Abstract
Measurement of vancomycin susceptibility has been shown to be highly predictive as a surrogate measure of oritavancin susceptibility among clinically indicated Gram-positive species. Results of studying over 30,000 pathogens (from 2011 to 2014) by cross-susceptibility analysis and determining the poor reproducibility of oritavancin-nonsusceptible results showed nearly perfect surrogate testing accuracy (99.86 to 99.94%). Any isolate of an indicated organism species with locally reproducible oritavancin-nonsusceptible results (extremely rare) should be referred to a reference laboratory for confirmation of the results and determination of the resistance mechanism.
TEXT
Oritavancin, a novel lipoglycopeptide, was recently approved for single-dose treatment of acute bacterial skin and skin structure infections (ABSSSIs) in the United States and Europe (1, 2). The compound (formerly LY333328) has a long history of in vitro evaluations and drug development, dating from the late 1990s (3–5) and documenting a broad anti-Gram-positive organism spectrum comparable to those of vancomycin and teicoplanin. Initial worldwide surveillance (5) suggested that oritavancin had activity similar to that of existing glycopeptides; reference methods were subsequently modified to recognize the greater oritavancin activity (8- to 16-fold superior) against Staphylococcus aureus and various streptococcal species (1, 2, 6, 7).
The physicochemical characteristics of lipoglycopeptides (oritavancin, dalbavancin, and telavancin) are particularly challenging for the development of standardized in vitro susceptibility testing methods. Poor drug diffusion through agar-based media limits the application of the agar disk diffusion method, as published by several international standards organizations (8), and the binding of drug to various plastics compromises dilution MIC testing (7, 9). Reference broth microdilution methods (10, 11) for oritavancin require the surfactant polysorbate-80 (P-80) (0.002%) to recognize full antimicrobial potency, and adaption of commonly used commercial devices appears to be uncertain. Such delays in the use of commercial susceptibility testing systems to direct oritavancin chemotherapy may necessitate alternative testing strategies, such as the use of vancomycin susceptibility testing results as a surrogate predictive measure (12). In this investigation, (i) we update the analysis of 2011-2013 oritavancin surveillance results (12) for validation of the use of vancomycin MIC results to infer oritavancin susceptibility, following retesting of strains with previous nonsusceptible results, and (ii) we present 2014 oritavancin surveillance data to confirm the high predictive value of surrogate vancomycin testing for oritavancin susceptibility.
European and U.S. oritavancin resistance surveillance isolates from 2011 to 2013 (26,993 isolates; 22,606 indicated species) and 2014 (10,002 isolates; 7,688 indicated species) were tested by validated reference broth microdilution methods (10). For species and antimicrobial-resistant subset details, refer to Tables 1 and 2, as well as to reference 12. The interpretive breakpoint criteria used for oritavancin were those selected by the U.S. Food and Drug Administration (FDA) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), and the criteria for vancomycin were those published by the Clinical and Laboratory Standards Institute (CLSI) (11, 13–15). All quality control (QC) results were within published ranges (13), using the following QC organisms: S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 49619.
TABLE 1.
Accuracy of vancomycin susceptibility test results (2011 to 2013) to infer susceptibility to oritavancin at U.S. FDA-approved breakpoints (14) for indicated Gram-positive pathogens (22,606 isolates)
| Speciesa | No. of isolates tested | Surrogate accuracy (%) |
|
|---|---|---|---|
| Jones et al. (12) | Current reanalysis | ||
| S. aureus | 17,717 | 98.8 | >99.9 |
| β-Hemolytic streptococcib | 2,357 | 98.1 | 99.8 |
| S. anginosus groupc | 368 | 100.0 | 100.0 |
| E. faecalis, vancomycin susceptible | 2,164 | 99.7 | 99.7 |
| Overall | 22,606 | 98.85 | 99.94 |
Indicated species with interpretive criteria only (14).
Includes Streptococcus pyogenes, S. agalactiae, and S. dysgalactiae.
Includes Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius.
TABLE 2.
Accuracy of vancomycin susceptibility test results (2014) to infer susceptibility to oritavancin at U.S. FDA-approved breakpoints (14) for indicated Gram-positive species groups (10,002 isolates)
| Species | No. of isolates tested | Surrogate accuracy (% [no. of nonsusceptible isolates]) |
|---|---|---|
| S. aureusa | 5,609 | >99.9 (3) |
| CoNSb | 641 | 100.0 (0) |
| β-Hemolytic streptococcia | 1,049 | 99.2 (8) |
| S. anginosus groupa | 178 | 100.0 (0) |
| S. pneumoniaeb | 1,673 | 100.0 (0) |
| Enterococcia | 852 | 100.0 (0) |
| Overalla | 7,688 | 99.86 (11) |
Includes the indicated species (Staphylococcus aureus, Streptococcus pyogenes, S. agalactiae, S. dysgalactiae, S. anginosus, S. constellatus, S. intermedius, and vancomycin-susceptible E. faecalis among all enterococci).
S. aureus interpretive criteria (susceptible, [0.12 μg/ml) were applied to coagulase-negative staphylococci (CoNS) and pneumococci for the vancomycin surrogate test comparison only; no errors were observed.
An earlier publication (12) quantified the predictive accuracy of using vancomycin susceptibility to infer susceptibility to oritavancin (Table 1). Across all U.S. FDA-indicated species for oritavancin treatment (22,606 isolates) (14), the surrogate accuracy was 98.85%, ranging from 98.7% (β-hemolytic streptococci) to 100.0% (Streptococcus anginosus group). Subsequent to that publication, organisms with oritavancin-nonsusceptible MIC values (10) were retested to determine MIC reproducibility. Among 215 retested isolates of S. aureus, only 1.4% (3 isolates) had reproducible oritavancin MIC results of ≥0.25 μg/ml. Similarly, repeated β-hemolytic streptococcal reproducibility ranged from nil (Streptococcus pyogenes) to 6.7% (Streptococcus agalactiae). The most frequently reproducible oritavancin-nonsusceptible results were recorded for Streptococcus dysgalactiae (MIC of 0.5 μg/ml, 1 doubling dilution above the breakpoint).
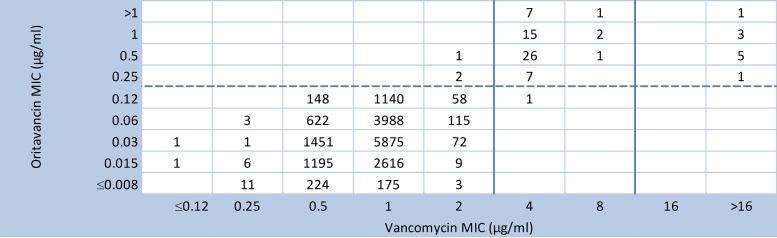
Figure 1 (modified from reference 12) provides updated vancomycin surrogate accuracy data for 17,717 S. aureus isolates following oritavancin MIC retesting. The vancomycin surrogate accuracy rate improved to >99.9%, and the overall acceptable cross-susceptibility rate for all indicated species increased to 99.94% (formerly 98.85%) (Table 1).
FIG 1.
Chart comparing vancomycin and oritavancin MIC results for 17,717 S. aureus isolates obtained between 2011 and 2013. Data for 60 vancomycin-intermediate S. aureus isolates (vancomycin MICs of 4 or 8 μg/ml) and 10 vancomycin-resistant S. aureus isolates (MICs of 64 to 1,024 μg/ml) are also shown (originally reported in reference 24). CLSI breakpoints (solid vertical line) and U.S. FDA breakpoints (dashed horizontal line) for vancomycin and oritavancin, respectively (13, 14), as modified from the report by Jones et al. (12), are shown.
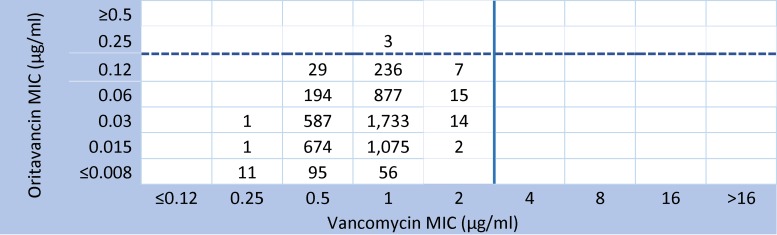
In the vancomycin surrogate cross-susceptibility validations using the most recent 2014 oritavancin surveillance isolates from the United States and Europe (Table 2), all oritavancin-nonsusceptible isolates were routinely retested. A similar pattern of very high cross-susceptibility (accuracy) between vancomycin and oritavancin was encountered, compared to the 2011-2013 statistics (Table 1). For the 7,688 Gram-positive isolates from the indicated species, the predictive accuracy of the surrogate vancomycin susceptibility values ranged from 99.32% (β-hemolytic streptococci) to 100.0% (S. anginosus group and vancomycin-susceptible E. faecalis); the overall accuracy was 99.86%. It should be noted that, among all enterococci tested (1,086 isolates), seven vancomycin-intermediate isolates had oritavancin MIC values of 0.004 to 0.015 μg/ml. Although oritavancin is not approved for the treatment of infections caused by vancomycin-resistant enterococci (VRE) (E. faecalis or Enterococcus faecium), 200 VRE isolates tested in this study had oritavancin MIC results ranging from 0.002 to 1 μg/ml and an MIC90 of only 0.12 μg/ml (data not shown). Figure 2 illustrates the high cross-susceptibility rate (>99.9%) among the 5,609 S. aureus isolates in 2014.
FIG 2.
Chart comparing vancomycin and oritavancin MIC results for 5,609 S. aureus surveillance program isolates obtained in the United States and Europe in 2014. Organisms with reproducible oritavancin-nonsusceptible results (MICs of 0.25 μg/ml) are shown (only 3 isolates [0.05%]).
Also found in Table 2 for the 2014 surveillance isolates are the cross-susceptibility statistics for coagulase-negative staphylococci (CoNS) (641 isolates) and S. pneumoniae (1,673 isolates); oritavancin is not indicated for either species (14). All of the isolates for these two pathogen groups were vancomycin susceptible (MICs of ≤1 or ≤2 μg/ml, respectively), except for one isolate of vancomycin-intermediate CoNS. Oritavancin was also very active, with all MIC results being ≤0.06 and ≤0.12 μg/ml for the pneumococci and CoNS, respectively.
Oritavancin has a remarkable combination of high antimicrobial activity against prevalent Gram-positive pathogens (1, 2) and a pharmacokinetic (PK)/pharmacodynamic (PD) profile that justifies single-dose intravenous therapy for ABSSSIs (14, 16, 17). Favorable results from the phase III SOLO I and SOLO II trials demonstrated comparable (noninferior) outcomes, compared to vancomycin-containing regimens (18, 19). Additional in vitro investigations support potential future studies regarding use against VRE infections (20), methicillin-resistant S. aureus (MRSA) infections with the novel mecC gene mechanism or infections emerging in the community setting (21, 22), some S. aureus strains with elevated vancomycin MIC values or heteroresistant variations (23, 24), and uncommonly isolated Gram-positive species (25). Furthermore, favorable in vitro drug combination (synergy) results have been reported for oritavancin testing against staphylococci (26).
With this favorable microbiological, PK/PD, and clinical trial background, oritavancin provides a valuable addition to our antimicrobial armamentarium to treat infections caused by resistant Gram-positive cocci. Unfortunately, direct measurements of oritavancin susceptibility in hospital clinical microbiology laboratories will be compromised by the technical difficulties of in vitro testing for this antimicrobial class (lipoglycopeptides) (7, 9). A highly accurate and simple solution would be to utilize the test results for another drug in the same class (vancomycin, a glycopeptide of lesser potency) to predict oritavancin susceptibility. The results of a 4-year cross-susceptibility analysis of surveillance isolates from the United States and Europe reported here (Tables 1 and 2) confirm the nearly perfect accuracy (99.86 to 99.94%) of this testing option across all species listed by regulators for oritavancin therapy (11, 14). The occurrences of nonsusceptibility to oritavancin among contemporary Gram-positive clinical isolates is exceedingly rare. When oritavancin in vitro tests become available and such oritavancin-nonsusceptible values are observed (a very low probability), the test should be repeated to ensure reproducibility.
Finally, the background data presented here in part have led to the following statements in the oritavancin U.S. FDA product labeling: “The current absence of resistant isolates precludes defining any results other than ‘Susceptible.’ Isolates yielding test results other than ‘Susceptible’ should be retested, and if the result is confirmed, the isolate should be submitted to a reference laboratory for further testing” (14). The recommendation that susceptibility to dalbavancin, oritavancin, and telavancin can be inferred from the vancomycin susceptibility results is also found in the USCAST breakpoint tables (27). Furthermore, the EUCAST has published the following three relevant guidelines regarding oritavancin tests and breakpoints (15). (i) Nonsusceptible isolates are rare or not yet reported. The identification and antimicrobial susceptibility test results for any such isolate must be confirmed and the isolate sent to a reference laboratory. (ii) MICs must be determined in the presence of polysorbate-80 (0.002% in the medium for broth dilution methods; agar dilution methods have not been validated). The manufacturers' instructions should be followed for commercial systems. (iii) S. aureus isolates susceptible to vancomycin can be reported as susceptible to (dalbavancin and) oritavancin. These recommendations have achieved great harmonization internationally regarding susceptibility testing for the lipoglycopeptides, especially oritavancin (11, 14, 15, 27).
ACKNOWLEDGMENTS
R.N.J., P.R.R., and R.E.M. are employees or subcontractors of JMI Laboratories, which coordinates an oritavancin international surveillance program for The Medicines Company. G.M., F.F.A., and M.N.D. are employees of The Medicines Company.
JMI Laboratories, Inc., has also received research and educational grants in 2014-2015 from Achaogen, Actavis, Actelion, Allergan, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Durata, Enteris, Exela, Forest Research Institute, Furiex, Genentech, GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Scynexis, Seachaid, Shionogi, Tetraphase, The Medicines Co., Theravance, ThermoFisher, VenatoRX, Vertex, Wockhardt, Zavante, and some other corporations. Some JMI employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, Cempra, and Theravance. With regard to speakers bureaus and stock options, the authors have nothing to declare.
R.N.J. is the guarantor of the data. We acknowledge the following JMI Laboratories employees for support via analysis and manuscript preparation: M. Janechek, R. Flamm, H. Sader, M. Castanheira, J. Oberholser, J. Schuchert, A. Fuhrmeister, and J. Streit.
REFERENCES
- 1.Syed YY, Scott LJ. 2015. Oritavancin: a review in acute bacterial skin and skin structure infections. Drugs 75:1891–1902. doi: 10.1007/s40265-015-0478-7. [DOI] [PubMed] [Google Scholar]
- 2.Saravolatz LD, Stein GE. 2015. Oritavancin: a long-half-life lipoglycopeptide. Clin Infect Dis 61:627–632. doi: 10.1093/cid/civ311. [DOI] [PubMed] [Google Scholar]
- 3.Nicas TI, Mullen DL, Flokowitsch JE, Preston DA, Snyder NJ, Zweifel MJ, Wilkie SC, Rodriguez MJ, Thompson RC, Cooper RD. 1996. Semisynthetic glycopeptide antibiotics derived from LY264826 active against vancomycin-resistant enterococci. Antimicrob Agents Chemother 40:2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RN, Barrett MS, Erwin ME. 1997. In vitro activity and spectrum of LY333328, a novel glycopeptide derivative. Antimicrob Agents Chemother 41:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeckel ML, Preston DA, Allen BS. 2000. In vitro activities of LY333328 and comparative agents against nosocomial Gram-positive pathogens collected in a 1997 global surveillance study. Antimicrob Agents Chemother 44:1370–1374. doi: 10.1128/AAC.44.5.1370-1374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. 2014. Oritavancin activity against Staphylococcus aureus causing invasive infections in U.S. and European hospitals: a five-year international surveillance program. Antimicrob Agents Chemother 58:2921–2924. doi: 10.1128/AAC.02482-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P, Sahm DF, Parr TR Jr, Moeck G. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 52:1597–1603. doi: 10.1128/AAC.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial disk susceptibility tests; approved standard—12th ed. M02-A12. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB, Goldstein BP. 2007. Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J Clin Microbiol 45:3151–3154. doi: 10.1128/JCM.02411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.EUCAST. 2015. New EUCAST breakpoints, April 2015: dalbavancin, oritavancin, and tedizolid. EUCAST, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/New_EUCAST_breakpoints_final.pdf. [Google Scholar]
- 12.Jones RN, Turnidge JD, Moeck G, Arhin FF, Mendes RE. 2015. Use of in vitro vancomycin testing results to predict susceptibility to oritavancin, a new long-acting lipoglycopeptide. Antimicrob Agents Chemother 59:2405–2409. doi: 10.1128/AAC.05098-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.The Medicines Company. 2014. Orbactiv™ package insert. The Medicines Company, Parsippany, NJ: http://www.orbactiv.com/pdfs/orbactiv-prescribing-information.pdf. [Google Scholar]
- 15.EUCAST. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. EUCAST, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. [Google Scholar]
- 16.Ambrose PG, Drusano GL, Craig WA. 2012. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin Infect Dis 54(Suppl 3):S220–S228. doi: 10.1093/cid/cis001. [DOI] [PubMed] [Google Scholar]
- 17.Rubino CM, Bhavnani SM, Moeck G, Bellibas SE, Ambrose PG. 2015. Population pharmacokinetic analysis for a single 1,200-milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother 59:3365–3372. doi: 10.1128/AAC.00176-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W. 2014. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 19.Corey GR, Good S, Jiang H, Moeck G, Wikler M, Green S, Manos P, Keech R, Singh R, Heller B, Bubnova N, O'Riordan W. 2015. Single-dose oritavancin versus 7–10 days of vancomycin in the treatment of Gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 60:254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 20.Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. 2012. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis 54(Suppl 3):S233–S238. doi: 10.1093/cid/cir924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arhin FF, Sarmiento I, Moeck G. 2014. In vitro activities of oritavancin and comparators against meticillin-resistant Staphylococcus aureus (MRSA) isolates harbouring the novel mecC gene. Int J Antimicrob Agents 44:65–68. doi: 10.1016/j.ijantimicag.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Arhin FF, Kurepina N, Sarmiento I, Parr TR Jr, Moeck G, Kreiswirth B. 2010. Comparative in vitro activity of oritavancin against recent, genetically diverse, community-associated meticillin-resistant Staphylococcus aureus (MRSA) isolates. Int J Antimicrob Agents 35:93–94. doi: 10.1016/j.ijantimicag.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Arhin FF, Sarmiento I, Parr TR Jr, Moeck G. 2009. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J Antimicrob Chemother 64:868–870. doi: 10.1093/jac/dkp286. [DOI] [PubMed] [Google Scholar]
- 24.Vidaillac C, Parra-Ruiz J, Rybak MJ. 2011. In vitro time-kill analysis of oritavancin against clinical isolates of methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin. Diagn Microbiol Infect Dis 71:470–473. doi: 10.1016/j.diagmicrobio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Mendes RE, Sader HS, Flamm RK, Jones RN. 2014. Activity of oritavancin tested against uncommonly isolated Gram-positive pathogens responsible for documented infections in hospitals worldwide. J Antimicrob Chemother 69:1579–1581. doi: 10.1093/jac/dku016. [DOI] [PubMed] [Google Scholar]
- 26.Lin G, Pankuch G, Appelbaum PC, Kosowska-Shick K. 2014. Antistaphylococcal activity of oritavancin and its synergistic effect in combination with other antimicrobial agents. Antimicrob Agents Chemother 58:6251–6254. doi: 10.1128/AAC.02932-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USCAST. 2015. Breakpoint tables for interpretations of MICs and zone diameters, version 1.0. USCAST, Silverton, OR: http://www.uscast.org/breakpoints.html. [Google Scholar]