin has been reported to be only chromosomally mediated. However, very recently, the finding of a transferable plasmid-mediated colistin resistance gene, mcr-1, suggests the possibility of quick acquisition of resistance (2, 3). If the mcr-1 gene is similar to the case of NDM-1, colistin-resistant bacteria may soon become endemic in the world.
Therefore, it is of paramount importance to prevent the dissemination of colistin resistance in an era that lacks new antibiotics against resistant Gram-negative pathogens. Besides, the finding and development of agents that effectively reverse resistance may be a promising strategy. Efflux pump inhibitors (EPIs) are potential agents in this category, and there have been initial reports on EPIs. A recent study showed that the addition of cyanide 3-chlorophenylhydrazone (CCCP) could decrease the MICs of colistin in Acinetobacter baumannii (4), but whether this phenomenon is strain specific or CCCP can reverse colistin resistance in Gram-negative bacteria deserves further investigation, and whether other commonly used EPIs have the same effects remains unknown. In order to answer these questions, we evaluated the effect of various putative EPIs on resistance to colistin in multidrug-resistant Gram-negative bacteria.
Nonduplicate colistin-susceptible and colistin-resistant clinical isolates of Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia that also show resistance to at least other three antibiotic classes were collected between May 2014 and October 2015 from separate patients in three tertiary hospitals of Beijing, China. Colistin, CCCP, phenyl-arginine-β-naphthylamide (PAβN), 1-(1-naphthylmethyl)-piperazine (NMP), omeprazole, and verapamil standards were purchased from Sigma-Aldrich (Shanghai, China). Reserpine standards were obtained from the National Institute for the Control of Pharmaceutical and Biological Products, China (Beijing, China).
MICs of EPIs, colistin, and colistin combined with EPIs were determined by the agar dilution method according to CLSI performance and interpretive guidelines (5). CCCP, NMP, and omeprazole were dissolved in dimethyl sulfoxide (DMSO) and prepared as a 5-mg/ml stock solution, PAβN was dissolved in sterile water and prepared as a 1.5-mg/ml stock solution, verapamil was dissolved in MeOH and prepared as a 50-mg/ml stock solution, and reserpine was dissolved in chloroform and prepared as a 1.5-mg/ml stock solution. Then Mueller-Hinton agar (MHA) plates containing a series of 2-fold-concentration increments of EPIs, colistin, or colistin in combination with either CCCP (10 mg/liter), PAβN (25 mg/liter), NMP (25 mg/liter), omeprazole (20 mg/liter), verapamil (80 mg/liter), or reserpine (20 mg/liter) were freshly prepared. Strains were thawed and inoculated on agar plates with 5% sheep blood and incubated for 18 to 24 h at 35°C. Subsequently, drug plates were inoculated with a suspension of a 0.5 McFarland standard. The plates were incubated for 18 to 24 h at 35°C in ambient air before values were read. All MIC tests were carried out in triplicate on separate days.
To confirm the effect of EPIs on reversing colistin resistance, we randomly selected one isolate of each bacterial species to perform in vitro time-kill assays of colistin in the presence or absence of EPIs. In brief, strains were grown overnight in Mueller-Hinton II broth (MHB) medium. Then stationary-phase cells were reinoculated into fresh MHB containing 3 mg/liter of colistin with or without EPIs (at the same concentrations used in colistin MIC tests) to yield a density of ∼5 × 105 CFU/ml. The cultures were incubated in a shaking bath at 35°C for 48 h. Samples were obtained at times zero, 3, 6, 12, 24, 36, and 48 h after inoculation and serially diluted in sterile 0.85% sodium chloride solution for determination of viable counts. The diluted samples, in 0.05-ml aliquots, were plated in duplicate on MHA plates. After the diluted samples were incubated at 35°C for 24 h in ambient air, formed colonies were counted, and the total bacterial log10 CFU per milliliter of the original sample was calculated. All of the in vitro time-kill experiments were performed in triplicate on different days. The statistical analysis was conducted using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA). Results are expressed as means ± standard deviation (SD).
Table 1 shows the MIC range of EPIs for each bacterial species. The MICs of CCCP for various isolates were lower than those of other EPIs. Moreover, compared with colistin-susceptible K. pneumoniae and S. maltophilia strains, the MICs of colistin-resistant strains were relatively lower. However, generally, all EPIs had no intrinsic inhibitory activity against clinical isolates at the concentrations used for the colistin MIC and time-kill assays. Table 2 shows the MIC range and the reduction in MICs of colistin for each species after the addition of EPIs. Particularly strong effects with CCCP were seen in colistin-resistant strains of all species. Besides, the addition of CCCP slightly reduced the MICs (MIC of ≤2 mg/liter) in colistin-susceptible strains of S. maltophilia. NMP had limited effects on MICs in both colistin-susceptible and colistin-resistant strains of S. maltophilia. In addition, PAβN could reduce the MICs by 4-fold or more in colistin-susceptible strains of P. aeruginosa, while having no effects on MICs in colistin-resistant strains. For the other three EPIs, no or minor effects on the MICs were observed, no matter whether in colistin-susceptible or colistin-resistant strains.
TABLE 1.
Intrinsic inhibitory effects of different EPIs on clinical isolates of 4 species of MDR Gram-negative bacteria
| Colistin susceptibility/resistance by species (n isolates) | MIC range (mg/liter) |
|||||
|---|---|---|---|---|---|---|
| CCCP | NMP | PAβN | Omeprazole | Verapamil | Reserpine | |
| Susceptible | ||||||
| A. baumannii (20) | 40–80 | 250–>250 | >200 | >100 | >400 | >100 |
| K. pneumoniae (20) | 100–>100 | >250 | >200 | >100 | >400 | >100 |
| P. aeruginosa (26) | >100 | >250 | >200 | >100 | >400 | >100 |
| S. maltophilia (7) | 40–100 | >250 | >200 | >100 | >400 | >100 |
| Resistant | ||||||
| A. baumannii (4) | 40–80 | >250 | >200 | >100 | >400 | >100 |
| K. pneumoniae (6) | 50–80 | >250 | >200 | >100 | >400 | >100 |
| P. aeruginosa (5) | >100 | >250 | >200 | >100 | >400 | >100 |
| S. maltophilia (16) | 30–80 | >250 | >200 | >100 | >400 | >100 |
TABLE 2.
Effects of different EPIs on the MICs of colistin in clinical isolates of 4 species of MDR Gram-negative pathogens
| Colistin susceptibility/resistance by species (n isolates) | MIC range (mg/liter) | MIC range in mg/liter (fold MIC reduction) after addition ofa: |
|||||
|---|---|---|---|---|---|---|---|
| CCCP | NMP | PAβN | Omeprazole | Verapamil | Reserpine | ||
| Susceptible | |||||||
| A. baumannii (20) | 0.25–1 | 0.25–1 (0–1) | 0.25–1 (0–1) | 0.25–1 (0) | 0.25–1 (0) | 0.25–1 (0) | 0.25–1 (0) |
| K. pneumoniae (20) | 0.125–1 | 0.125–1 (0–1) | 0.125–1 (0–1) | 0.125–1 (0) | 0.125–1 (0) | 0.125–1 (0) | 0.125–1 (0) |
| P. aeruginosa (26) | 0.5–2 | 0.5–2 (0) | 0.5–2 (0) | 0.03–0.25 (4–16) | 0.5–2 (0) | 0.5–2 (0) | 0.5–2 (0) |
| S. maltophilia (7) | 0.5–2 | 0.125–0.5 (1–4) | 0.25–1 (1–4) | 0.5–2 (0) | 0.5–2 (0) | 0.5–2 (0) | 0.5–2 (0) |
| Resistant | |||||||
| A. baumannii (4) | 64–256 | 0.25–0.5 (128–512) | 64–256 (0–1) | 64–128 (0–1) | 64–256 (0) | 64–256 (0) | 64–256 (0) |
| K. pneumoniae (6) | 64–256 | 0.25–1 (128–512) | 64–256 (0) | 64–256 (0) | 64–256 (0) | 64–256 (0–1) | 32–128 (0–1) |
| P. aeruginosa (5) | 16–64 | 0.5–2 (16–32) | 16–64 (0) | 16–64 (0) | 16–64 (0) | 16–64 (0) | 16–64 (0) |
| S. maltophilia (16) | 16–256 | 0.125–1 (64–1,024) | 1–128 (1–16) | 16–256 (0) | 8–256 (0–1) | 16–256 (0–1) | 4–256 (0–4) |
Particularly strong effects are highlighted in boldface.
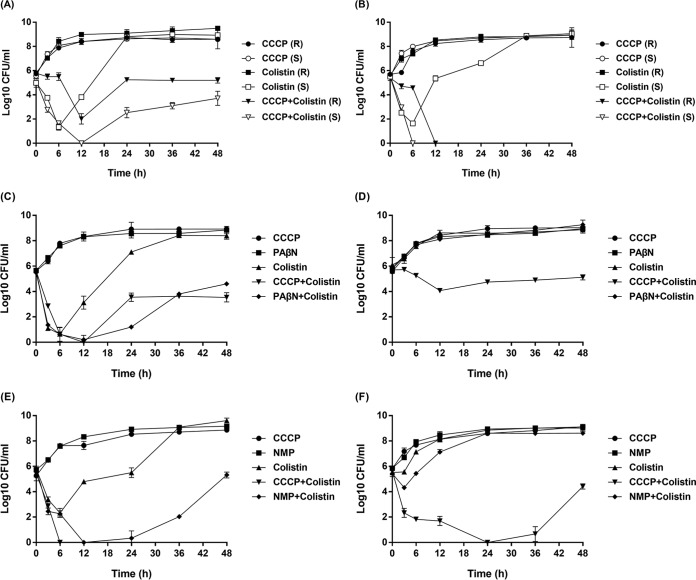
The results of in vitro time-kill assays are displayed in Fig. 1. For all colistin-susceptible strains, bacterial cell counts were reduced in the first 6 h of testing when they were exposed to colistin alone, but then regrowth was observed. After exposure to both colistin and CCCP, the bacterial cell counts of colistin-susceptible A. baumannii and S. maltophilia strains fell below the lower threshold of detection at 6 h and remained so throughout the 48-h testing period. The bacterial cell counts of colistin-susceptible K. pneumoniae and P. aeruginosa strains also fell below the lower threshold of detection at 12 h, but regrowth occurred. For colistin-resistant A. baumannii strains, a single regimen of colistin showed no inhibitory effects on bacterial growth, while the addition of CCCP could completely suppress the growth. For colistin-resistant K. pneumoniae, P. aeruginosa, and S. maltophilia strains, reduction of bacterial cell numbers was observed after exposure to colistin and CCCP, but various degrees of regrowth were observed, and the effects of CCCP on P. aeruginosa were less pronounced, which was in accordance with the results of MIC tests. Besides, the addition of PAβN displayed inhibitory effects on colistin-susceptible P. aeruginosa strains but had no effects on the colistin-resistant strain. The effects of NMP on S. maltophilia were similar to those of PAβN for P. aeruginosa. After the addition of NMP, the bacterial cell counts of colistin-susceptible S. maltophilia strains could fall below the lower threshold of detection at 12 h, while the cell counts of colistin-resistant S. maltophilia strains were only slightly reduced in the first 3 h of testing, indicating the negligible inhibitory effects.
FIG 1.
In vitro time-kill curves of colistin (3 mg/liter) alone and combined with EPIs against colistin-susceptible and colistin-resistant MDR Gram-negative bacteria. (A) Colistin-resistant K. pneumoniae (R) with MICcolistin of 256 mg/liter and MICcolistin+CCCP of 1 mg/liter and colistin-susceptible K. pneumoniae (S) with MICcolistin of 0.25 mg/liter and MICcolistin+CCCP of 0.25 mg/liter. (B) Colistin-resistant A. baumannii (R) with MICcolistin of 128 mg/liter and MICcolistin+CCCP of 0.25 mg/liter and colistin-susceptible A. baumannii (S) with MICcolistin of 0.5 mg/liter and MICcolistin+CCCP of 0.5 mg/liter. (C) Colistin-susceptible P. aeruginosa with MICcolistin of 1 mg/liter, MICcolistin+CCCP of 1 mg/liter, and MICcolistin+PAβN of 0.25 mg/liter. (D) Colistin-resistant P. aeruginosa with MICcolistin of 64 mg/liter, MICcolistin+CCCP of 2 mg/liter, and MICcolistin+PAβN of 64 mg/liter. (E) Colistin-susceptible S. maltophilia with MICcolistin of 1 mg/liter, MICcolistin+CCCP of 0.5 mg/liter, and MICcolistin+NMP of 0.5 mg/liter. (F) Colistin-resistant S. maltophilia with MICcolistin of 64 mg/liter, MICcolistin+CCCP of 0.5 mg/liter, and MICcolistin+NMP of 8 mg/liter.
Our in vitro studies showed that the addition of low-dose CCCP to the test medium could significantly decrease the MICs of colistin-resistant strains and partially or completely inhibit the regrowth of the resistant subpopulation during the time-kill assays. Among other EPIs, only PAβN and NMP could partially inhibit the regrowth of the resistant subpopulation in P. aeruginosa and S. maltophilia, respectively. These findings suggest CCCP can suppress and reverse colistin resistance in MDR Gram-negative bacteria, especially in A. baumannii and S. maltophilia. CCCP is an uncoupler of oxidative phosphorylation, which can disrupt the proton gradient of the bacterial membranes (6). In addition, colistin with a positive charge targets lipopolysaccharide (LPS) of the membranes, displaces Mg2+ or Ca2+, and binds the lipid A component, resulting in the destabilization and disruption of the bacterial membrane (7). The most common mechanism of colistin resistance is its decreased binding to the bacterial outer membrane because of the decreased negative charge by LPS modifications (8). Therefore, CCCP may restore the negative charge of the membranes through the disruption of proton motive force. Nevertheless, the concrete mechanisms by which CCCP influences colistin resistance in Gram-negative bacteria require further investigation.
In summary, colistin resistance of Gram-negative bacteria can be suppressed and reversed by CCCP. Although CCCP cannot be directly applied in the clinic due to its intrinsic cytotoxicity, the results of our study point out the way for the development of new agents to protect and potentiate the activity of colistin.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 2.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 18 November 2015. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 3.Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, Cavaco LM, Hansen DS, Aarestrup FM, Skov RL. 10 December 2015. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 4.Park YK, Ko KS. 2015. Effect of carbonyl cyanide 3-chlorophenylhydrazone (CCCP) on killing Acinetobacter baumannii by colistin. J Microbiol 53:53–59. doi: 10.1007/s12275-015-4498-5. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement (M100-S25). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Mitchell P. 2011. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta 1807:1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Rafailidis PI, Matthaiou DK. 2010. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat 13:132–138. doi: 10.1016/j.drup.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]