Abstract
Gram-negative bacteria are evolving to produce β-lactamases of increasing diversity that challenge antimicrobial chemotherapy. OP0595 is a new diazabicyclooctane serine β-lactamase inhibitor which acts also as an antibiotic and as a β-lactamase-independent β-lactam “enhancer” against Enterobacteriaceae. Here we determined the optimal concentration of OP0595 in combination with piperacillin, cefepime, and meropenem, in addition to the antibacterial activity of OP0595 alone and in combination with cefepime, in in vitro time-kill studies and an in vivo infection model against five strains of CTX-M-15-positive Escherichia coli and five strains of KPC-positive Klebsiella pneumoniae. An OP0595 concentration of 4 μg/ml was found to be sufficient for an effective combination with all three β-lactam agents. In both in vitro time-kill studies and an in vivo model of infection, cefepime-OP0595 showed stronger efficacy than cefepime alone against all β-lactamase-positive strains tested, whereas OP0595 alone showed weaker or no efficacy. Taken together, these data indicate that combinational use of OP0595 and a β-lactam agent is important to exert the antimicrobial functions of OP0595.
INTRODUCTION
The global increase in antibiotic-resistant Gram-negative bacteria in recent years is posing a serious medical problem. In many cases, the mechanism underlying bacterial resistance involves the production of β-lactamase. As of 2012, roughly 1,300 β-lactamases had been identified, including many that are not inhibited by established β-lactamase inhibitors such as tazobactam and clavulanic acid and several that hydrolyze carbapenems, which were previously regarded as “β-lactamase stable” (1).
β-Lactamases can be classified according to several schemes. The most fundamental one is the Ambler classification, which is based on amino acid sequence and subdivides the serine β-lactamases into class A, including the common TEM, SHV, CTX-M, and KPC types, class C (e.g., AmpC, CMY, etc.), and class D (OXA). Class B comprises metalloenzymes (e.g., IMP, NDM, etc.) that require divalent cations, generally zinc, for substrate hydrolysis (2).
The spread of extended-spectrum TEM, SHV, and CTX-M β-lactamases has led to increased clinical dependence on carbapenems; however, carbapenemase-producing Enterobacteriaceae spp. are spreading rapidly worldwide (3). The most prevalent types of carbapenemase vary geographically; for example, KPC types are dominant in North and South America, China, Israel, and parts of southern Europe, whereas OXA-48 is prevalent in the Middle East (except Israel) and NDM is common in south Asia (4). Bacteria expressing these enzymes can cause severe infections, and the fatality rate in cases of bloodstream infections involving carbapenemase-producing Klebsiella pneumoniae is high (5).
As a result, new and effective β-lactamase inhibitors are urgently needed, and diazabicyclooctanes such as avibactam, relebactam (MK-7655), and OP0595 are under clinical development for this role (6, 7). Diazabicyclooctanes represent a new class of β-lactamase inhibitor and show strong inhibitory activity against both class A β-lactamases, including KPC types, and class C AmpC enzymes. The initial partner β-lactam agents for avibactam and relebactam have been identified as ceftazidime and imipenem, respectively, whereas that for OP0595 has not been decided. Essentially, avibactam and relebactam restore the antibiotic activity of substrate antibiotics against strains producing serine β-lactamases (8–11).
Differing from avibactam and relebactam, OP0595 acts in three ways: (i) as a β-lactamase inhibitor, (ii) as an antibiotic agent against Enterobacteriaceae, and (iii) as an “enhancer” of the activity of various β-lactam agents (7). As a first approach to characterization of OP0595, its antibacterial activity, alone and in combination with other β-lactam agents, was evaluated here to determine the optimal concentration of OP0595 for combination use. The effect of OP0595 at this concentration was then tested in in vitro time-kill studies and an in vivo model of infection; in these studies, cefepime was selected as the main partner β-lactam agent for OP0595 because the three actions of OP0595 are known to be effective in this combination (7). CTX-M-15-positive Escherichia coli and KPC-positive Klebsiella pneumoniae were tested as key examples of problematic serine β-lactamase-expressing Enterobacteriaceae.
MATERIALS AND METHODS
Compounds.
OP0595 was synthesized by Meiji Seika Pharma Co., Ltd. (Tokyo, Japan), and was evaluated as an anhydride. The antibacterial compounds were obtained from the following sources: piperacillin from Sigma-Aldrich (St. Louis, MO); meropenem from U.S. Pharmacopeial Convention (Rockville, MD); and cefepime from U.S. Pharmacopeial Convention and GlaxoSmithKline K. K. (Tokyo, Japan).
Bacterial strains.
Five strains of CTX-M-15-positive E. coli, MSC20613, MSC20627, MSC20653, MSC20662, and MSC20670, which tested positive for blaCTX-M-15 (GenBank accession number AY044436) by PCR, were clinical isolates from Japan. Screening was conducted to detect the CTX-M type using the method of Yagi et al. (12). CTX-M-15 genes were amplified for sequencing purposes by using in-house-designed primers CTXM15-1 (5′-GTTACAATGTGTGAGAAGCA) and CTXM15-2 (5′-GGAACCACGGAGCTTATGGC) from heat-denatured cells and were detected by using CTXM15-1, CTXM15-2, and CTXM15-3 (5′-TACAGCGGCACACTTCCTAA). PCR products were purified with a MultiScreenHTS (Merck, Darmstadt, Germany), and sequence identification was conducted with a 3730 DNA Analyzer (Applied Biosystems, Carlsbad, CA). Five KPC-positive K. pneumoniae strains, ATCC BAA-1705, ATCC BAA-1898, ATCC BAA-1899, ATCC BAA-1900, and ATCC BAA-1904, were obtained from the American Type Culture Collection.
Susceptibility testing.
The MIC of each compound was determined by broth microdilution, performed according to CLSI guidelines (13, 14). The test inoculum was approximately 5 × 104 CFU/well. The MIC was defined as the lowest concentration to prevent visible growth after incubation at 35°C for 18 to 20 h.
Time-kill experiments.
Time-dependent bactericidal activity was determined with the time-kill method standardized by CLSI guidelines as follows (15). Well-isolated bacterial colonies were added to cation-adjusted Mueller-Hinton broth (CAMHB; Becton, Dickinson and Company, Franklin Lakes, NJ) and cultured for approximately 16 h at 35°C. The cultured bacterial suspensions were diluted with CAMHB and cultured for 2 h while being shaken by 100 rpm at 35°C. An aliquot of 4.9 ml of bacterial suspension was added to a test tube and mixed with 0.1 ml of the test compound solution. The suspension was then cultured at 35°C with shaking at 100 rpm. At time points of 0, 2, 4, 6, and 24 h after addition of the test compound, a sample was collected from the culture medium, serially diluted, spread on a Mueller-Hinton agar (MHA; Becton, Dickinson and Company) plate, and cultured at 35°C. The number of colonies grown on the plate was counted after approximately 24 h. The detection limit was set at <1.3 log10 CFU/ml; if no colonies were detected, the value of 1.3 log10 CFU/ml was adopted. The data were expressed as means ± standard deviations (SD) of log10 CFU per milliliter. A bactericidal effect was defined as a decrease of ≥3 log10 CFU/ml relative to the level seen with the control sample obtained at 0 h.
Neutropenic murine thigh infection model.
All animal studies and protocols were approved by the Animal Experiment Management Committee, Pharmaceutical Research Center, Meiji Seika Pharma Co., Ltd., and were based on Guidelines on the Management of Animal Experiments established by the Pharmaceutical Research Center. Four-week-old, specific-pathogen-free, male Crlj:CD1 (ICR) mice (Charles River Laboratories Japan, Inc., Kanagawa, Japan) weighing 18 to 22 g were used for all tests. These mice were used because a murine model of thigh infection with E. coli and K. pneumoniae has been established for this species (16). The mice were kept in an animal room under controlled conditions (temperature, 21 to 25°C; humidity, 50% to 70%; lighting times, 07:00 to 19:00) and were allowed to acclimatize for 1 week before the study. During the acclimation and study periods, feed (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) and water were available ad libitum.
The mice were rendered neutropenic by intraperitoneal administration of cyclophosphamide (Sigma-Aldrich) 4 days before infection (150 mg/kg of body weight) and the day before infection (100 mg/kg of body weight). Six mice per group were infected with E. coli or K. pneumoniae by the injection of ∼105–6 CFU into the thigh. The mice were treated by subcutaneous administration of the test compound at 1, 3, and 5 h after infection and were euthanized 24 h after infection by cervical dislocation. The thigh was then removed and homogenized. Each homogenate was diluted 10-fold serially with physiological saline solution, and an aliquot of each initial homogenate and dilution series was smeared onto a plate of MHA, which was then cultured at 35°C. The number of colonies grown on the plate was counted after approximately 22 h. The detection limit was set at <2.15 log10 CFU/thigh; if no colonies were detected in the initial homogenate, the value of 2.15 log10 CFU/thigh was adopted. The data were expressed as means ± SD log10 CFU/thigh. The numbers of viable cells in the vehicle and administration groups were compared by using Steel's test.
RESULTS
In vitro combinational concentration determination.
To assess the functional concentration of OP0595 for use with β-lactam agents, the MICs of piperacillin, cefepime, and meropenem in combination with different concentrations of OP0595 were determined against five strains of CTX-M-15-positive E. coli and five strains of KPC-positive K. pneumoniae. The log averages of MICs were plotted as the geometric mean MICs (Fig. 1). For all β-lactam agents, the geometric mean MICs decreased as the OP0595 concentrations increased, regardless of the type of partner β-lactam agent or the bacterial species. In each case, the MIC reached a plateau at 2 to 4 μg/ml of OP0595. These results suggest that a concentration of 4 μg/ml of OP0595 against these strains is sufficient to result in maximal MIC reduction in in vitro studies.
FIG 1.
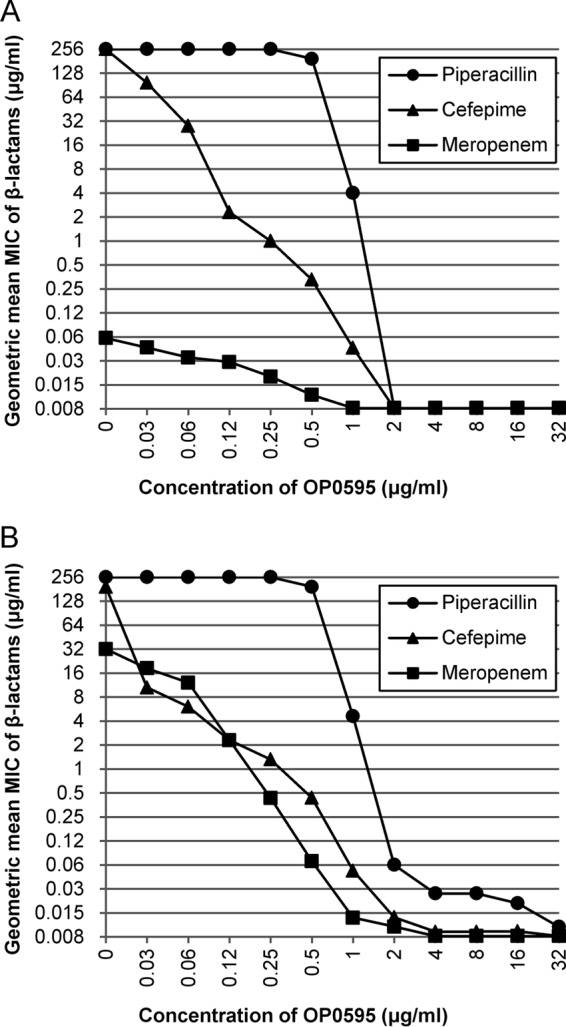
Antimicrobial activity of β-lactam agents in combination with various concentrations of OP0595 against five strains of CTX-M-15-positive E. coli (A) and five strains of KPC-positive K. pneumoniae (B). A MIC of >128 μg/ml was counted as 256 μg/ml in calculating the geometric mean and a MIC of ≤0.008 as 0.008 μg/ml.
Bactericidal activity against β-lactamase-positive Enterobacteriaceae.
Because OP0595 shows antibacterial activity against several strains of Enterobacteriaceae, the MIC of a β-lactam agent is easily masked by its combination with OP0595 (7). To clarify the combinational antibacterial activity of cefepime and OP0595, an assay of bactericidal activity was performed using five strains of CTX-M-15-positive E. coli and five strains of KPC-positive K. pneumoniae. The MICs of OP0595, cefepime, and cefepime-OP0595 against these strains are summarized in Table 1, the bactericidal activities are summarized in Tables 2 and 3, and the time-kill curves of E. coli MSC20653 and K. pneumoniae ATCC BAA-1904 are shown as examples in Fig. 2. The MIC of cefepime-OP0595 was found to be ≤0.004 for all strains at 4 μg/ml of OP0595, but the OP0595 MIC of 4 μg/ml is the MIC value against many strains of Enterobacteriaceae except for K. pneumoniae ATCC BAA-1899. It is difficult to distinguish the antibacterial activity of OP0595 alone from the cefepime-OP0595 combination MIC; as a result, the concentrations of cefepime in this assay were fixed at 0.25/ml and 1 μg/ml, which are 1/16 and 1/2 of the CLSI-designated cefepime susceptibility concentration against Enterobacteriaceae (2 μg/ml) (13). And the concentrations of OP0595 were fixed at 1 and 4 μg/ml.
TABLE 1.
In vitro antibacterial activity of cefepime and OP0595 against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae
| Organism | β-Lactamase | MIC (μg/ml) |
||
|---|---|---|---|---|
| OP0595 | Cefepime | Cefepime-OP0595a | ||
| E. coli MSC20613 | CTX-M-15 | 2 | >64 | ≤0.004 |
| E. coli MSC20627 | CTX-M-15 | 2 | >64 | ≤0.004 |
| E. coli MSC20653 | CTX-M-15, OXA-1 | 2 | >64 | ≤0.004 |
| E. coli MSC20662 | CTX-M-15 | 2 | >64 | ≤0.004 |
| E. coli MSC20670 | CTX-M-15, OXA-1 | 1 | >64 | ≤0.004 |
| K. pneumoniae ATCC BAA-1705 | KPC-2 | 2 | >64 | ≤0.004 |
| K. pneumoniae ATCC BAA-1898 | KPC-2 | 2 | 64 | ≤0.004 |
| K. pneumoniae ATCC BAA-1899 | KPC-2 | 16 | >64 | ≤0.004 |
| K. pneumoniae ATCC BAA-1900 | KPC-3 | 2 | 64 | ≤0.004 |
| K. pneumoniae ATCC BAA-1904 | KPC-3 | 4 | >64 | ≤0.004 |
The combined concentration of OP0595 was fixed at 4 μg/ml.
TABLE 2.
Effect of OP0595 on the bactericidal activity of cefepime against CTX-M-15-positive E. coli
| Concn of cefepime (μg/ml) | Concn of OP0595 (μg/ml) | No. of strains reaching the indicated decrease in CFU/ml after the period shown |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h |
4 h |
6 h |
24 h |
||||||||||
| 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | ||
| Growth control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0.25 | 4 | 3 | 3 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 1 | 1 | 4 | 2 | 1 | 5 | 5 | 4 | 5 | 5 | 5 | 4 | 4 | 4 |
| 1 | 4 | 4 | 2 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 4 | 1 | 0 | 0 | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | |
TABLE 3.
Effect of OP0595 on the bactericidal activity of cefepime against KPC-positive K. pneumoniae
| Concn of cefepime (μg/ml) | Concn of OP0595 (μg/ml) | No. of strains reaching the indicated decrease in CFU/ml after the period shown |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h |
4 h |
6 h |
24 h |
||||||||||
| 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | ||
| Growth control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0.25 | 4 | 5 | 5 | 2 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| 1 | 1 | 5 | 5 | 3 | 5 | 5 | 4 | 5 | 5 | 5 | 3 | 3 | 2 |
| 1 | 4 | 4 | 4 | 3 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 1 | 0 | 0 | 5 | 2 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | |
FIG 2.
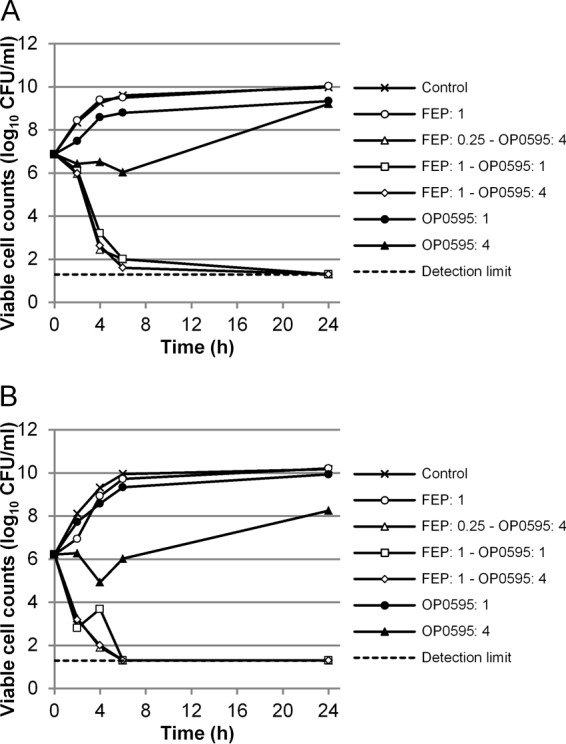
Time-kill curve of a combination of OP0595 and cefepime against CTX-M-15-positive E. coli MSC20653 (A) and KPC-positive K. pneumoniae ATCC BAA-1904 (B). Abbreviation: FEP, cefepime. The detection limit was 1.30 log10 CFU/ml.
With regard to the MIC of cefepime, neither 0.25/ml nor 1 μg/ml of cefepime reduced the viable cell counts of the test strains. When OP0595 was combined with cefepime, however, a bactericidal effect was observed. In particular, 4 μg/ml of OP0595 showed a strong bactericidal effect in combination with 0.25/ml and 1 μg/ml of cefepime. All five strains of CTX-M-15-positive E. coli and four of the five strains of KPC-positive K. pneumoniae showed a decrease of 3 log10 CFU/ml after 24 h compared with the starting inoculum. The regrowth strain was K. pneumoniae ATCC BAA-1900, but the number of such regrowth cells was 1 log10 CFU/ml lower than the initial cell number. Therefore, further studies were not conducted on this regrowth strain. This bactericidal activity was stronger than that observed for 1 μg/ml of OP0595 in combination with cefepime.
OP0595 showed antibacterial activity against these strains of β-lactamase-positive Enterobacteriaceae, but incubation with 4 μg/ml of OP0595 did not lead to a reduction in counts of viable cells. The viable cell count decreased for 4 or 6 h, and then regrowth occurred (Fig. 2). The cells that regrew were resistant to the antibacterial activity of OP0595, and the MIC of OP0595 was ≥32 μg/ml. Furthermore, a combination using 1 μg/ml of OP0595 did not reduce the viable cell count, and the growth curves were similar to those in the control.
Neutropenic murine thigh infection model.
A neutropenic murine model of thigh infection was used to clarify the efficacy of OP0595 alone and in combination with cefepime in vivo. The strains tested were CTX-M-15-positive E. coli MSC20653 and MSC20662 and KPC-positive K. pneumoniae ATCC BAA-1705 and ATCC BAA-1904, which have been confirmed to grow in the thigh of neutropenic Crlj:CD1 (ICR) mice.
The viable cell counts of CTX-M-15-positive E. coli MSC20653 or MSC20662 in control mice and in mice after administration of the test compounds are shown in Fig. 3A. The viable cell count in mice treated with cefepime (10 mg/kg) or OP0595 (2.5 mg/kg) was not significantly lower than that in mice treated with vehicle at 23 h after the start of treatment. However, mice in the cefepime-OP0595 groups (5/1.25 mg/kg and 10/2.5 mg/kg) had significantly (P < 0.05) lower bacterial counts than those in the saline vehicle group.
FIG 3.
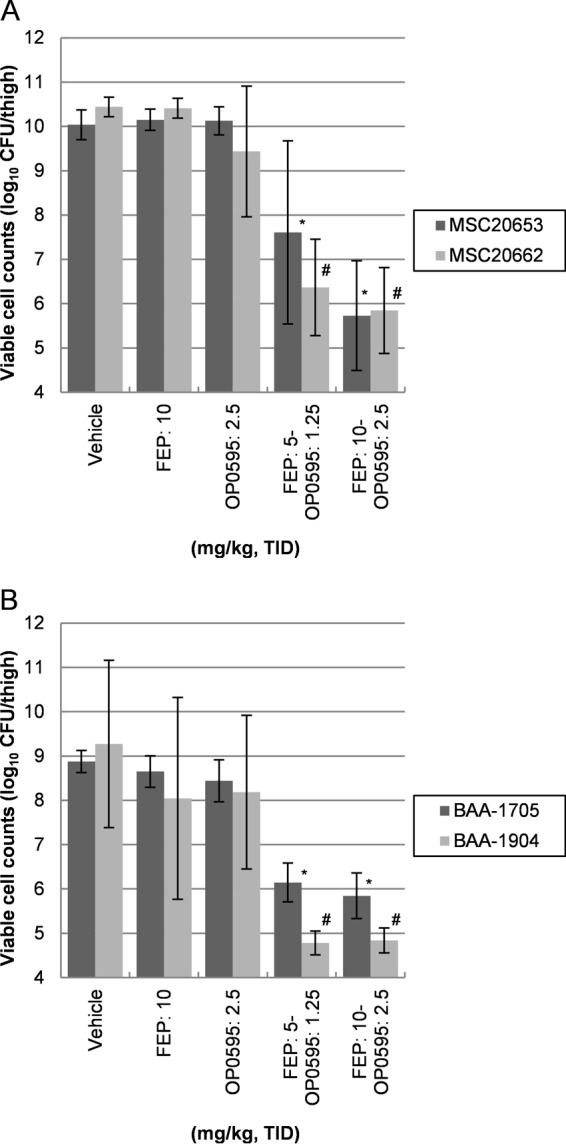
Comparison of the efficacies of sole and combined administration of β-lactam agents in a neutropenic murine model of thigh infection with CTX-M-15-positive E. coli (A) and KPC-positive K. pneumoniae (B). Abbreviations: FEP, cefepime; TID, three times a day. Six mice per group were rendered neutropenic, and 6.28 log10 CFU/mouse of E. coli MSC20653, 6.10 log10 CFU/mouse of E. coli MSC20662, 5.60 log10 CFU/mouse of K. pneumoniae ATCC BAA-1705, or 5.49 log10 CFU/mouse of K. pneumoniae ATCC BAA-1904 was injected into the thigh, followed by subcutaneous administration of the test compounds at 1, 3, and 5 h after infection. The mean log10 CFU per thigh recovered from the infected thigh after 24 h is shown; error bars represent the SD. A P value of <0.05 indicates significance relative to the untreated control (vehicle) and is indicated by an asterisk (*) or a hash mark (#).
The viable cell counts of KPC-positive K. pneumoniae ATCC BAA-1705 or ATCC BAA-1904 in control mice and in mice after administration of the test compounds are shown in Fig. 3B. The viable cell count in mice treated with cefepime (10 mg/kg) or OP0595 (2.5 mg/kg) was not significantly lower than that in the saline vehicle group at 23 h after the start of treatment. However, mice in the cefepime-OP0595 groups (5/1.25 mg/kg and 10/2.5 mg/kg) had significantly (P < 0.05) lower bacterial counts than those in the saline vehicle group. As a result, the therapeutic effect of cefepime-OP0595 coadministration was found to be considerably superior to that of saline vehicle in all tests.
DISCUSSION
The worldwide spread of potent β-lactamases, including extended-spectrum β-lactamases (ESBLs) and carbapenemases, is creating the need and opportunity for new β-lactamase inhibitors. In this study, we demonstrated that OP0595, a new diazabicyclooctane molecule, has potency in both in vitro time-kill studies and an in vivo model of infection when used in combination with cefepime against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae strains.
Recently, β-lactamase inhibitors have been tested at a concentration of 4 μg/ml in combinational studies of ceftazidime-avibactam, imipenem-relebactam, and ceftolozane-tazobactam, among other combinations (6, 9, 17). Here, to confirm the optimal combinational concentration of OP0595, the effects of different concentrations of OP0595 in combination with piperacillin, cefepime, or meropenem were evaluated. The antibacterial activity of each β-lactam agent against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae increased with the rising combinational concentration of OP0595, reaching a plateau at 2 to 4 μg/ml of OP0595. These results are similar to those of Livermore et al. (18) and suggested that a combinational concentration of 4 μg/ml of OP0595 was sufficient for in vitro tests. Clearly, the in vitro tests were conducted under stationary conditions and this concentration would not be suitable for in vivo studies; nevertheless, these data are needed for appropriate selection of a suitable drug in the clinical setting. They also raise a further issue about the antibacterial activity of the combination of the β-lactam agent and OP0595. It is known that OP0595 at a concentration of 4 μg/ml or under has antibacterial activity against several strains of Enterobacteriaceae caused by the inhibition of PBP2 (7). This fact adds complexity to evaluating the antibacterial activity of the combination of an β-lactam agent and OP0595.
Time-kill studies were used to gather information on the activity of OP0595 combined with β-lactam agents. In these studies, cefepime was used as a partner β-lactam agent. Cefepime is one of the best partner β-lactam agents, because OP0595 can show β-lactamase inhibitory activity and a β-lactam enhancer effect in combined use with cefepime against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae (7, 18). Time-kill studies are usually done as a multiple of the MIC. But OP0595 itself has antibacterial activity, and MIC basis concentrations in combination with OP0595 at 4 μg/ml were not possible to determine. CLSI designates that the cefepime susceptibility concentration against Enterobacteriaceae is 2 μg/ml (13). If the cefepime-OP0595 combination showed bactericidal activity at lower concentrations than the cefepime susceptibility concentration, we thought that the efficacy of cefepime-OP0595 could be confirmed. Accordingly, the concentration experiment was set up using the cefepime susceptibility concentration basis. When OP0595 was combined with cefepime, a bactericidal effect was observed against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae strains, which were resistant to cefepime in terms of MIC and bactericidal activity. These results suggested that OP0595 restored the bactericidal effect of cefepime by its antibacterial activity, β-lactamase inhibitory activity, and β-lactam agent enhancer effect. In contrast, OP0595 alone showed some antibacterial activity against these β-lactamase-positive Enterobacteriaceae, but the reduction in viable cell count was not bactericidal and cells that were resistant to the antibacterial activity of OP0595 regrew. These findings suggested that some strains of Enterobacteriaceae were resistant to the antibacterial activity of OP0595 due to PBP2 inhibition and that these OP0595-resistant strains of Enterobacteriaceae subsequently became the major population. This resistance to PBP2 inhibitors occurs readily, but the fitness cost for the resistant clones that survive is high, as shown by a study by Doumith et al. and a study of mutants with resistance to amdinocillin, another PBP2 inhibitor (19, 20). In addition, OP0595-resistant Enterobacteriaceae spp. show high susceptibility to the combination of the β-lactam agent and OP0595, owing to both the β-lactamase inhibitory activity and the β-lactam agent enhancer effect of OP0595, neither of which is influenced by resistance to the antibacterial activity of OP0595 (21). Taken together, these results indicate that the antibacterial activity of OP0595 is insufficient on its own but that it contributes to the bactericidal activity of cefepime. The β-lactamase inhibitory activity and the β-lactam agent enhancer effect of OP0595 also strongly contributed to the bactericidal activity of cefepime in the time-kill studies against β-lactamase-positive Enterobacteriaceae.
This article presents the first in vivo data for OP0595, where cefepime was used as the partner β-lactam agent against CTX-M-15-positive E. coli and KPC-positive K. pneumoniae infection. Treatment with either cefepime alone or OP0595 alone did not decrease the bacterial count in the murine thigh; however, combinational treatment with cefepime-OP0595 decreased the count to 2.5 to 4 log10 CFU per thigh. This bacterial count was significantly lower than that seen with mice treated with saline vehicle. These results suggested that the antibacterial activity of OP0595 alone was insufficient but that its β-lactamase inhibitory activity and β-lactam agent enhancer effect were exerted in vivo when it was combined with cefepime. To clarify the features of OP0595 in the in vivo study, dose and administration settings for reproducible human pharmacokinetics (PK) and pharmacokinetic/pharmacodynamic (PK/PD) studies are needed. However, the MIC value of the β-lactam agent–OP0595 combination against many strains of Enterobacteriaceae would be difficult to determine for calculation of the PK/PD parameters because the antibacterial activity and β-lactam agent enhancer effect of OP0595 were observed at a concentration of 4 μg/ml, which is the combined concentration for MIC (7, 18). Recently, several PK/PD studies of β-lactam agent–β-lactamase inhibitor combinations, such as ceftaloline-avibactam, aztreonam-avibactam, and ceftolozane-tazobactam, have been carried out in vitro and in vivo (16, 22–24). These studies clarified the influence of the β-lactamase inhibitory activity by using the stationary concentration of the β-lactamase inhibitor with the human PK value of the β-lactam agent in an in vitro hollow-fiber infection model. However, the use of OP0595 is difficult for this type of experiment due to its antibacterial activity and β-lactam agent enhancer effect (7), which overrides the β-lactamase inhibitory activity. These issues must be solved in future research.
In conclusion, the antibacterial activity of OP0595 alone was found to be insufficient but a cefepime-OP0595 combination showed stronger efficacy than cefepime alone in both in vitro time-kill studies and an in vivo model of infection. These data indicate that combinational use of OP0595 and β-lactam agents is important to exert the antimicrobial functions of OP0595.
ACKNOWLEDGMENTS
We thank David M. Livermore of the University of East Anglia and Takuji Yoshida, Mototsugu Yamada, and Nobuyoshi Baba of Meiji Seika Pharma Co., Ltd., for productive discussion. We also thank Eiki Shitara of Meiji Seika Pharma Co., Ltd., for productive discussion and compound supply and Yumiko Suzuki and Tomomi Koide for technical assistance with the experiments.
This work was internally funded.
All of us are employees of Meiji Seika. A.M., K.Y., T.T., T.A., T.F., Y.S., and T.I. have relevant shareholdings (Meiji Holdings) amounting to <10% of portfolio value.
REFERENCES
- 1.Bush K. 2013. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 2.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 6.Watkins RR, Papp-Wallace KM, Drawz SM, Bonomo RA. 2013. Novel β-lactamase inhibitors: a therapeutic hope against the scourge of multidrug resistance. Front Microbiol 4:392. doi: 10.3389/fmicb.2013.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morinaka A, Tsutsumi Y, Yamada M, Suzuki K, Watanabe T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Mitsuhashi N, Ida T, Livermore DM. 2015. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J Antimicrob Chemother 70:2779–2786. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- 8.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch EB, Ledesma KR, Chang KT, Schwartz MS, Motyl MR, Tam VH. 2012. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria. Antimicrob Agents Chemother 56:3753–3757. doi: 10.1128/AAC.05927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore DM, Warner M, Mushtaq S. 2013. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 68:2286–2290. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 12.Yagi T, Kurokawa H, Shibata N, Shibayama K, Arakawa Y. 2000. A preliminary survey of extended-spectrum beta-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Japan. FEMS Microbiol Lett 184:53–56. doi: 10.1111/j.1574-6968.2000.tb08989.x. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—ninth edition: approved standard M7-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Craig WA, Andes DR. 2013. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum β-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother 57:1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melchers MJ, van Mil AC, Mouton JW. 2015. In vitro activity of ceftolozane alone and in combination with tazobactam against extended-spectrum-β-lactamase-harboring Enterobacteriaceae. Antimicrob Agents Chemother 59:4521–4525. doi: 10.1128/AAC.04498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM, Mushtaq S, Warner M, Woodford N. 25 August 2015. Activity of OP0595/β-lactam combinations against Gram-negative bacteria with extended-spectrum, AmpC and carbapenem-hydrolysing β-lactamases. J Antimicrob Chemother doi: 10.1093/jac/dkv239. [DOI] [PubMed] [Google Scholar]
- 19.Doumith M, Mushtaq S, Livermore DM, Woodford N. 2015. Molecular mechanisms raising MICs of the new diazabicyclooctane, OP0595, for Escherichia coli, abstr C-120. Abstr 55th Intersci Conf Antimicrob Agents Chemother-28th Intersci Congr Chemother Meet. American Society for Microbiology and International Society of Chemotherapy, San Diego, CA. [Google Scholar]
- 20.Thulin E, Sundqvist M, Andersson DI. 2015. Amdinocillin (Mecillinam) resistance mutations in clinical isolates and laboratory-selected mutants of Escherichia coli. Antimicrob Agents Chemother 59:1718–1727. doi: 10.1128/AAC.04819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore DM, Warner M, Mushtaq S, Woodford N. 2014. Activity of OP0595-β-lactam combinations vs. OP0595-resistant Enterobacteriaceae mutants, abstr F-943. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S, O'Donnell JP, Bradford PA, Eakin AE. 2015. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. doi: 10.1093/jac/dkv132. [DOI] [PubMed] [Google Scholar]
- 23.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of β-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of β-lactamases. Antimicrob Agents Chemother 56:258–270. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanScoy B, Mendes RE, Nicasio AM, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2013. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob Agents Chemother 57:2809–2814. doi: 10.1128/AAC.02513-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


