Abstract
This study investigated the ability of the probiotic Bifidobacterium breve strain Yakult (BbY) to protect against infection, as well as the potentiation of BbY activity by the synbiotic combination of BbY and prebiotic galactooligosaccharides (GOS). The study employed a mouse model of lethal intestinal multidrug-resistant Acinetobacter baumannii (MDRAb) infection. The endogenous intestinal microbiota was disrupted by the administration of multiple antibiotics, causing the loss of endogenous Bifidobacterium. Oral infection of these mice with MDRAb resulted in marked growth of this organism. Additional treatment of the infected mice with a sublethal dose of 5-fluorouracil (5-FU) induced systemic invasion by MDRAb and subsequent animal death. The continuous oral administration of BbY increased the survival rate and inhibited the intestinal growth and invasion by MDRAb in the infection model. Disruptions of the intestinal environment and barrier function in the infected mice were attenuated by BbY. Protection against the MDRAb infection was markedly potentiated by a synbiotic combination of BbY and GOS, although GOS by itself did not provide protection. Negative correlations were observed between intestinal MDRAb and BbY counts or acetic acid levels; positive correlations were observed between acetic acid levels and intestinal epithelium expression of tight-junction-related genes. These results demonstrated that the probiotic and synbiotic markedly potentiated protection against fatal intestinal infection caused by a multidrug-resistant bacterium. Probiotics and synbiotics are presumed to provide protection by compensation for the disrupted indigenous populations, thereby maintaining the intestinal environments and barrier functions otherwise targeted during opportunistic infection by MDRAb.
INTRODUCTION
Infections caused by multidrug-resistant bacteria are currently regarded as an important issue worldwide. Hospital infections by multidrug-resistant Acinetobacter baumannii (MDRAb) represent a serious threat for immunocompromised hosts, including patients in intensive care units (ICUs) and those who have undergone surgery (1–6). Colonization of the digestive tract in ICU patients is an important epidemiological reservoir for MDRAb infections in hospital outbreaks (7).
Extensive efforts have been made to eliminate multidrug-resistant pathogens, including the concomitant administration of peptide antibiotics (including colistin) and β-lactamase inhibitors with β-lactam antibiotics, and the development of novel antibiotics (1). Nonetheless, sufficiently effective measures are not yet available, and alternative approaches to antibiotics continue to be sought.
Probiotics have been defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (8). Anaerobic bifidobacteria previously were reported to be useful in the treatment of a disturbed intestinal microbiota and diarrheal diseases (9). Prebiotics have been defined as nondigestive food constituents that selectively alter the growth and/or activity of one or a limited number of bacteria in the colon, thereby potentially improving the health of the host (10, 11). The combined use of probiotics and prebiotics is called synbiotics (11).
In the present study, to investigate a synbiotic strategy for prophylaxis of antimicrobial-induced dysbiosis in immunocompromised hosts, we employed a mouse model of a lethal intestinal MDRAb infection under treatment with multiple antibiotics to examine the protective ability of Bifidobacterium breve strain Yakult (BbY), with and without synbiotic potentiation, against MDRAb infection.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 6-week-old male BALB/c mice were purchased from Charles River Japan, Inc. (Kanagawa, Japan). Animals were house at 5 or 6 per cage in polypropylene cages (CLEA Japan, Tokyo, Japan) containing sterilized bedding. Cages were placed in individual isolator units that were air-conditioned with a HEPA filter, and the cages were maintained under controlled lighting (12-h light/12-h dark cycle), temperature (24°C), and relative humidity (55%) conditions. Mice (16 per group) were provided with ad libitum access to MF Diet chow (Oriental Yeast, Tokyo) and sterilized (126°C for 30 min) water containing Cl2 at a final concentration of 1.5 ppm. Kanamycin sulfate (KM; Sigma Chemical, St. Louis, MO), metronidazole (MTN; Sigma), cefotiam (CTM; Takeda Pharmaceutical, Osaka, Japan), and lomefloxacin (LOM; Sigma) were dissolved in the drinking water at concentrations of 1 mg/ml, 0.2 mg/ml, 0.1 mg/ml, and 0.01 mg/ml, respectively. Water bottles were exchanged with freshly prepared bottles every 3 days. In addition, 0.025 mg/kg (of body weight) of imipenem-cilastatin (IPM, Banyu Pharmaceutical, Tokyo, Japan) was administered intraperitoneally every 2 days. All experimental procedures were performed in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals (12). All animal use procedures were approved by the Institutional Animal Care and Use Committee of Yakult Central Institute.
Murine model of MDRAb infection.
MDRAb ATCC BAA-1799 (YIT12470) was used in the present study. This strain is resistant to KM (MIC: >512 μg/ml), MTN (MIC: >512 μg/ml), CTM (MIC: >512 μg/ml), LOM (MIC: >512 μg/ml), and IPM (MIC: 128 μg/ml). MDRAb was cultured overnight at 30°C in Trypticase soy broth (BD Diagnostic Systems, Sparks, MD). After washing with sterile phosphate-buffered saline (PBS, pH 7.3) by centrifugation, MDRAb cells were resuspended in PBS and adjusted to approximately 1 × 105 CFU/ml. This suspension then was administered at 100 μl (104 CFU) per mouse by oral gavage using a gastric sonde (Fuchigami Kikai, Kyoto, Japan); infection was performed on nominal day 0, corresponding to 7 days after starting treatment with KM, MTN, CTM, LOM, and IPM. Antibiotics were administered to mice from day −7 until day 28. On day 4 after the MDRAb infection, animals were injected intraperitoneally with 5-fluorouracil (5-FU; Kyowa Hakko Kogyo, Tokyo, Japan) at a dose of 400 mg/kg of body weight.
Six Somnopentyl (Kyoritsuseiyaku Co. Tokyo)-anesthetized mice per group per period were killed by cervical dislocation. In order to assess MDRAb viable counts in various body compartments (including feces, cecal contents, blood, liver, and mesenteric lymph nodes [MLNs]), samples were removed aseptically from mice and then homogenized in 1 ml (5 ml for the liver) of sterile PBS at pH 7.3 using a Teflon grinder. Viable counts of MDRAb were determined using a selective medium consisting of a 1:1 mixture of DHL agar (Nissui Pharmaceutical, Tokyo, Japan) and Trypticase soy agar (BD Diagnostic Systems) supplemented with 10 μg/ml of ceftriaxone (CTRX; Sigma Chemical). The media were cultured aerobically at 37°C for 24 h, and the colonies on the plates were counted. Colonies that grew on the selective medium were identified as MDRAb by a PCR method using OXA-51-like gene-specific primers (13). In vivo invasion by bacteria other than MDRAb was investigated by plating aliquots of the same specimens to brucella agar medium containing 5% hemolyzed rabbit blood (a nonselective medium used to detect obligate anaerobes; Nikken Bio Medical Laboratory Inc., Tokyo, Japan) and to Trypticase soy agar medium containing 5% sheep blood agar (a nonselective medium used to detect facultative anaerobes and aerobes; Nikken Bio Medical Laboratory Inc.). The lower limit of bacterial detection with this procedure was 100 CFU/g for feces, cecal contents, liver, and MLNs or 1 CFU/ml for blood.
Probiotics.
Bifidobacterium breve strain Yakult (YIT12272; BbY) was used as the probiotic bacterial strain (14). BbY is resistant to KM (MIC: 256 μg/ml), MTN (MIC: 32 μg/ml), CTM (MIC: >512 μg/ml), LOM (MIC: 128 μg/ml), and IPM (MIC: 8 μg/ml). BbY was cultivated in GAM broth (Nissui Pharmaceutical, Tokyo, Japan) at 37°C for 24 h and washed twice with PBS at pH 7.3. Cells then were suspended in PBS at pH 7.3 to a concentration of 109 CFU/ml. Colonization by BbY was established by once-daily oral gavage administration of freshly grown probiotics (1 to 3 × 108 CFU/mouse/day) from day −7 until day 28 in mice being treated with antibiotics in their drinking water. Periodic determinations of the viable counts of BbY in stool specimens were performed in subsets of 6 mice from each group. Briefly, fresh stool specimens (within 30 s after a bowel movement, 1 or 2 pellets/mouse) were recovered, weighed, placed in a Microfuge tube containing 1 ml of sterilized anaerobic buffer solution (15) and then homogenized with a pestle. Aliquots of the homogenates were plated to T-CBPC agar (15). Plates were cultured anaerobically in an atmosphere of 7% H2 and 5% CO2 in N2 at 37°C for 72 h, and the resulting colonies were counted. Colonies grown on T-CBPC agar were confirmed to be BbY by a PCR method using strain-specific primers (16).
Prebiotic and synbiotic.
Galactooligosaccharides (GOS; purity, >99%; Yakult Pharmaceutical Industry, Tokyo, Japan) were prepared as described previously (17). GOS were dissolved at 100 mg/ml in distilled water and administered by once-daily oral gavage at 0.1 ml (10 mg) per mouse from day −7 until day 28. For the synbiotic administration, the combined BbY and GOS dose was administered by once-daily oral gavage for the same interval. The preparations of BbY and GOS were made separately and mixed immediately before administration. Saline-treated infected control animals (infected but not treated with pro-, pre-, or synbiotic) were administered (once daily, oral gavage) with an equivalent volume of saline for the same interval.
Gut microbiota analysis.
At necropsy, a portion of the cecal contents from each animal was transferred to a tared tube (76 by 20 mm; Sarstedt AG & Co., Germany) containing 2 ml of RNAlater (Ambion, Inc., Austin, TX). After the tube containing the cecal sample was weighed, a 9-fold volume of RNAlater was added to prepare a cecal suspension. An aliquot (40 μl) of the resulting cecal homogenate was diluted into 1 ml of sterilized PBS, and the mixture then was centrifuged at 5,000 × g for 10 min. The supernatant was discarded, and the pellet was stored at −80°C until pending extraction of RNA. RNA was isolated using a modification of the acid guanidinium thiocyanate-phenol-chloroform extraction method (18, 19). The nucleic acid fraction was suspended in 1 ml of nuclease-free water (Ambion). In order to quantify the bacteria present in the fecal samples, total RNA fractions from feces were extracted by the method described above. The microbiota composition was analyzed using the Yakult Intestinal Flora-SCAN (YIF-SCAN) version of a 16S rRNA-targeted reverse transcription-quantitative PCR (RT-qPCR) system (18, 19). Three serial dilutions of the extracted RNA sample were used for bacterial rRNA-targeted RT-qPCR (18, 19), and the threshold cycle values in the linear range of the assay were applied to the standard curve in order to obtain the corresponding bacterial cell count in each nucleic acid sample. These data then were used to calculate bacterial counts per sample. The specificity of the RT-qPCR assay using group-, genus-, or species-specific primers was determined as described previously (17, 19).
Detection of organic acids in cecal contents.
The concentrations of organic acids in the cecal contents were determined according to previously described methods (20), with slight modifications. Briefly, the sample was homogenized in 4 volumes of 0.15-μmol/liter perchloric acid and allowed to stand at 4°C for 12 h. The suspension was centrifuged at 20,400 × g at 4°C for 10 min. The resulting supernatant was passed through a filter with a pore size of 0.45 μm (Millipore Japan, Tokyo, Japan). The sample was analyzed for organic acids using a high-performance liquid chromatography system (432 conductivity detector; Waters Co., Milford, MA) equipped with two columns (Shodex Rspack KC-811; Showa Denko, Tokyo, Japan) (20). Organic acid concentrations were calculated using external standards. The pH of the cecal contents was measured by the direct insertion of an IQ 150 pH meter (Spectrum Technologies, Inc., Plainfield, IL) into the cecum through a cecal wall incision immediately after the sacrifice of the mice. The mean of 3 measurements was adopted as the measured value.
Measurement of endotoxin levels in cecal contents and blood.
After blood had been collected from the heart under Somnopentyl anesthesia, the cecal contents were also collected from mice. Fresh blood collected in a tube was coagulated in a refrigerator at 4°C and centrifuged at 1,000 × g at 4°C for 10 min. The serum obtained was used as a sample for blood endotoxin measurements. Cecal contents were diluted 10-fold with endotoxin-free water and centrifuged at 15,000 × g at 4°C for 10 min. The obtained supernatant was used as a sample. Endotoxin levels in the cecal contents and blood samples were measured using a Lonza QCL-1000 endotoxin quantitation kit (LONZA Japan, Tokyo, Japan; catalog no. 50-647U). Endotoxin levels in the cecal contents and blood of untreated normal mice were also measured as negative controls.
Measurement of DAO activity.
Serum diamine oxidase (DAO) activity was used as a marker of intestinal integrity (21). Blood was collected from the heart, coagulated at 4°C in a refrigerator, and centrifuged at 1,000 × g at 4°C for 10 min. The supernatant was collected, centrifuged again under the same conditions, and then stored at −80°C for later measurements. Serum DAO activity was determined using the method described by Takagi et al. (22).
Measurement of expression of tight-junction-related genes in the intestinal epithelium.
The mucosal tissues of the ileum, cecum, and colon were extirpated from untreated normal mice and from MDRAb-infected mice euthanized at 14 days after infection. These tissues were then washed in PBS, which had been cooled on ice, and cut into pieces measuring 0.5 to 1 cm in length. Intestinal epithelial cells prepared as described by Matsumoto et al. (23) were dissolved in 1.5 ml of TRIzol reagent (Life Technologies Corporation, Carlsbad, CA) and promptly stored at −80°C. Each epithelial cell suspension was mixed with 300 μl of chloroform, allowed to stand for 5 min, and then centrifuged at 12,000 × g for 15 min. Each resulting supernatant was mixed with 700 μl of isopropanol, allowed to stand for 5 min, and then centrifuged at 12,000 × g for 15 min to remove the supernatant. Each precipitate was washed twice in 1.5 ml of 75% ethanol, dried under reduced pressure, and dissolved in 50 μl of RNA-free water. The resulting solutions were used as the RNA samples for RT-qPCR.
Reverse transcription was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). RT-qPCR was performed using SYBR Premix Ex Taq (TaKaRa Bio Inc., Otsu, Shiga, Japan) in an ABI 7500 real-time PCR system (Applied Biosystems). Samples were assessed for expression of the genes encoding claudin-1, occludin, and zona occludens-1 (ZO-1) using target-specific primers as previously reported (24–26). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-encoding gene was used as an endogenous control to normalize the expression of each individual gene.
Statistical analysis.
Statistical analyses were performed using IBM SPSS Statistics Desktop version 22.0 software (IBM Japan Ltd., Tokyo, Japan). Differences between the treatment and control groups were analyzed by two-tailed Dunnett's test, Fisher's exact probability test, or Tukey's honestly significant difference (HSD) test, where applicable. Pearson's correlation coefficient was used to analyze the relationship between bacterial counts and cecal organic acid concentrations. In all tests, a P value of <0.05 was regarded as significant. For principal-component analysis (PCA), the log-transformed bacterial count was used. For samples in which bacteria were not detected (ND), the bacterial counts were regarded to be half the detection limits of the corresponding primer sets. PCA was applied to the data sets by using the statistical program R3.1.1. The result of the PCA was visualized using the ade4 package provided in the program R3.1.1.
RESULTS
Anti-infectious activity of the synbiotic in a novel mouse model of intestinal MDRAb infection.
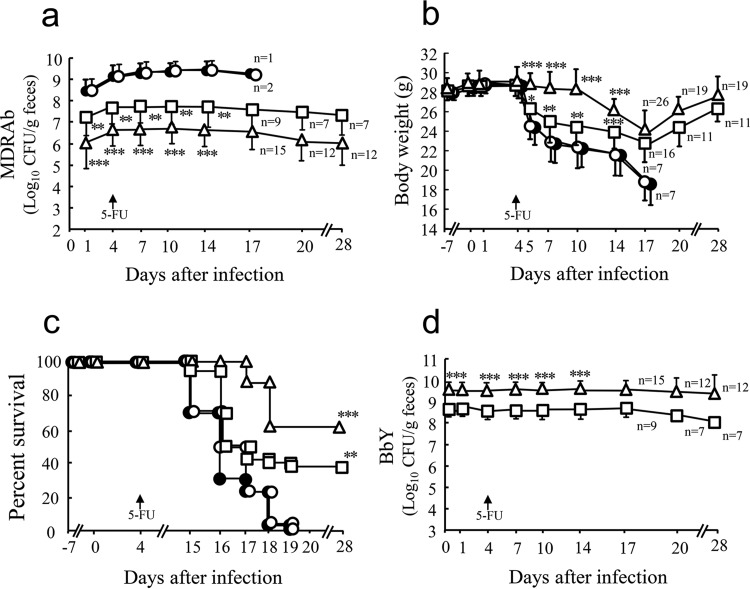
In the saline-treated infected control group, a rapid and marked increase in the population levels of MDRAb was observed 1 day after infection, which was maintained until day 14 (Fig. 1a). Body weight began to decrease after the 5-FU treatment on day 4 after the MDRAb infection (Fig. 1b), and all mice died within 15 to 19 days after infection (Fig. 1c).
FIG 1.
Protective effects of the synbiotic against infection in a mouse model of intestinal MDRAb infection. KM, CTM, LOM, MTN, and IPM were administered (orally or intraperitoneally) to mice (48 mice/group) from day −7 until day 28. Mice were infected orally with MDRAb (1 × 104 to 2 × 104 CFU/mouse) on day 0. B. breve strain Yakult (BbY; 1 × 108 to 2 × 108 CFU/mouse/day) and/or galactooligosaccharides (GOS; 10 mg/mouse/day) were administered orally once daily from day −7 until day 28. On day 4 after the MDRAb infection, animals were injected intraperitoneally with 5-FU at a dose of 400 mg/kg of body weight. Thirty mice out of 48 mice per group (saline-treated infected control, BbY, GOS, or BbY plus GOS) were observed for body weight (b) and survival (c) (28 days after the MDRAb challenge), and the other 18 mice from each group were assessed by bacteriological analysis; the counts of viable MDRAb (a) and BbY (d) were determined using selective media. Results are expressed as means ± SD for the log10 CFU per gram of feces. The same experiment was repeated three times in total. Symbols: ●, saline-treated infected control; □, BbY; ○, GOS; △, BbY plus GOS. Where indicated, significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) were observed between mice treated with the experimental agents and saline-treated infected control mice.
In the probiotic-treated group, both weight loss (Fig. 1b) and mortality (Fig. 1c) were significantly attenuated compared to those in the saline-treated infected control group. In the group treated with BbY and GOS, both weight loss and mortality were further attenuated, with the 63% survival rate achieving a P value of <0.001 compared to the value for the saline-treated infected control group (Fig. 1b and c). In contrast, treatment with prebiotics (GOS) alone did not attenuate either weight loss or death. The intestinal colonization level of MDRAb was significantly attenuated in the BbY-treated group (P < 0.01) and in the synbiotic-treated group (P < 0.001) compared to that in the saline-treated infected control group (Fig. 1a). GOS alone did not affect the MDRAb intestinal colonization level (Fig. 1a).
In the BbY-treated group, BbY was recovered at a level of 108 CFU/g from the feces throughout the experimental period (Fig. 1d). The administration of the synbiotic (GOS with BbY) further increased the fecal BbY colonization, to 109 CFU/g (P < 0.001 versus the value for BbY alone).
The systemic spread of MDRAb to various organs, including the MLNs (Fig. 2a) and liver (Fig. 2b), was clearly observed in the saline-treated infected control group. Administration of BbY significantly inhibited systemic infection (Fig. 2). These inhibitory effect was enhanced by the synbiotic administration of BbY and GOS (Fig. 2).
FIG 2.
Inhibition of systemic infection of MDRAb by the synbiotic. KM, CTM, LOM, MTN, and IPM were administered to mice (18 mice/group) from day −7 until day 28. Mice were infected orally with MDRAb (1 × 104 to 2 × 104 CFU/mouse) on day 0. BbY (1 × 108 to 3 × 108 CFU/mouse/day) and/or GOS (10 mg/mouse/day) was administered orally once daily from day −7 until day 14. On day 4 after the MDRAb infection, animals were injected intraperitoneally with 5-FU at a dose of 400 mg/kg of body weight. Seventy-two mice were dissected 14 days after being infected with MDRAb. Results are expressed as the means ± SD for log10 CFU per gram of mesenteric lymph nodes (MLNs) (a) and livers (b) of mice. The experiment at the same procedure was repeated three times in total. Black bars, saline-treated infected control; white bars, BbY; light gray bars, GOS; dark gray bars, BbY plus GOS. Where indicated, significant differences (**, P < 0.01; ***, P < 0.001) were observed between mice treated with the experimental agents and saline-treated infected control mice. Data are presented as means, error bars indicate SD, and animal numbers are as indicated.
Pro- and synbiotics do not improve antibiotic-induced dysbiosis.
The multiple antibiotic treatments disrupted intestinal microbial populations (Table 1), as clearly shown by the dramatic reduction in the density of the dominant anaerobes (day 0). In the BbY-treated group, BbY achieved a high population level but did not improve antibiotic-induced dysbiosis (Table 1, day 0). In the group treated with BbY and GOS, the population level of BbY was 10-fold higher than that in the BbY group, but no improvement of dysbiosis was observed (day 0 and day 14). Even without MDRAb infection, the BbY population level was higher in the group treated with BbY and GOS than in that treated with BbY alone, but no improvement in dysbiosis was seen (see Table S1 in the supplemental material). MDRAb infection did not affect the antibiotic-induced dysbiosis aside from the rapid increase and subsequent stable colonization by itself (Table 1, day 0 and day 14). A principal-component analysis of intestinal microbiota data was performed for the 8 experimental groups (Table 1; see also Table S1) obtained from the YIF-SCAN. The analysis showed that they could be divided broadly into 4 groups, hereby referred to as A to D (A, saline-treated infected control [day 0], GOS [day 0], antibiotics [AB] plus 5-FU, and AB plus GOS plus 5-FU; B, saline-treated infected control [day 14] and GOS [day 14]; C, BbY [day 0], BbY plus GOS [day 0], AB plus BbY plus 5-FU, and AB plus BbY plus GOS plus 5-FU; and D, BbY [day 14] and BbY plus GOS [day 14]) (see Fig. S1 in the supplemental material). The differences between groups A and B and between groups C and D were in whether or not BbY had been administered (whether or not BbY established itself in the intestine). The differences between groups A and C and between groups B and D were in whether or not there was intestinal infection with MDRAb.
TABLE 1.
Effects of the synbiotic on indigenous cecal microbiota in mice treated with antibiotics
| Organisms | No. of bacteria (mean ± SD) in cecal contentsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal mice (nontreated, uninfected) | Saline-treated infected controlb |
BbYb,d |
GOSb,e |
BbY + GOSb,d,e |
|||||
| Day 0 | Day 14c | Day 0 | Day 14c | Day 0 | Day 14c | Day 0 | Day 14c | ||
| Total bacteria | 10.5 ± 0.2 | 8.1 ± 0.2 | 9.3 ± 0.8 | 8.8 ± 0.4 | 9.0 ± 0.3 | 8.2 ± 0.8 | 9.2 ± 0.4 | 9.4 ± 0.4 | 9.6 ± 0.4 |
| Clostridium coccoides group | 10.1 ± 0.1 | 6.3 ± 0.8 | 6.9 ± 1.8 | 6.4 ± 0.7 | 6.6 ± 1.1 | 6.3 ± 0.8 | 6.7 ± 1.6 | 6.4 ± 1.0 | 6.7 ± 1.0 |
| C. leptum subgroup | 10.1 ± 0.2 | 6.0 ± 0.4 | 6.3 ± 0.9 | 6.0 ± 0.6 | 5.9 ± 0.6 | 6.2 ± 0.5 | 5.8 ± 0.5 | 5.9 ± 0.5 | 6.2 ± 0.5 |
| Bacteroides fragilis group | 9.0 ± 0.3 | 6.5 ± 0.6 | 6.2 ± 0.5 | 6.6 ± 1.0 | 6.3 ± 0.5 | 6.4 ± 0.4 | 6.2 ± 0.4 | 6.4 ± 0.5 | 6.5 ± 0.3 |
| Bifidobacterium | 6.7 ± 0.3 | <5.0 | <5.0 | 8.3 ± 0.3 | 8.2 ± 0.5 | <5.0 | <5.0 | 9.3 ± 0.3 | 9.4 ± 0.4 |
| Atopobium cluster | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| Prevotella | 5.6 ± 0.5 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| Total Lactobacillus | 9.7 ± 0.3 | 5.9 ± 0.5 | 5.9 ± 1.0 | 6.0 ± 0.5 | 5.8 ± 0.9 | 5.7 ± 0.4 | 5.8 ± 0.8 | 5.6 ± 0.5 | 5.5 ± 0.8 |
| Enterobacteriaceae | 5.5 ± 0.2 | <4.0 | <4.0 | <4.0 | <4.0 | <4.0 | <4.0 | <4.0 | <4.0 |
| Enterococcus | 8.7 ± 0.3 | 7.9 ± 0.3 | 8.0 ± 0.7 | 8.1 ± 0.9 | 8.3 ± 0.7 | 8.1 ± 0.9 | 8.0 ± 0.5 | 8.1 ± 0.8 | 8.3 ± 0.6 |
| Staphylococcus | 5.1 ± 0.5 | 4.4 ± 0.6 | 4.2 ± 0.6 | 4.2 ± 0.4 | 3.9 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.4 | 4.0 ± 0.4 | 3.8 ± 0.3 |
| BbY | <2.0 | <2.0 | <2.0 | 8.0 ± 0.6 | 8.3 ± 0.7 | <2.0 | <2.0 | 9.1 ± 0.4 | 9.4 ± 0.3 |
| MDRAb | <2.0 | <2.0 | 9.1 ± 0.6 | <2.0 | 7.5 ± 1.3 | <2.0 | 9.1 ± 0.4 | <2.0 | 6.8 ± 0.4 |
Mice were dissected on day 0 (6 mice/group) and day 14 (6 mice/group) after being infected with MDRAb. Results are expressed as log10 viable cells per gram in cecal contents (n = 6).
The antibiotics (KM, CTM, LOM, MTN, and IPM) were given to mice from day −7 until day 14.
Mice were infected orally with MDRAb (1.9 × 104 CFU/mouse) on day 0. On day 4 after the MDRAb infection, animals were injected intraperitoneally with 5-FU at a dose of 400 mg/kg of body weight.
BbY (2 × 108 to 3 × 108 CFU/mouse/day) was administered to mice once daily from day −7 until day 14.
GOS (10 mg/mouse/day) was administered to mice once daily from day −7 until day 14.
Effects of the synbiotic on the intestinal environment in MDRAb-infected mice.
In the saline-treated infected control group, acetic acid (AA) levels in the cecal contents were one-fifth of those in normal mice, consistent with the reduction of total organic acid concentrations; the pH of the cecal contents was elevated in the antibiotic-treated animals compared to that in normal mice (Table 2). These reductions were attenuated by treatment with BbY (P < 0.01) and by combined administration of BbY and GOS (P < 0.001) (Table 2).
TABLE 2.
Influences of the concomitant administration of the synbiotic on the intestinal environment
| Groupa | pH of cecal contents (mean ± SD)b |
Concn (mean ± SD) of organic acids in the cecal contents |
||||
|---|---|---|---|---|---|---|
| Total organic acids |
Acetic acid |
|||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| Normal mice | 6.5 ± 0.2 | 94 ± 11 | 80 ± 13 | |||
| Saline-treated infected control mice | 7.9 ± 0.1 | 7.9 ± 0.2 | 19 ± 5 | 20 ± 6 | 17 ± 5 | 18 ± 5 |
| BbY-treated mice | 7.3 ± 0.5** | 7.2 ± 0.3** | 45 ± 14** | 43 ± 11** | 40 ± 13** | 40 ± 11** |
| GOS-treated mice | 7.9 ± 0.2 | 7.9 ± 0.2 | 19 ± 6 | 21 ± 6 | 18 ± 7 | 19 ± 5 |
| Mice treated with BbY + GOS | 6.9 ± 0.2*** | 6.8 ± 0.2*** | 62 ± 11*** | 66 ± 13*** | 57 ± 11*** | 58 ± 10*** |
Mice were infected with MDRAb, treated with BbY and GOS, and treated with 5-FU, as described in the legend to Fig. 2.
Mice (same mice as for Fig. 3) were dissected on day 0 (18 mice/group) and day 14 (18 mice/group) after the MDRAb infection; concentrations are expressed in micromoles per gram of cecal contents. The same experiment was repeated three times in total. Significant differences between treated and saline-treated control mice are shown as follows: **, P < 0.01; and ***, P < 0.001.
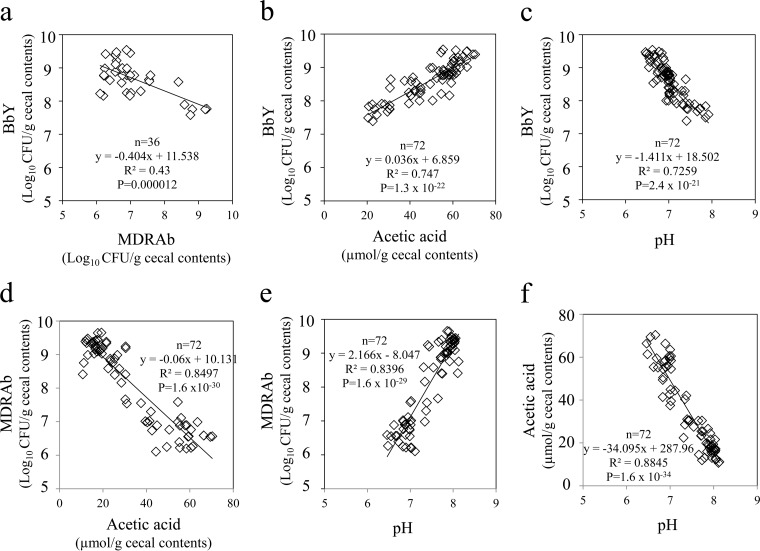
Relationships between MDRAb and BbY population levels and the intestinal environment.
The cecal counts of BbY exhibited inverse correlation with the cecal counts of MDRAb (P < 0.001) and with the pH of the cecal contents (P < 0.001) and showed positive correlation with cecal content AA levels (P < 0.001) (Fig. 3a to c). In contrast, the cecal counts of MDRAb exhibited inverse correlation with AA levels (P < 0.001) and positive correlation with pH of the cecal contents (P < 0.001) (Fig. 3d and e). Cecal content AA levels showed inverse correlation with pH (P < 0.001) (Fig. 3f).
FIG 3.
Relationships between cecal MDRAb counts and BbY counts, acetic acid levels, and pH and between acetic acid levels and BbY counts and pH (day 14 after MDRAb infection). Mice were infected with MDRAb, treated with BbY plus GOS, and treated with 5-FU, as described in the legend to Fig. 2. Mice were dissected on day 0 (18 mice/group) and day 14 (mice used in Fig. 2; 18 mice/group) after being infected with MDRAb. Comparisons were between MDRAb and BbY counts (a), acetic acid levels and BbY counts (b), pH and BbY counts (c), acetic acid levels and MDRAb counts (d), pH and MDRAb counts (e), and pH and acetic acid levels (f). The experiment was repeated three times in total.
Effects of the synbiotic on endotoxin levels and epithelial damage in MDRAb-infected mice.
Endotoxin concentrations in the cecal contents and blood samples in the saline-treated infected control group were higher than in untreated normal mice (Table 3). Administration of BbY attenuated the endotoxin levels in cecal contents (P < 0.05) but not in the blood (compared to saline-treated infected control group). Synbiotic administration of BbY and GOS attenuated the endotoxin levels in both matrices (P < 0.01 compared to the value for the saline-treated infected control group). GOS alone was not effective.
TABLE 3.
Influences of the concomitant administration of the synbiotic on endotoxin levels and epithelial damage in MDRAb-infected mice
| Groupa | Concn of endotoxin (mean ± SD)b,d |
Serum DAO activity level (mean ± SD)c,d |
||||
|---|---|---|---|---|---|---|
| Day 0 |
Day 14 |
Day 0 | Day 14 | |||
| Blood | Cecal contents | Blood | Cecal contents | |||
| Normal mice | ND | 3.6 ± 0.8 | 30.9 ± 4.5 | |||
| Saline-treated infected control mice | ND | 3.8 ± 0.8 | 3.2 ± 3.2 | 26.4 ± 3.0 | 30.0 ± 4.0 | 5.4 ± 3.8 |
| BbY-treated mice | ND | 3.6 ± 0.8 | 2.2 ± 1.2 | 12.7 ± 11.2* | 29.6 ± 3.7 | 15.6 ± 6.9* |
| GOS-treated mice | ND | 3.9 ± 0.7 | 3.0 ± 2.6 | 24.1 ± 1.8 | 28.9 ± 4.3 | 6.3 ± 4.3 |
| Mice treated with BbY + GOS | ND | 3.8 ± 0.7 | 1.1 ± 1.0** | 5.1 ± 4.9** | 29.3 ± 3.5 | 22.0 ± 7.5** |
Mice (24 mice/group) were infected orally with MDRAb (1.2 × 104 CFU) at an inoculum of 0.1 ml/mouse on day 0. MDRAb-infected mice were treated intraperitoneally with 5-FU (400 mg/kg) 4 days after the MDRAb infection. BbY (2 × 108 to 3 × 108 CFU/mouse/day) and GOS (10 mg/mouse/day) were administered to mice once daily from day −7 until day 14. Mice were dissected on day 0 (12 mice/group) and day 14 (12 mice/group) after the MDRAb infection.
Endotoxin levels in the blood and cecal contents 14 days after the infection are presented as endotoxin units per milliliter and per milligram, respectively (6 mice/group). ND, not detected.
Serum diamine oxidase (DAO) activity levels are presented as units per gram (6 mice/group).
Significant differences between treated and saline-treated control mice are shown as follows: *, P < 0.05; and **, P < 0.01.
No significant differences in serum DAO activity at baseline were observed among the experimental groups (Table 3). DAO activity was markedly lowered by the MDRAb infection, and administration of BbY with or without GOS attenuated this effect. GOS alone was not effective.
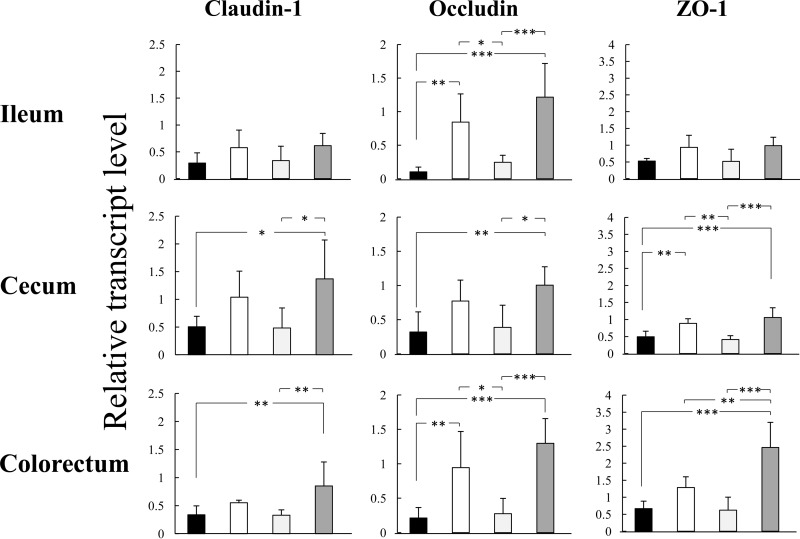
Improvements in tight-junction-related gene expression in the intestinal epithelium by the synbiotic.
The expression levels of genes encoding tight-junction-related proteins claudin-1, occludin, and ZO-1 were markedly lower in the ileal, cecal, and colorectal epithelia of MDRAb-infected animals than in uninfected normal mice (Fig. 4). Treatment with BbY or with the synbiotic (BbY plus GOS) attenuated these decreases in gene expression.
FIG 4.
Improvements in tight-junction-related gene expression in the intestinal epithelium by synbiotic treatment of MDRAb-infected mice. Mice (6/group) were infected orally with MDRAb (1.8 × 104 CFU) at an inoculum of 0.1 ml/mouse on day 0. MDRAb-infected mice were treated intraperitoneally with 5-FU (400 mg/kg) 4 days after the MDRAb infection. BbY (2 × 108 to 3 × 108 CFU/mouse/day) and GOS (10 mg/mouse/day) were administered to mice once daily from day −7 until day 14. Mice (6/group) were dissected 14 days after being infected with MDRAb to examine the mRNA expression of tight-junction-related genes in the intestinal epithelium using a quantitative RT-PCR method. The relative amounts of claudin-1-, occludin-, and ZO-1-encoding transcripts were calculated and normalized with respect to the amounts of the GAPDH transcript, and the relative gene expression values were compared to those of normal mice (nontreated, uninfected; n = 6). Data are presented as means ± SD. Black bars, saline-treated infected control; white bars, BbY; light gray bars, GOS; dark gray bars, BbY plus GOS. Where indicated, significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) were observed between mice treated with the experimental agents and saline-treated infected control mice.
A positive correlation was noted between fecal AA levels and tight-junction-related gene expression in the intestinal epithelium (Table 4).
TABLE 4.
Correlations between the fecal acetic acid levels and tight-junction-related gene expression in the intestinal epithelium on day 14 after MDRAb infection
| Site and gene producta | Correlation between fecal acetic acid levels and tight-junction-related gene expression (n = 24) |
|
|---|---|---|
| Pearson correlation coefficient | P | |
| Ileum | ||
| Claudin-1 | 0.470 | <0.05 |
| Occludin | 0.695 | <0.001 |
| ZO-1 | 0.559 | <0.01 |
| Cecum | ||
| Claudin-1 | 0.503 | <0.05 |
| Occludin | 0.597 | <0.01 |
| ZO-1 | 0.730 | <0.001 |
| Colorectum | ||
| Claudin-1 | 0.659 | <0.001 |
| Occludin | 0.761 | <0.001 |
| ZO-1 | 0.856 | <0.001 |
Mice were infected with MDRAb, treated with BbY plus GOS, and treated with 5-FU, as described in the legend to Fig. 4. Feces were collected immediately before dissection of the mice (6 mice/group) on day 14 after infection with MDRAb, and the organic acid concentration was measured.
DISCUSSION
Iatrogenic risk factors for carrying MDRAb and being infected include surgery, the use of a ventilator, tracheotomy, admission to the ICU (or neonatal ICU), catheter placement in the central vein, tube feeding, cancer therapy, and the administration of cephems, quinolones, and carbapenems (1). MDRAb then causes postoperative, wound, and vascular catheter-related blood flow infections as well as respirator-related pneumonia in hosts with reduced resistance to infections. Previous studies reported that the intestine in perioperative patients may serve as a hotbed for hospital infections and is the main site for the colonization and growth of MDRAb (7, 27, 28). Animal experimental MDRAb infection models include those for pneumonia (29, 30), femoral region infection (31), burns (32), sepsis (33), severe diabetes (34), and infectious endophthalmitis (35); however, to our knowledge, no animal model currently exists for intestinal infections. In the infection model in the present study, mice were treated with the 5 antibiotics widely used perioperatively in clinical practice (36, 37), and marked intestinal growth of MDRAb was observed within 24 h of administration of a small oral inoculum. Subsequent immunosuppression (via 5-FU administration) induced lethal systemic invasion by MDRAb. Therefore, lethal intestinal MDRAb infection can be induced by the combination of multiple-antibiotic treatment and host immunosuppression.
When mice treated with antibiotics were colonized prophylactically with orally dosed probiotics (BbY) with natural resistance to the administered antibiotics, the intestinal growth of orally inoculated MDRAb, 5-FU-induced MDRAb invasion after the MDRAb infection, and the host mortality were markedly attenuated. These protective effects against infection appeared to be potentiated by the synbiotic, specifically the combination dosing with BbY and GOS (11). Furthermore, we demonstrated that the maintenance of a high intestinal colonization level of BbY exhibited an inverse correlation with the intestinal MDRAb growth, and treatment with the synbiotic yielded a higher level of colonization by BbY than seen in infected animals treated with BbY alone.
In patients with dysbiosis of the intestinal microbiota due to antibiotics (38), surgery (39), and trauma (40), drug-resistant bacteria and endogenous pathogens (such as methicillin-resistant Staphylococcus aureus [MRSA] and Clostridium difficile) accumulate to abnormal levels in the intestine, leading to enteritis and infectious diseases. The multiple-antibiotic treatment used in this study reduced the intestinal levels of dominant obligate and facultative anaerobes (i.e., induced dysbiosis) while also permitting the accumulation of intestinal MDRAb to abnormally high densities. Treatment of infected animals with BbY or with BbY and GOS did not attenuate this dysbiosis. However, these treatments did yield stable intestinal colonization by BbY, along with significant reduction of colonization by MDRAb. These results strongly suggested that the administered BbY colonized the intestine as the most dominant bacteria and compensated for the missing indigenous population, improving colonization resistance against the opportunistic pathogen. No infection-protective effect was noted in the mice treated with a prebiotic (GOS) alone, a result that may reflect the marked reduction of indigenous Bifidobacterium by the multiple-antibiotic exposure.
Antibiotic-induced aberration of the intestinal environment was improved by the pro- and synbiotic. AA is the main metabolite of BbY (15), and previous studies demonstrated that AA exhibited a strong bactericidal effect on various pathogenic Gram-negative rods in vitro; this bactericidality was suggested to be exerted by AA in its nondissociated state (41–43). The positive correlation between AA and the BbY count, the inverse correlation between AA and the MDRAb count, and the inverse correlation between AA and pH all suggested that the mechanism of action of BbY and the synbiotic BbY colonizing at a high population levels (even with ongoing exposure to multiple antibiotics) produces significant levels of AA, leading to reduction of cecal pH and elevation of the concentration of nondissociated AA. Thus, administration of GOS in combination with BbY inhibits intestinal MDRAb growth more strongly than does administration of BbY alone. BbY is presumed to actively metabolize the coadministered prebiotic (GOS), resulting in higher AA production and stronger anti-infectious activity against MDRAb.
The etiology of MDRAb infection includes endotoxin production by the bacterium (3). Accordingly, when local endotoxin levels increase due to MDRAb infections, chemokines, adhesion molecules, and inducible nitric oxide synthase are induced; these products are perceived as inflammatory signals via the endotoxin receptor (Toll-like receptor [TLR]), resulting in multiple organ failure, shock, and disseminated intravascular coagulation, phenomena that aggravate infections and increase mortality (44, 45). Consistent with this etiology, the synbiotic attenuated the elevation of intestinal and blood endotoxin levels observed in our MDRAb infection model.
Recent work using a gnotobiotic mouse model of intestinal enterohemorrhagic Escherichia coli O157:H7 infection demonstrated the intestinal epithelium-protective effects of AA produced by Bifidobacterium (46). In vitro permeability studies using intestinal mucoepithelial cell tight junctions have shown that a specific probiotic bacterial strain restored tight junctions damaged by H2O2 (47), that Bifidobacterium-produced AA strengthened tight junctions (48), and that an increase in AA levels within the physiological range (and associated pH reductions) improved permeability (49, 50). The present study demonstrated that infection-related decreases in serum DAO activity (an index of the health and maturity of the intestinal mucosa) and tight-junction-related gene expression levels in the intestinal mucoepithelium were markedly attenuated by synbiotic treatment. A strong correlation was noted between intestinal AA concentration and tight-junction-related gene expression levels; the infection-protective mechanism of the synbiotic is presumed to include the maintenance of the barrier function of the intestinal epithelium, thereby inhibiting the systemic spread of MDRAb. In animal experiments it is important to verify reproducibility by performing repeat experiments for all data. The results in Fig. 4 and Table 4 are those from one experiment, but the results in all other figures and tables are the results from three repeated experiments. In any event, it will be necessary to accumulate further experimental results in the future to elucidate the mechanism.
There are many previous reports on the usefulness of synbiotics in clinical practice. This takes the form of defense against bacterial translocation and infectious complications in seriously ill patients, including perioperative patients with bile duct cancer (21, 39), esophageal cancer (51, 52), hepatic cirrhosis (53), and colorectal cancer (54), patients undergoing cancer chemotherapy (55), systemic inflammatory response syndrome (SIRS) patients (40), and patients on long-term mechanical ventilation (56). Moreover, research has suggested that improvement of dysbiosis and activity to improve the intestinal environment (pH and organic acid [acetic acid] level) are important in the mechanism for this protective effect against infections.
In conclusion, we demonstrated that a pro- and synbiotic markedly potentiated protection against fatal intestinal infections caused by a multidrug-resistant bacterium. BbY appears to compensate for the disruption of the indigenous bacterial population, thereby maintaining the intestinal environments and barrier function of the intestinal epithelium and reducing fatal opportunistic infections by MDRAb. This protective mechanism is expected to be of value in certain clinical settings, particularly in the cases of iatrogenic dysbiosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hirokazu Tsuji for his instructions on statistical analysis.
This research was not supported by a specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02928-15.
REFERENCES
- 1.Ayraud-Thévenot S, Huart C, Mimoz O, Taouqi M, Laland C, Bousseau A, Castel O. 2012. Control of multi-drug-resistant Acinetobacter baumannii outbreaks in an intensive care unit: feasibility and economic impact of rapid unit closure. J Hosp Infect 82:290–292. doi: 10.1016/j.jhin.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Ogutlu A, Guclu E, Karabay O, Utku AC, Tuna N, Yahyaoglu M. 2014. Effects of carbapenem consumption on the prevalence of Acinetobacter infection in intensive care unit patients. Ann Clin Microbiol Antimicrob 13:7. doi: 10.1186/1476-0711-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towner KJ. 2009. Acinetobacter: an old friend, but a new enemy. J Hosp Infect 73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Young LS, Sabel AL, Price CS. 2007. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol 28:1247–1254. doi: 10.1086/521660. [DOI] [PubMed] [Google Scholar]
- 7.Corbella X, Pujol M, Ayats J, Sendra M, Ardanuy C, Domínguez MA, Liñares J, Ariza J, Gudiol F. 1996. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin Infect Dis 23:329–334. doi: 10.1093/clinids/23.2.329. [DOI] [PubMed] [Google Scholar]
- 8.Reid G, Food Agricultural Organization of the United Nations and the WHO. 2005. The importance of guidelines in the development and application of probiotics. Curr Pharm Des 11:11–16. doi: 10.2174/1381612053382395. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe C, Larsen CN, Fonde'n R, Svensson U, Ouwehand A, Lahtinen S, Kiwaki M, Nomoto K, Kimura K, Eskesen D, Saxelin M, Kajander K, Reid G, Bruce AW, Nurminen P, Korpela R, Tsuji H, Xiao JZ, Salminen S. 2009. Commercially available human probiotic microorganisms, p 441–532. In Lee YK, Salminen S (ed), Handbook of probiotics and prebiotics, 2nd ed John Wiley and Sons, Inc, New York, NY. [Google Scholar]
- 10.Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 11.Colins MD, Gibson GR. 1999. Probiotics, prebiotics, and synbiotics approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69:S1052–S1057. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85-23. National Institutes of Health, Bethesda, MD. [Google Scholar]
- 13.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SGB, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Kano M, Masuoka N, Kaga C, Sugimoto S, Iizuka R, Manabe K, Sone T, Oeda K, Nonaka C, Miyazaki K, Ishikawa F. 2013. Consecutive intake of fermented milk containing Bifidobacterium breve strain Yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Biosci Microbiota Food Health 32:33–39. doi: 10.12938/bmfh.32.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun 72:2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto J, Tanigawa K, Kudo Y, Makino H, Watanabe K. 2011. Identification and quantification of viable Bifidobacterium breve strain Yakult in human faeces by using strain-specific primers and propidium monoazide. J Appl Microbiol 110:209–217. doi: 10.1111/j.1365-2672.2010.04873.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa F, Takayama H, Matsumoto K, Ito M, Chonan O, Deguchi Y, Kikuchi-Hayakawa H, Watanuki M. 1995. Effects of β1-4 linked galactooligosaccharides on human fecal microflora. Bifidus 9:5–18. [Google Scholar]
- 18.Matsuda K, Tsuji H, Asahara T, Kado Y, Nomoto K. 2007. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl Environ Microbiol 73:32–39. doi: 10.1128/AEM.01224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda K, Tsuji H, Asahara T, Matsumoto K, Takada T, Nomoto K. 2009. Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules. Appl Environ Microbiol 75:1961–1969. doi: 10.1128/AEM.01843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikuchi H, Yajima T. 1992. Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J Sci Food Agric 60:139–146. doi: 10.1002/jsfa.2740600202. [DOI] [Google Scholar]
- 21.Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. 2006. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 244:706–714. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takagi K, Nakao M, Ogura Y, Nabeshima T, Kunii A. 1994. Sensitive colorimetric assay of serum diamine oxidase. Clin Chim Acta 226:67–75. doi: 10.1016/0009-8981(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Setoyama H, Imaoka A, Okada Y, Amasaki H, Suzuki K, Umesaki Y. 1995. Gamma delta TCR-bearing intraepithelial lymphocytes regulate class II major histocompatibility complex molecule expression on the mouse small intestinal epithelium. Epithelial Cell Biol 4:163–170. [PubMed] [Google Scholar]
- 24.Kang YJ, Otsuka M, van den Berg A, Hong L, Huang Z, Wu X, Zhang DW, Vallance BA, Tobias PS, Han J. 2010. Epithelial p38alpha controls immune cell recruitment in the colonic mucosa. PLoS Pathog 6:e1000934. doi: 10.1371/journal.ppat.1000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roxas JL, Koutsouris A, Bellmeyer A, Tesfay S, Royan S, Falzari K, Harris A, Cheng H, Rhee KJ, Hecht G. 2010. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest 90:1152–1168. doi: 10.1038/labinvest.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song HL, Lv S, Liu P. 2009. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol 9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agustí C, Pujol M, Argerich MJ, Ayats J, Badía M, Domínguez MA, Corbella X, Ariza J. 2002. Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother 49:205–208. doi: 10.1093/jac/49.1.205. [DOI] [PubMed] [Google Scholar]
- 28.Timsit JF, Garrait V, Misset B, Goldstein FW, Renaud B, Carlet J. 1993. The digestive tract is a major site for Acinetobacter baumannii colonization in intensive care unit patients. J Infect Dis 168:1336–1337. doi: 10.1093/infdis/168.5.1336. [DOI] [PubMed] [Google Scholar]
- 29.Pachón-Ibáññez ME, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jiménez-Mejias ME, García-Curiel A, Pichardo C, Jiménez L, Pachón J. 2010. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada K, Yanagihara K, Kaku N, Harada Y, Migiyama Y, Nagaoka K, Morinaga Y, Nakamura S, Imamura Y, Miyazaki T, Izumikawa K, Kakeya H, Hasegawa H, Mikamo H, Kohno S. 2013. Azithromycin attenuates lung inflammation in a mouse model of ventilator-associated pneumonia by multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 57:3883–3888. doi: 10.1128/AAC.00457-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson MG, Black CC, Pavlicek RL, Honnold CL, Wise MC, Alamneh YA, Moon JK, Kessler JL, Si Y, Williams R, Yildirim S, Kirkup BC Jr, Green RK, Hall ER, Palys TJ, Zurawski DV. 2014. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother 58:1332–1342. doi: 10.1128/AAC.01944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. 2014. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis 209:1963–1971. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Rojas R, McConnell MJ, Jiménez-Mejías ME, Domínguez-Herrera J, Fernández-Cuenca F, Pachón J. 2013. Colistin resistance in a clinical Acinetobacter baumannii strain appearing after colistin treatment: effect on virulence and bacterial fitness. Antimicrob Agents Chemother 57:4587–4589. doi: 10.1128/AAC.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo G, Spellberg B, Gebremariam T, Bolaris M, Lee H, Fu Y, French SW, Ibrahim AS. 2012. Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother 67:1439–1445. doi: 10.1093/jac/dks050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talreja D, Kaye KS, Yu FS, Walia SK, Kumar A. 2014. Pathogenicity of ocular isolates of Acinetobacter baumannii in a mouse model of bacterial endophthalmitis. Invest Ophthalmol Vis Sci 55:2392–2402. doi: 10.1167/iovs.13-13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka A, Sadahiro S, Suzuki T, Okada K, Saito G. 2014. Randomized controlled trial comparing subcuticular absorbable suture with conventional interrupted suture for wound closure at elective operation of colon cancer. Surgery 155:486–492. doi: 10.1016/j.surg.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Brook I. 2016. Spectrum and treatment of anaerobic infections. J Infect Chemother 22:1–13. doi: 10.1016/j.jiac.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Kanamori Y, Hashizume K, Kitano Y, Tanaka Y, Morotomi M, Yuki N, Tanaka R. 2003. Anaerobic dominant flora was reconstructed by synbiotics in an infant with MRSA enteritis. Pediatr Int 45:359–362. doi: 10.1046/j.1442-200X.2003.01728.x. [DOI] [PubMed] [Google Scholar]
- 39.Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. 2005. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 390:104–113. doi: 10.1007/s00423-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Matsushima A, Tasaki O, Fujita K, Hosotsubo H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. 2009. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci 54:1071–1078. doi: 10.1007/s10620-008-0460-2. [DOI] [PubMed] [Google Scholar]
- 41.Ostling CE, Lindgren SE. 1993. Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol 75:18–24. [DOI] [PubMed] [Google Scholar]
- 42.Eklund T. 1983. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J Appl Bacteriol 54:383–389. doi: 10.1111/j.1365-2672.1983.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 43.Brocklehurst TF, Lund BM. 1990. The influence of pH, temperature and organic acids on the initiation of growth of Yersinia enterocolitica. J Appl Bacteriol 69:390–397. doi: 10.1111/j.1365-2672.1990.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 44.Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. 2007. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol 56:165–171. doi: 10.1099/jmm.0.46823-0. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda N, Hattori Y. 2006. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci 101:189–198. doi: 10.1254/jphs.CRJ06010X. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 47.Miyauchi E, O'Callaghan J, Buttó LF, Hurley G, Melgar S, Tanabe S, Shanahan F, Nally K, O'Toole PW. 2012. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: strain dependence and attenuation by bacteriocin production. Am J Physiol Gastrointest Liver Physiol 303:G1029–G1041. doi: 10.1152/ajpgi.00003.2012. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. 2015. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep 3:e12327. doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariadason JM, Barkla DH, Gibson PR. 1997. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol 272:G705–G712. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T, Yoshida S, Hara H. 2008. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 100:297–305. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka K, Yano M, Motoori M, Kishi K, Miyashiro I, Ohue M, Ohigashi H, Asahara T, Nomoto K, Ishikawa O. 2012. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: a prospective randomized controlled trial. Surgery 152:832–842. doi: 10.1016/j.surg.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama Y, Nishigaki E, Abe T, Fukaya M, Asahara T, Nomoto K, Nagino M. 2014. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy. Br J Surg 101:189–199. doi: 10.1002/bjs.9385. [DOI] [PubMed] [Google Scholar]
- 53.Usami M, Miyoshi M, Kanbara Y, Aoyama M, Sakaki H, Shuno K, Hirata K, Takahashi M, Ueno K, Tabata S, Asahara T, Nomoto K. 2011. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. J Parenter Enteral Nutr 35:317–328. doi: 10.1177/0148607110379813. [DOI] [PubMed] [Google Scholar]
- 54.Komatsu S, Sakamoto E, Norimizu S, Shingu Y, Asahara T, Nomoto K, Nagino M. 2016. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: a randomized controlled trial. Surg Today 46:479–490. doi: 10.1007/s00595-015-1178-3. [DOI] [PubMed] [Google Scholar]
- 55.Motoori M, Yano M, Miyata H, Sugimura K, Saito T, Omori T, Fujiwara Y, Miyoshi N, Akita H, Gotoh K, Takahashi H, Kobayashi S, Noura S, Ohue M, Asahara T, Nomoto K, Ishikawa O, Sakon M. 25 November 2015. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin Nutr. doi: 10.1016/j.clnu.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Hayakawa M, Asahara T, Ishitani T, Okamura A, Nomoto K, Gando S. 2012. Synbiotic therapy reduces the pathological gram-negative rods caused by an increased acetic acid concentration in the gut. Dig Dis Sci 57:2642–2649. doi: 10.1007/s10620-012-2201-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.