Abstract
Experimental and clinical studies have indicated that the antileprosy drug clofazimine may contribute treatment-shortening activity when included in tuberculosis treatment regimens. Clofazimine accumulates to high levels in tissues, has a long half-life, and remains in the body for months after administration is stopped. We hypothesized that in tuberculosis treatment, accumulated clofazimine may contribute sustained antimicrobial activity after treatment cessation, and we used the BALB/c mouse model of chronic tuberculosis chemotherapy to address this hypothesis. Mycobacterium tuberculosis-infected mice were treated for 4 weeks or 8 weeks with either isoniazid alone, clofazimine alone, the first-line regimen rifampin-isoniazid-pyrazinamide-ethambutol, or a first-line regimen where clofazimine was administered in place of ethambutol. To evaluate posttreatment antimicrobial activity, bacterial regrowth in the lungs and spleens was assessed at the day of treatment cessation and 2, 4, 6, and 8 weeks after treatment was stopped. Bacterial regrowth was delayed in all mice receiving clofazimine, either alone or in combination, compared to the mice that did not receive clofazimine. This effect was especially evident in mice receiving multidrug therapy. In mice not receiving clofazimine, bacterial regrowth began almost immediately after treatment was stopped, while in mice receiving clofazimine, bacterial regrowth was delayed for up to 6 weeks, with the duration of sustained antimicrobial activity being positively associated with the time that serum clofazimine levels remained at or above the 0.25-μg/ml MIC for M. tuberculosis. Thus, sustained activity of clofazimine may be important in the treatment-shortening effect associated with this drug.
INTRODUCTION
Clofazimine is a lipid-soluble phenazine dye developed in the 1950s by Vincent Barry and colleagues as a new drug to treat tuberculosis (TB) (1). Although experimentally highly active against Mycobacterium tuberculosis (1, 2), clofazimine was not advanced for TB treatment but later became a key component in the multidrug treatment of leprosy (3). Clofazimine has been used for the treatment of drug-resistant TB, for which it is currently classified by the World Health Organization (WHO) as a group 5 drug (i.e., a drug with unclear efficacy that is not recommended for routine use) (4). However, interest in clofazimine for the treatment of TB was rejuvenated following its inclusion in the so-called “Bangladesh regimen” that was found to cure nearly 90% of patients with multidrug-resistant TB in 9 months (5), representing a significant improvement from the WHO-recommended 20+-month regimen that cured <50% of patients (6). This finding was subsequently confirmed in a larger study in Bangladesh (7), and similar results were also reported from observational studies in Niger and Cameroon (8, 9). The specific bactericidal and treatment-shortening activity of clofazimine was experimentally evidenced in mouse models of drug-susceptible (10) and drug-resistant (11) TB as well as clinically evidenced in a controlled clinical trial conducted in China (12). Thus, clofazimine appears to have potential as a repurposed drug for improving, i.e., significantly shortening, the treatment of TB.
Clofazimine has several notable pharmacokinetic characteristics, most of which have been appreciated since it was first synthesized in the 1950s (1, 2). It has an extremely long half-life, measured in weeks to months, that is dependent on the duration of administration (13–16), which may be related to its lipid dissolution and appropriation into macrophages as crystal-like structures (2, 17–20). Clofazimine accumulates to extremely high levels in tissues (sometimes up to milligrams per gram of tissue), and after administration of clofazimine is stopped, the high tissue levels are maintained for extended periods of time (again, weeks to months, depending on the duration of administration) (13, 15, 16). Serum levels of clofazimine are relatively low and steady compared to tissue levels, ∼1.0 μg/ml in mice receiving 25 mg/kg of body weight/day, a dose with exposures considered equivalent to the 100-mg/day dose usually used in patients with multidrug-resistant TB (5, 16, 21). Serum clofazimine levels are also maintained above the 0.25-μg/ml MIC for M. tuberculosis for weeks to months after treatment cessation, depending on the duration of clofazimine administration (11, 13, 15, 16). We hypothesized that the sustained levels of this drug may contribute sustained antimicrobial activity after treatment is stopped and may be associated with the significant treatment-shortening activity of clofazimine. To address this hypothesis, we conducted an experimental study in the mouse model of TB chemotherapy to measure the clofazimine-specific antimicrobial activity after treatment cessation.
MATERIALS AND METHODS
All experiments involving cultures of M. tuberculosis were performed in the biosafety level 3 facilities at the KwaZulu-Natal Research Institute for TB-HIV, Durban, South Africa. Experiments involving infected animals were performed in the animal biosafety level 3 facilities at the Biomedical Resources Unit, College of Health Sciences, University of KwaZulu-Natal (UKZN), Westville, South Africa. Ethical clearance was received from the UKZN Animal Ethics Subcommittee (050/13/Animal and 025/14/Animal), and all procedures were performed according to approved standard operating procedures of the UKZN.
Animals.
Two hundred forty-two female BALB/c mice aged 4 to 6 weeks (18 to 20 g), bred in-house, were used in the experiment. The mice were housed in ventilated cages of 5 mice per cage, with access to food and water ad libitum. At sacrifice time points, mice were first anesthetized by using the drop method (Isofor; Safeline Pharmaceuticals) and then killed by either cervical dislocation or cardiac exsanguination. Lungs and spleens were collected from all mice, and blood was collected from mice that had been administered clofazimine.
Infection.
The mouse model of chronic TB was used in this experiment to ensure that all mice (treated or untreated) survived for the duration of the study (22). Mice were infected by aerosol with M. tuberculosis strain H37Rv (the broth used for infection contained 6.24 log10 CFU/ml) in two infection runs by using a full-size inhalation exposure system (Glas-Col) as previously described (16). The day after infection, six mice (three from each infection run) were sacrificed to assess the number of CFU implanted in the lungs.
Drug preparation.
All drugs were purchased from Sigma-Aldrich Inc. Rifampin was prepared as a solution in distilled water for administration at 10 mg/kg in a single dose of 0.2 ml. Isoniazid (10 mg/kg), pyrazinamide (150 mg/kg), and ethambutol (100 mg/kg) were prepared together as a solution in distilled water to be administered as a single dose in 0.2 ml. Clofazimine was prepared as a suspension in 0.05% (wt/vol) agarose for administration at 25 mg/kg as a single dose in 0.2 ml.
Treatment.
Treatment began 4 weeks after infection; on the day of treatment initiation, six mice (three from each infection run) were sacrificed to determine the pretreatment lung and spleen CFU counts. Mice were randomized into one of the following regimens: (i) no-drug control (30 mice); (ii) isoniazid alone (isoniazid has a short half-life and no tissue binding and thus has no sustained tissue concentrations) (50 mice); (iii) clofazimine alone (50 mice); (iv) the standard, first-line regimen control rifampin-isoniazid-pyrazinamide-ethambutol (RHZE) (50 mice); or (v) the 4-drug test combination of rifampin-isoniazid-pyrazinamide-clofazimine (RHZC) (50 mice). Treatment was administered daily (5 days per week, Monday to Friday), and all drugs were administered by oral gavage. To avoid adverse pharmacokinetic interactions (23, 24), rifampin was administered at least 1 h before the isoniazid-pyrazinamide combination or isoniazid-pyrazinamide-ethambutol combination. Because clofazimine was prepared separately as a suspension, it was also administered separately, at least 30 min after isoniazid-pyrazinamide. The mice were treated for either 4 or 8 weeks (25 mice from each treatment group for each treatment duration) and not for a longer duration because mice treated with a clofazimine-containing first-line regimen can be cured after 3 months of treatment (10). In addition, groups of mice were maintained for up to 8 weeks after treatment was stopped for the assessment of sustained antimicrobial activity. The experimental scheme is illustrated in Fig. S1 in the supplemental material.
Assessment of antimicrobial activity.
Antimicrobial activity was measured by determining the decline in CFU counts in the lungs and spleens after 4 or 8 weeks of treatment (five mice from each treatment group were sacrificed at each of these time points). The sustained antimicrobial activity was measured by monitoring the change in CFU counts in the lungs and spleens after treatment was stopped (five mice from each treatment group were sacrificed 2, 4, 6, and 8 weeks after cessation of treatment that was administered for 4 or 8 weeks). Quantitative CFU counts were performed by plating lung and spleen homogenates onto 7H11 selective agar as previously described (16). To detect and minimize the effect of drug carryover, the lung and spleen homogenates of clofazimine-treated mice were additionally plated onto 7H11 selective agar supplemented with 0.4% (wt/vol) activated charcoal as previously described (16). The dilution that yielded CFU counts closest to 50 was used to calculate the total CFU in the organs, with the CFU counts from either the plain or charcoal-containing agar plate (whichever plate had the highest count) being used for the calculation. Raw CFU count data are presented in Tables S1 (lungs) and S2 (spleens) in the supplemental material; the specific dilution and plate counts used to determine the bacterial burden in each organ in each mouse are also indicated in these tables.
Liquid chromatography-mass spectrometry.
Clofazimine levels in serum were measured by liquid chromatography-mass spectrometry (LC-MS), as previously described (10).
Statistical analyses.
Statistical analyses were performed by using GraphPad Prism version 6.05. CFU counts were log transformed before analysis. Group means were compared by using two-way analysis of variance with Tukey's multiple-comparison test.
RESULTS
Establishment of infection.
The day after aerosol infection, the mean CFU count in the lungs was 2.87 log10 CFU (standard deviation [SD], 0.16 log10 CFU). Four weeks later, on the day of treatment initiation, the mean CFU counts were 7.04 log10 CFU (SD, 0.33 log10 CFU) and 4.97 log10 CFU (SD, 0.37 log10 CFU) in the lungs and spleens, respectively. In untreated control mice, the log10 CFU counts in the lungs and the spleens remained relatively stable at ∼7 log10 CFU (range, 6.82 to 7.45 log10 CFU) and 5 log10 CFU (range, 4.77 to 5.24 log10 CFU), respectively, throughout the course of the experiment (Fig. 1; see also Tables S1 and S2 in the supplemental material), confirming that chronic M. tuberculosis infection was established in the mice.
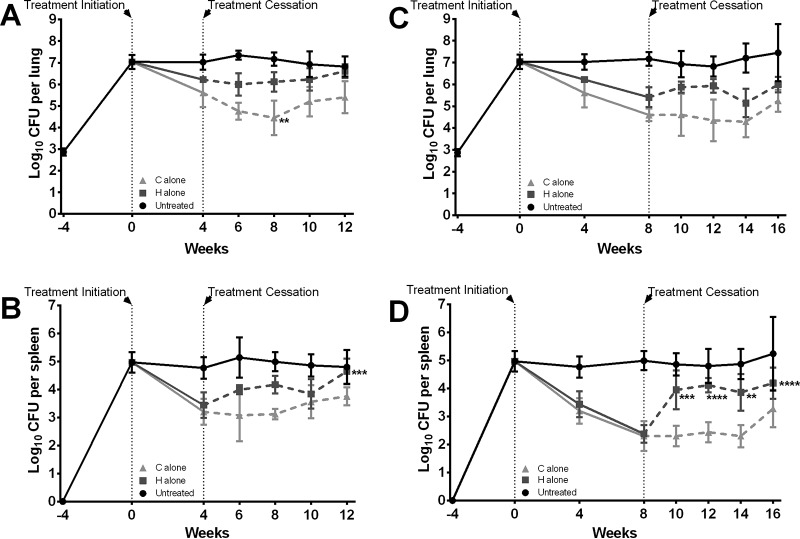
FIG 1.
Assessment of CFU counts in the organs before, during, and after treatment with isoniazid or clofazimine alone. Treatment was administered for 4 weeks (A and B) or 8 weeks (C and D), and CFU counts were determined in the mouse lungs (A and C) and spleens (B and D) at the indicated time points. Data represent the means (4 to 6 mice per group per time point), and the error bars represent the standard deviations. CFU counts are presented in Tables S1 and S2 in the supplemental material. C, clofazimine administered at 25 mg/kg; H, isoniazid administered at 10 mg/kg. Asterisks represent statistically significant differences in CFU counts posttreatment compared to those at the time of treatment cessation (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Antimicrobial activity during treatment.
The declines in mean CFU counts in the lungs and spleens of mice treated for 4 weeks with isoniazid alone were 0.8 and 1.5 log10 CFU, respectively (Fig. 1A and B; see also Tables S1 and S2 in the supplemental material), and the declines in the lungs and spleens of mice treated for 8 weeks were 1.6 and 2.6 log10 CFU, respectively (Fig. 1C and D; see also Tables S1 and S2 in the supplemental material). In mice treated for 4 weeks with clofazimine alone, the declines in mean log10 CFU counts in the lungs and spleens were 1.4 and 1.8 log10 CFU, respectively, and in mice treated for 8 weeks, the declines were 2.4 log10 CFU (lungs) and 2.7 log10 CFU (spleens).
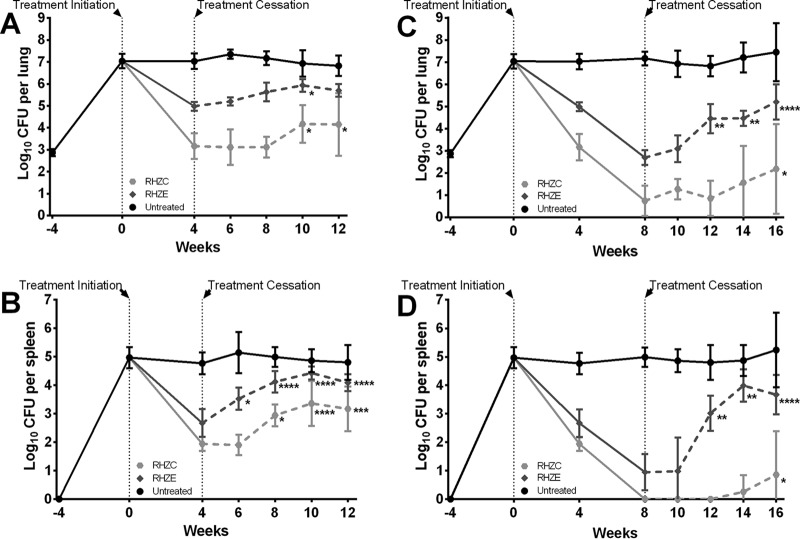
In mice treated for 4 weeks with the standard RHZE regimen, the declines in the mean CFU counts in the lungs and spleens were 2.1 log10 CFU and 2.3 log10 CFU, respectively (Fig. 2A and B; see also Tables S1 and S2 in the supplemental material), and in mice treated for 8 weeks, the declines were 4.4 log10 CFU (lungs) and 4.0 log10 CFU (spleens) (Fig. 2C and D; see also Tables S1 and S2 in the supplemental material). In mice treated with the same regimen, except with clofazimine in place of ethambutol, the declines in mean CFU counts in the lungs and spleens were 3.9 and 3.0 log10 CFU, respectively, after 4 weeks of drug administration and 6.3 and >5.0 log10 CFU, respectively, after 8 weeks of drug administration. Differences were not observed in the gross pathological appearances of the lungs or the spleens of mice during treatment (see Fig. S2 and S3 in the supplemental material).
FIG 2.
Assessment of CFU counts in the organs before, during, and after treatment with combination regimens. Treatment was administered for 4 weeks (A and B) or 8 weeks (C and D), and CFU counts were determined in mouse lungs (A and C) and spleens (B and D) at the indicated time points. Data represent the means (4 to 6 mice per group per time point), and the error bars represent the standard deviations. CFU counts are presented in Tables S1 and S2 in the supplemental material. R, rifampin administered at 10 mg/kg; H, isoniazid administered at 10 mg/kg; Z, pyrazinamide administered at 150 mg/kg; E, ethambutol administered at 100 mg/kg; C, clofazimine administered at 25 mg/kg. Asterisks represent statistically significant differences in CFU counts posttreatment compared to those at the time of treatment cessation (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
CFU counts after treatment cessation.
In mice treated for 4 weeks (Fig. 1A and B; see also Tables S1 and S2 in the supplemental material) with isoniazid alone, there was no or a limited increase of the CFU counts in lungs and spleens during the 8 weeks after stopping treatment; however, the mean spleen CFU counts at 8 weeks posttreatment (at week 12 in Fig. 1B) were significantly higher than those at the end of treatment (P ≤ 0.001). In mice treated for 4 weeks with clofazimine alone, CFU counts in the lungs continued to decline for as long as 4 weeks after treatment was stopped, with the mean CFU count at week 8 being significantly lower than the CFU count at the end of treatment (P ≤ 0.01) (Fig. 1A). The CFU counts began to increase by 6 weeks posttreatment, and by 8 weeks posttreatment, the lung CFU counts were significantly higher than those at 4 weeks posttreatment (P ≤ 0.05). In the spleens, the CFU counts did not decline after treatment was stopped, and there was no or a limited increase in CFU counts throughout the 8-week follow-up period.
In mice treated for 8 weeks (see Tables S1 and S2 in the supplemental material) with isoniazid alone, the CFU counts in the lungs remained relatively stable (Fig. 1C), while they increased in the spleens immediately after treatment was stopped (Fig. 1D), becoming significantly higher than those at the end of treatment as soon as 2 weeks later (P ≤ 0.01, at most, for each posttreatment time point). In mice treated for 8 weeks with clofazimine alone, the CFU counts in the lungs and spleens were not significantly different than the CFU counts at the end of treatment during the entire 8 weeks of follow-up, although the data suggest that the bacteria were starting to multiply at the final time point (8 weeks after treatment was stopped).
In mice treated for 4 weeks with the RHZE standard regimen, the CFU counts in the lungs (Fig. 2A) began to slightly increase immediately after treatment was stopped, becoming significantly higher (than those at the end of treatment) by 6 weeks posttreatment (P ≤ 0.05). The CFU counts in the spleens (Fig. 2B) also began to rise immediately after treatment was stopped, becoming significantly higher by 2 weeks posttreatment than those at the end of treatment (P ≤ 0.05, at most, for each posttreatment time point). In mice treated for 4 weeks with RHZC, the lung CFU counts remained stable for at least 4 weeks after treatment was stopped; by 6 weeks posttreatment (at week 10 in Fig. 2A), there was evidence of bacterial growth. In the spleens, bacterial growth was evident by 4 weeks (at week 6 in Fig. 2B) after treatment cessation.
In mice treated for 8 weeks with RHZE, the CFU counts in both the lungs and spleens increased starting 4 weeks after treatment was stopped (at week 12 in Fig. 2C and D). In mice treated with RHZC for 8 weeks, bacterial regrowth was suppressed for as long as 6 weeks (at week 16 in Fig. 2C and D) after treatment was stopped. No differences were observed in the gross pathological appearance in the lungs or the spleens of mice after treatment was stopped (see Fig. S2 and S3 in the supplemental material).
Drug carryover.
The drug carryover of clofazimine released from homogenized tissues can prevent the growth of M. tuberculosis on standard 7H11 agar, resulting in artificially low CFU counts (10, 11). To detect and minimize the impact of any clofazimine carryover, the lung and spleen homogenates from clofazimine-treated mice were plated in parallel onto standard (plain) 7H11 selective agar and onto 7H11 selective agar supplemented with 0.4% activated charcoal to adsorb the drug. Evidence of clofazimine carryover in the neat lung homogenates was observed at all time points in this study (during treatment and throughout the 8-week posttreatment follow-up) in mice that received clofazimine alone or in combination for either 4 or 8 weeks (see Table S1 in the supplemental material). Carryover was not observed in any 10-fold dilutions of lung homogenates. Clofazimine carryover was also observed in the neat spleen homogenates during treatment and up 2 weeks posttreatment in mice that were treated with either clofazimine-containing regimen for either 4 or 8 weeks (see Table S2 in the supplemental material).
Clofazimine concentrations in serum.
The average clofazimine concentrations in the serum of mice treated for 4 weeks with clofazimine alone or in combination with rifampin-isoniazid-pyrazinamide were similar, at ∼1.0 μg/ml. Two weeks after treatment cessation, clofazimine concentrations in these mice were near 0.25 μg/ml (the MIC for M. tuberculosis [11]). Thereafter, the serum levels fell below 0.25 μg/ml but remained close to 0.10 μg/ml during the remainder of the 8 weeks of follow-up (Table 1). In mice treated for 8 weeks with clofazimine alone or in combination, the clofazimine concentrations in serum were just above 1.5 μg/ml. The serum levels remained above the MIC for M. tuberculosis for up to 4 weeks after treatment was stopped. Thereafter, the clofazimine concentrations fell below 0.25 μg/ml but remained at ∼0.10 μg/ml up to the end of the 8-week follow-up period.
TABLE 1.
Total clofazimine concentrations during and after treatment in sera of mice that were administered clofazimine (alone or in combination)a
| Regimen | Mean (SD) serum clofazimine concn (μg/ml) at time point(s) (wk treatment + wk posttreatment) of: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 4 + 2 | 4 + 4 | 4 + 6 | 4 + 8 | 8 | 8 + 2 | 8 + 4 | 8 + 6 | 8 + 8 | |
| C alone | 1.19 (0.48) | 0.21 (0.28) | 0.16 (0.08) | 0.07 (0.04) | 0.09 (0.12) | 1.55 (0.85) | 0.38 (0.08) | 0.40 (0.24) | 0.13 (0.07) | 0.06 (0.03) |
| RHZC | 1.01 (0.33) | 0.48 (0.55) | 0.20 (0.09) | 0.18 (0.21) | 0.10 (0.04) | 1.73 (0.52) | 0.65 (0.30) | 0.28 (0.10) | 0.13 (0.07) | 0.10 (0.08) |
H, isoniazid at 10 mg/kg; R, rifampin at 10 mg/kg; Z, pyrazinamide at 150 mg/kg; E, ethambutol at 100 mg/kg; C, clofazimine at 25 mg/kg.
DISCUSSION
When clofazimine was first described by Vincent Barry in the 1950s, it was suggested by Barry and subsequently by others that the tissue accumulation and long half-life of this drug could potentially be exploited to provide antimicrobial activity after treatment is stopped (2, 25, 26). Here, we have demonstrated that, indeed, clofazimine, given alone or in combination with other drugs, contributes sustained antimicrobial activity postadministration in the mouse model of TB chemotherapy. In this study, the duration of clofazimine's sustained antimicrobial activity was positively associated with both the duration of administration and the postadministration time that the serum clofazimine concentration remained near or above the MIC for M. tuberculosis, two factors which likely are tightly correlated.
A key finding of this study is this apparent relationship between serum clofazimine levels and antimicrobial activity. Although the tissue clofazimine concentrations were not specifically assessed in the current study, it is well appreciated that the drug accumulates to extremely high levels in the tissues. For example, in an extensive pharmacokinetic study of clofazimine conducted in BALB/c mice, the clofazimine concentrations were ∼50 μg/g tissue in the lungs and liver and ∼100 μg/g tissue in the spleen after 4 to 8 weeks of administration of 25 mg/kg/day clofazimine (16). Six months after treatment was stopped, the clofazimine concentration in each of these tissues remained at or above the MIC value for M. tuberculosis. Thus, in the 8-week follow-up period after treatment cessation in this study, we expect that clofazimine concentrations in the tissues remained quite high; however, bacterial multiplication was suppressed only when the serum clofazimine concentration was at or above the MIC for M. tuberculosis. Thus, our data suggest that the accumulated clofazimine in the tissues, whether dissolved in fat or sequestered in macrophages, does not exhibit antimicrobial activity. However, the accumulated clofazimine may contribute to the sustained antimicrobial activity via release of the drug from the tissues into the blood. This hypothesis is further supported by the observation that the carryover of clofazimine released in neat lung homogenates was able to prevent bacterial growth on non-charcoal-containing plates throughout the 8-week posttreatment follow-up period (see Table S1 in the supplemental material), indicating that the considerable amount of drug that remained in the tissues was not able to prevent bacterial multiplication in the lungs.
If clofazimine exerts similar sustained antimicrobial activity in humans, the benefit of treatment with this drug may clinically extend over the “official” treatment duration. It is possible that the treatment-shortening potential observed with the 3-month clofazimine-containing first-line regimen in the mouse model (10) is related in part to the sustained antimicrobial activity of clofazimine after treatment cessation. It is also possible that clofazimine administration could be discontinued after the second month in a 3-month clofazimine-containing first-line regimen without compromising the overall antimicrobial activity of the drug combination because of the observed 4-week-long sustained antimicrobial activity after treatment is stopped. Even in the case of the other drugs being discontinued, the sustained activity would be occurring at a point in treatment when the bacterial burden would be extremely low (when only a few persisting organisms remain) and the selection of clofazimine-resistant bacteria would not be expected to occur, a situation analogous to the use of isoniazid alone as preventive therapy for individuals with latent TB.
It is unknown if carryover of clofazimine occurs in sputum samples from patients receiving this drug, which would be an important consideration in any clinical evaluation of clofazimine in patients. One indication that carryover may occur is the finding that patients with multidrug-resistant TB who have received clofazimine as part of their treatment regimen tend to experience sputum culture conversion before sputum smear conversion (9, 27). As sputum smear microscopy is well known to be less sensitive for the detection of M. tuberculosis than sputum culture, it is possible that the earlier culture conversion may in fact be a false-negative culture because clofazimine in the sputum is preventing the growth of the bacteria in culture. A similar phenomenon has been reported in TB patients who have received bedaquiline (28), a drug also known to accumulate to high levels in tissues and to be associated with carryover in experimental chemotherapy studies (29). One may, however, suppose that the clofazimine concentration in bronchial secretions and sputum is the result of clofazimine diffusing from the serum and therefore is relatively low but microbiologically active. A study specifically designed to assess carryover in human sputum will be necessary to truly address this key issue.
The relatively short duration of the sustained antimicrobial activity of clofazimine may be of practical interest for the methods used in the laboratory to limit to the greatest possible extent the undesirable impact of the long half-life and tissue accumulation of the drug. In previous studies assessing clofazimine-containing regimens, relapse was assessed 6 months after cessation of treatment to ensure that all or most of the clofazimine was cleared from the tissues to allow the greatest opportunity for persisting M. tuberculosis to regrow (10, 11). Here, we have shown that persisting bacteria regrow just 4 weeks after treatment cessation, suggesting that a 3-month follow-up period after cessation of treatment may be sufficient for relapse assessment. However, cultures would still need to be done on charcoal-containing selective agar to neutralize the effect of clofazimine carryover from tissues.
A second key finding of this study is the potent bactericidal activity that clofazimine contributes to the first-line drug combination of rifampin-isoniazid-pyrazinamide. Compared to treatment with the standard RHZE regimen, mice that received the clofazimine-containing RHZC regimen experienced ∼2 log10 CFU more killing in the lungs and ∼1 log10 CFU more killing in the spleens after 4 or 8 weeks of treatment (Fig. 1 and 2). These data are consistent with previously reported findings in which the RHZC regimen, in comparison with RHZE, exhibited high bactericidal activity of the same magnitude in the lungs and spleens of M. tuberculosis-infected BALB/c mice, and this increase in bactericidal activity was evident by the second week of treatment (16). Considering the results from these two studies, it is reasonable to expect a treatment-shortening effect of combining clofazimine with first-line drugs in the treatment of patients with TB if the main pharmacokinetic parameters of clofazimine in the mouse are applicable to humans. The decades of experience utilizing clofazimine for multidrug treatment of leprosy patients at daily doses of 50 to 100 mg demonstrate that clofazimine can be safely administered to humans for long periods of time, with the most common adverse reaction being reversible skin discoloration and ichthyosis (12). Thus, clofazimine is extremely unique in that it is an off-patent, available drug that could immediately be evaluated as a repurposed drug for the treatment of TB, and it seems advisable that the contribution of clofazimine as a first-line drug for the treatment of patients with TB should be assessed.
Supplementary Material
ACKNOWLEDGMENT
We thank Elaine M. Bautista for assistance with the figures in the supplemental material.
Funding Statement
This work was funded by the KwaZulu-Natal Research Institute for Tuberculosis and HIV (K-RITH).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00177-16.
REFERENCES
- 1.Barry VC, Conalty ML, Gaffney EE. 1956. Antituberculosis activity in the phenazine series; isomeric pigments obtained by oxidation of o-phenylenediamine derivatives. J Pharm Pharmacol 8:1089–1096. doi: 10.1111/j.2042-7158.1956.tb12238.x. [DOI] [PubMed] [Google Scholar]
- 2.Barry VC, Belton JG, Conalty ML, Denneny JM, Edward DW, O'Sullivan JF, Twomey D, Winder F. 1957. A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature 179:1013–1015. doi: 10.1038/1791013a0. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2004. Multidrug therapy against leprosy: development and implementation over the past 25 years. WHO/CDS/CPE/CEE/2004.46. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Dooley KE, Obuku EA, Durakovic N, Belitsky V, Mitnick C, Nuermberger EL, Efficacy Subgroup, RESIST-TB . 2013. World Health Organization group 5 drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J Infect Dis 207:1352–1358. doi: 10.1093/infdis/jis460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2013. Multidrug-resistant tuberculosis (MDR-TB). 2013 update. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL. 2014. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 8.Piubello A, Harouna SH, Souleymane MB, Boukary I, Morou S, Daouda M, Hanki Y, Van Deun A. 2014. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 18:1188–1194. doi: 10.5588/ijtld.13.0075. [DOI] [PubMed] [Google Scholar]
- 9.Kuaban C, Noeske J, Rieder HL, Ait-Khaled N, Abena Foe JL, Trébucq A. 2015. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 19:517–524. doi: 10.5588/ijtld.14.0535. [DOI] [PubMed] [Google Scholar]
- 10.Tyagi S, Ammerman NC, Li SY, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li SY, Ammerman NC, Bishai WR, Enarson D, Trébucq A. 2013. Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188:608–612. doi: 10.1164/rccm.201304-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang S, Yao L, Hao X, Liu Y, Zeng L, Liu G, Li M, Li F, Wu M, Zhu Y, Sun H, Gu J, Wang X, Zhang Z. 2015. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis 60:1361–1367. doi: 10.1093/cid/civ027. [DOI] [PubMed] [Google Scholar]
- 13.Levy L. 1974. Pharmacologic studies of clofazimine. Am J Trop Med Hyg 23:1097–1109. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield RE. 1974. Tissue concentrations of clofazimine (B663) in man. Am J Trop Med Hyg 23:1116–1119. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee DK, Ellard GA, Gammon PT, Waters MF. 1974. Some observations on the pharmacology of clofazimine (B663). Am J Trop Med Hyg 23:1110–1115. [DOI] [PubMed] [Google Scholar]
- 16.Swanson RV, Adamson J, Moodley C, Ngcobo B, Ammerman NC, Dorasamy A, Moodley S, Mgaga Z, Tapley A, Bester LA, Singh S, Grosset JH, Almeida DV. 2015. Pharmacokinetics and pharmacodynamics of clofazimine in the mouse model of tuberculosis. Antimicrob Agents Chemother 59:3042–3051. doi: 10.1128/AAC.00260-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougall AC. 1974. Electron microscope studies of the antileprosy drug B663 (clofazimine; Lamprene). Int J Lepr Other Mycobact Dis 42:1–12. [PubMed] [Google Scholar]
- 18.Aplin RT, McDougall AC. 1975. Identification of crystals of the rimino-phenazine compound B663 (Lamprene: clofazimine) in mouse spleen macrophages by thin layer chromatography and mass spectrum analysis. Experientia 31:468–469. doi: 10.1007/BF02026384. [DOI] [PubMed] [Google Scholar]
- 19.Baik J, Rosania GR. 2012. Macrophages sequester clofazimine in an intracellular liquid crystal-like supramolecular organization. PLoS One 7:e47494. doi: 10.1371/journal.pone.0047494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baik J, Stringer KA, Mane G, Rosania GR. 2013. Multiscale distribution and bioaccumulation analysis of clofazimine reveals a massive immune system-mediated xenobiotic sequestration response. Antimicrob Agents Chemother 57:1218–1230. doi: 10.1128/AAC.01731-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosset J, Ji B. 1998. Experimental chemotherapy of mycobacterial diseases, p 51–97. In Gangadharam PRJ, Jenkins PA (ed), Mycobacteria, vol 2 Chemotherapy. Chapman & Hall, New York, NY. [Google Scholar]
- 23.Dickinson J, Guy A, Mitchison DA. 1992. Bioavailability of rifampin in experimental murine tuberculosis. Antimicrob Agents Chemother 36:2066–2067. doi: 10.1128/AAC.36.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noufflard H, Berteaux S. 1958. Activité antituberculeuse du produit B-663. I. Action in vitro et sur la tuberculose expérimentale de la souris en traitement immédiat (test de protection). Ann Inst Pasteur (Paris) 95:449–455. [PubMed] [Google Scholar]
- 26.Grumbach F. 1960. Activité antituberculeuse expérimentale de deux dérivés de phénazine pigmentée (B 663 et B 720) seuls et associés a d'autres antituberculeux (isoniazide et éthioniamide). Ann Inst Pasteur (Paris) 99:567–585. [PubMed] [Google Scholar]
- 27.Union Internationale contre la Tuberculose et les Maladies Respiratoires. 2016. Bulletin de la tuberculose en Afrique francophone. No. 6. Union Internationale contre la Tuberculose et les Maladies Respiratoires, Paris, France. [Google Scholar]
- 28.Guglielmetti L, Le Dû D, Jachym M, Henry B, Martin D, Caumes E, Veziris N, Métivier N, Robert J, MDR-TB Management Group of the French National Reference Center for Mycobacteria, and the Physicians of the French MDR-TB Cohort. 2015. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 60:188–194. doi: 10.1093/cid/ciu786. [DOI] [PubMed] [Google Scholar]
- 29.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.