Abstract
In our previous report, we showed that astrakurkurone, a triterpene isolated from the Indian mushroom Astraeus hygrometricus (Pers.) Morgan, induced reactive oxygen species, leading to apoptosis in Leishmania donovani promastigotes, and also was effective in inhibiting intracellular amastigotes at the 50% inhibitory concentration of 2.5 μg/ml. The aim of the present study is to characterize the associated immunomodulatory potentials and cellular activation provided by astrakurkurone, leading to effective antileishmanial activity in vitro and in vivo. Astrakurkurone-mediated antileishmanial activity was evaluated by real-time PCR and flow cytometry. The involvement of Toll-like receptor 9 (TLR9) was studied by in vitro assay in the presence of a TLR9 agonist and antagonist and by in silico modeling of a three-dimensional structure of the ectodomain of TLR9 and its interaction with astrakurkurone. Astrakurkurone caused a significant increase in TLR9 expression of L. donovani-infected macrophages along with the activation of proinflammatory responses. The involvement of TLR9 in astrakurkurone-mediated amastigote killing has been evidenced from the fact that a TLR9 agonist (CpG, ODN 1826) in combination with astrakurkurone enhanced the amastigote killing, while a TLR9 antagonist (bafilomycin A1) alone or in combination with astrakurkurone curbed the amastigote killing, which could be further justified by in silico evidence of docking between mouse TLR9 and astrakurkurone. Astrakurkurone was found to reduce the parasite burden in vivo by inducing protective cytokines, gamma interferon and interleukin 17. Moreover, astrakurkurone was nontoxic toward peripheral blood mononuclear cells of immunocompromised patients with visceral leishmaniasis. Astrakurkurone, a nontoxic antileishmanial, enhances the immune efficiency of host cells, leading to parasite clearance in vitro and in vivo.
INTRODUCTION
Leishmania occurs in the tropical and subtropical regions of the world and cause severe symptoms, with a huge socioeconomical impact to millions of poor people. The diseases are endemic in more than 80 countries, and 350 million people are considered to be at risk (1). Among the three clinical forms of leishmaniasis—visceral (VL), cutaneous (CL), and mucocutaneous (MCL)—VL is the most severe form and is fatal if untreated. The disease is highly endemic in the Indian subcontinent and in East Africa. An estimated 200,000 to 400,000 new cases of VL occur worldwide each year. Over 90% of new cases occur in 6 countries: India, Nepal, Bangladesh, Brazil, Ethiopia, and Sudan (1). Use of pentavalent antimonials for the treatment of different forms of leishmaniasis was introduced more than 7 decades ago and now has been forced to be checked for its limited efficacy, toxicity, and resistance (1). Efficacy of the first-line oral treatment, miltefosine, has declined rapidly over the past decade due to treatment failure and relapse, and miltefosine is also associated with gastrointestinal toxicity and teratogenesis (2, 3). Paramomycin had shown a promising 94% cure rate but is associated with systemic hepatic toxicity; the extended treatment schedule is also a major disadvantage for routine clinical use (2). Recently, the use of liposomal amphotericin B has been suggested by the WHO Advisory Panel for Leishmaniasis Control as the first choice to treat and eliminate VL from the Indian subcontinent (4). The aim for elimination of VL from the Indian subcontinent, bringing down the number of cases to fewer than one diseased person per 10,000 people in the districts of endemicity by 2015 (5), still remains a dream, even after the unremitting efforts of clinicians and scientists. Every magic bullet has a certain path and then drops down, but the incidence of leishmaniasis, which has been estimated to cause the ninth largest disease burden among individual infectious diseases, continues to increase (6, 7), so the pursuit for a more efficient drug takes a compelling urgency. Despite the best efforts to introduce enhanced therapy, the cost of drugs is a major concern. In this context, mushrooms, which are traditionally revered as sources of medicines by ethnic groups in India, can provide alternative sources for antileishmanial medicines. The homeostasis of healthy organisms grown unattended in nature produces a diverse range of metabolites to fight against the adverse condition. Immunomodulation through natural substances may be considered an alternative for the prevention and cure of diseases. In the past decade, discovery of bioactive molecules from the mushroom-type fungi (Basidiomycetes and Ascomycetes) has renewed the interest in the quest for natural immunomodulatory and therapeutic substances. Easy availability, along with cheap industrial-scale production, has made these organisms the major targets for research as well as for industrial exploitation. The total number of mushroom-forming species on Earth has been estimated at between 53,000 and 110,000. There are approximately 20,000 described species of mushroom, which mostly correspond to Basidiomycetes. This would suggest that only 18% to 38% of all the mushrooms have been documented (8). More than 778 species of macrofungi are available in India (9), and further investigation for the pharmacological importance of macrofungi is anticipated. To date, a very limited approach has been made to establish the mushrooms or mushroom-derived metabolites as therapeutic or immunostimulatory agents against Leishmania infection. As few as only seven reports, including three from our group, have been documented up to 2015 regarding the antileishmanial effect of extracts or active constituents of wild mushrooms (10–16). The first breakthrough was made by Jin and Zjawiony in 2006, when they isolated a novel compound, 5-heptadeca-8′Z,11′Z,16-trienylresorcinol, from a polypore mushroom, Merulius incarnatus Schweinitz 1822 (Corticiaceae), which inhibited the Leishmania growth in vitro (50% inhibitory concentration [IC50] of 3.6 μg/ml) with no toxicity on Vero cells up to 25 μg/ml (10). Souza-Fagundes et al., in 2010, reported that two terpenoids, hypnophilin and panepoxydone, isolated from Lentinus strigosus (Pegler 1983) (Polyporaceae, a basidiomycete), inhibited the growth of Leishmania (Leishmania) amazonensis (amastigote-like) by 67% in vitro at a concentration of 1.25 μg/ml (11). Coelho and colleagues in 2011 and 2012 continued their work with Agaricus blazei Murill extract and showed its activity against L. amazonensis, Leishmania chagasi, and Leishmania major in vitro and against Leishmania amazonensis in vivo (12, 13). We initiated our work with the active extracts of Astraeus hygrometricus (Pers.) Morgan and reported the differential antileishmanial effect against L. donovani promastigotes and intracellular amastigotes in vitro (15). In continuation with our line of investigation on antileishmanial leads, we also claimed simultaneously the isolation and structure elucidation of a novel triterpene molecule, astrakurkurone, from an active fraction of A. hygrometricus, with a significant effect against promastigotes (14). We have also reported the involvement of reactive oxygen species (ROS) and mitochondrial dysfunction in astrakurkurone-mediated cell death in promastigotes (16). Astrakurkurone was also found effective against clinically important intracellular amastigotes, with a significantly low IC50 of 2.5 μg/ml and a high selective index of 100 (16). To further strengthen our investigation on inventive antileishmanial leads, we attempted in this study to answer three major questions: First, what role does astrakurkurone play in the activation of the innate arm of homeostasis, including the generation of nitric oxide? Second, what, if any, is the involvement of Toll-like receptors (TLRs)? Third, how can we characterize cellular events to identify effector cytokines, in vitro and in vivo, responsible for disease eradication?
MATERIALS AND METHODS
Ethics statement.
All of the animal experiments were performed with the prior approval of the institutional animal ethical committee (IAEC), West Bengal State University (WBSU). IAEC, WBSU, approval CP-KA-SM/WBSU/2010-11/2 was dated 23 March 2012. The IAEC has been constituted and registered as per the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA), Ministry of Environment and Forests, Government of India (Registration 1394/ac/10/CPCSEA, dated 16 November 2010). Peripheral blood mononuclear cells (PBMCs) of VL patients were collected from the Canning Subdivisional Hospital, West Bengal. Protocols regarding the VL patient–related work have also been approved (reference no. WBSU-IEC/06, dated 24 April 2012), as per the guidelines of the Indian Council of Medical Research, Government of India. All adult subjects provided informed written consent, and no child participant was enrolled in the study. The study of mushrooms as ethnic medicine in tribal populations in West Bengal was approved (reference no. 7337-BCW, 6M-51/2010, dated 15 December 2010, Government of West Bengal).
Animals and Leishmania parasites.
Male BALB/c mice (average weight, 25 to 30 gm) were procured from the National Center for Laboratory Animal Sciences, Hyderabad, India. Animals were maintained at a standard temperature (25°C ± 5°C) on a 12-h day/night cycle, fed a standard pellet diet, and provided water ad libitum in the animal facility of West Bengal State University, as per the guidelines of IAEC, WBSU.
L. donovani (MHOM/IN/1983/AG83) was maintained in BALB/c mice in an animal facility, as per the guidelines of IAEC, WBSU. Amastigotes were prepared from the spleen of a BALB/c mouse infected with L. donovani, as described previously (17). Promastigotes were differentiated from amastigotes and maintained in vitro in an M-199 medium containing 10% fetal calf serum (FCS). For in vivo infection, BALB/c mice were injected with 5 × 106 amastigotes in 0.5 ml of normal saline via the intravenous route (18).
L. donovani infection in macrophages and astrakurkurone treatment.
BALB/c mice were injected with 2 ml of 4% thioglycolate intraperitoneally. The peritoneal cells were harvested 5 days after the thioglycolate injection and kept in rest for 24 h before any treatments on these cells to achieve resting peritoneal macrophages. Meanwhile, the nonadherent cells were washed out. The thioglycolate-elicited macrophages thus isolated were cultured in 8-well culture slides in complete RPMI 1640 media (supplemented with 1% l-glutamine, 1% penicillin-streptomycin, 50 μM 2-mercaptoethanol, 1% essential amino acids, and 10% FCS). Macrophages were infected with promastigotes at a macrophage-to-promastigote ratio of 1:10 for 4 h. After washing, the cultures were then incubated for another 60 h without treatment for established infection. After treatment with a 50% inhibitory concentration of astrakurkurone (2.5 μg/ml) with or without N-monomethyl-l-arginine (l-NMMA; Sigma-Aldrich), the cultures were continued for 48 h, culture supernatants were collected, and the nitrite concentration was measured by the use of Griess reagent (Sigma-Aldrich), as described earlier (19). To check the cooperation of Toll-like receptor 9 (TLR9), infected macrophages were pretreated with a TLR9 antagonist (bafilomycin A1; Invivogen) and a TLR9 agonist (CpG, ODN 1826; Invivogen) and incubated without or with the 50% inhibitory concentration of astrakurkurone (2.5 μg/ml). The cultures were continued for 48 h and then fixed with methanol and stained with Giemsa for determination of parasite load under a Zeiss phase-contrast microscope. The parasite load was calculated as the number of amastigotes per hundred macrophages, as described earlier (16, 17).
Reverse transcriptase and real-time PCR.
To reconfirm the effect of astrakurkurone on induction of intracellular cytokines interleukin 12 (IL-12), interleukin 10 (IL-10), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β) in L. donovani-infected and uninfected macrophages, cells were treated with a 50% inhibitory concentration of astrakurkurone (2.5 μg/ml) for 6 h. Total RNA was extracted using TRIzol (Sigma). For cDNA synthesis, 2 μg of total RNA from each sample was incubated with random primer, 0.1 M dithiothreitol, 500 pM deoxynucleoside triphosphates, 40 U RNase inhibitor, and 1 μl (3 U) of Moloney murine leukemia virus reverse transcriptase (Bangalore Genei). Samples were incubated at 37°C for 1 h, followed by 10 min incubation at 70°C. cDNA from each sample was amplified with Taq DNA polymerase (Bangalore Genei) in 50 μl under following conditions: 95°C for 2 min, 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min, for a total of 35 cycles. Specific primers were designed to amplify the mouse IL-12 (forward: 5′-CACGCCTGAAGAAGATGACA-3′; reverse: 5′-GACAGAGACGCCATTCCACA-3′), IL-10 (forward: 5′-CTGCTATGCTGCCTGCTCTT-3′; reverse: 5′-CTCTTCACCTGCTCCACTGC-3′), TNF-α (forward: 5′-CCACCACGCTCTTCTGTCTA-3′; reverse: 5′-CTTGGGCAGATTGACCTCAG-3′), and TGF-β (forward: 5′-GCAACAACGCCATCTATAGAG-3′; reverse: 5′-CCTGTATTCCGTCTCCTTGG-3′). Each sample was amplified for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward: 5′-GAGCCAAACGGGTCATCATC-3′; reverse: 5′-CCTGCTTCACCACCTTCTTG-3′) to ensure equal cDNA input. The PCR products were run in a 1.2% agarose gel, and densitometry calculation was carried out. Real-time PCR was performed to check the expression of TLR2, TLR4, and TLR9 and also to check inducible nitric oxide synthase 2 (iNOS2). Specific primers were designed for iNOS2 (forward: 5′-CTCCTCCCAGGACCACAC-3′; reverse: 5′-ACGCTGAGTACCTCATTGGC-3′), TLR2 (forward: 5′-AAGAGGAAGCCCAAGAAAGC-3′; reverse: 5′-CGATGGAATCGATGATGTTG-3′), TLR4 (forward: 5′-ACCTGGCTGGTTTACACGTC-3′; reverse: 5′-CTGCCAGAGACATTGCAGAA-3′), and TLR9 (forward: 5′-ACTGAGCACCCCTGCTTCTA 3′; reverse: 5′-AGATTAGTCAGCGGCAGGAA-3′). Real-time PCR was done in the Applied Biosystems 7500 fast real-time PCR using the power SYBR green PCR master mix. For the real-time reaction, 10 ng of cDNA template, 2 ng of forward primer, and 2 ng of reverse primer were used, and the reaction was performed on a 0.1-ml MicroAmp fast optical 96-well reaction plate (Applied Biosystems). Using the comparative threshold method (ΔΔCT), quantification was done, and mRNA expressions of target genes were normalized against GAPDH and represented as the relative fold change compared with untreated controls (19).
Flow cytometric analysis.
Expression of relevant costimulatory molecules in macrophages and dendritic cells in vitro, and induction of intracellular cytokines in L. donovani-infected and uninfected macrophages in vitro, in the presence of astrakurkurone was estimated by flow cytometry, as described earlier (19). The in vivo expansion pattern of CD4+ T cells in respect to Th1, Th2, and Th17 cytokines in L. donovani-infected and uninfected BALB/c mice with or without treatment of astrakurkurone was also analyzed (20). Anti-mouse IA/IE (isoform of mouse MHC II), CD40, CD80, CD86, gamma interferon (IFN-γ), TNF-α, TGF-β, and IL-17 were purchased from Biolegend (San Diego, CA). Anti-mouse IL-10 and IL-12 were procured from BD Pharmingen (San Diego, CA). A fluorescence-activated cell sorter (FACS) permeabilizing solution was obtained from BD Biosciences (Mountain View, CA). Brefeldin A was obtained from Sigma-Aldrich (St. Louis, MO). Cells were acquired in a flow cytometer (BD FACSVerse; BD Biosciences, CA, USA) and analyzed by Flowing software, version 2.5 (http://www.flowingsoftware.com; Perttu Terho, Centre for Biotechnology, Turku, Finland).
Regulation of kinases and NF-κB as determined by Western blotting.
L. donovani-infected and uninfected macrophages were treated with a 50% inhibitory concentration of astrakurkurone (2.5 μg/ml) for 15 min and were lysed using lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1 mM EGTA, and 1% Nonidet P-40), protease inhibitor mixture (Roche Applied Science, Mannheim, Germany), and phosphatase inhibitor mixture (Pierce) by incubation on ice for 1 h. After centrifugation at 12,000 rpm for 15 min, the supernatants were quantified by a bininhoninic acid kit (Pierce, Rockford, Michigan). Equal protein amounts were loaded on SDS-PAGE and resolved. The resolved protein was transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). After transfer, the membrane was blocked with 5% non-dried fat milk in Tris-buffered saline and Tween 20 (TBST; 25 mM Tris [pH 7.6], 137 mM NaCl, and 0.2% Tween 20) and incubated with primary antibody (Ab) at 4°C for 4 h at 22°C, washed with TBST, and incubated with horseradish peroxidase-conjugated secondary antibodies. Luminol reagent was used to visualize the immunoactive bands. Densitometric analyses of bands were performed using Quantity One software (21).
Assessment of toxicity on PBMCs of VL patients.
PBMCs of VL patients were collected from the Canning Subdivisional Hospital, West Bengal, India, as per the guidelines of the ethics committee. Proliferation of CD4+ T cells was estimated from in vitro culture of PBMCs of immunocompromised VL patients in the presence or absence (control) of astrakurkurone by appropriate staining with fluorochrome-tagged antibodies. Cells were acquired in a flow cytometer (BD FACSVerse; BD Biosciences, USA) and analyzed by Flowing software, version 2.5 (http://www.flowingsoftware.com; Perttu Terho, Centre for Biotechnology, Turku, Finland).
In silico modeling of three-dimensional structure of ectodomain of TLR9 and interaction with astrakurkurone.
The model of mouse TLR9 was generated using the crystal structure of the B chain of human TLR8 complexed with the agonist CL097 (PBD identification 3W3J) solved at 2.0-Å resolution as the template. The ectodomains of mouse TLR9 and human TLR8 share a sequence identity of 35%. The two-dimensional (2D) chemical structure of astrakurkurone was sketched using Discovery Studio version 3.5 (Accelrys Discovery Studio visualizer version 3.5.0.12158; Accelrys Software, Inc., San Diego) and converted into the corresponding standard three-dimensional (3D) structure. Docking of the inhibitor astrakurkurone to one of the possible binding sites of the receptor molecule was performed. The possible binding sites on the receptor molecule were identified based on the shape of the receptor using the program Discovery Studio version 3.5. Docking calculations were carried out with program LibDock (22). The top 70 poses were collected from 6 binding sites, with the best docked score values associated with a favorable binding conformation. Each pose was selected for calculation of binding energy between the receptor and ligand. In situ ligand-receptor minimization was performed on the complexes to remove any ligand van der Waals clashes prior to calculating the binding energy. The TLR9-astrakurkurone complex with the best binding energy was kept for further calculations and analyses. The docked complex of TLR9 with astrakurkurone was solvated with the 3-site transferable intermolecular potential (TIP3P) water molecules (23) in an explicit spherical boundary condition (24) for molecular dynamic simulation study using Discovery Studio version 3.5. The properly optimized assembly was subjected to simulation study, which was carried out by the standard dynamics cascade protocol. The minimized structures were subjected to heating from 50 K to 300 K for 120 ps and equilibrated for 250 ps. Long-range electrostatic interactions were treated with a spherical cutoff method. The receptor-inhibitor complex was further simulated for 5 ns at 300 K with a time step of 2 fs for the production run. This was carried in the constant-temperature, constant-volume ensemble (NVT). During the simulations, all covalent bonds involving hydrogen were constrained using the SHAKE algorithm (25). The trajectories were saved for further analysis.
Statistical analysis.
All experiments were performed with at least five independent replicates. Results were analyzed using Student's t test or one-way analysis of variance followed by Tukey's post hoc test. Differences were considered to be statistically significant if the P value was less than 0.05.
RESULTS
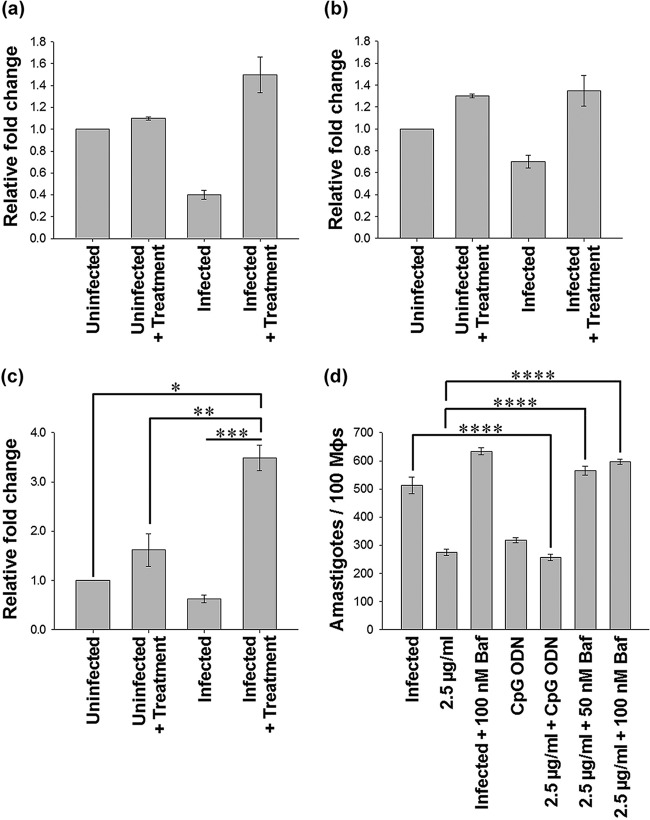
Astrakurkurone-induced nitric oxide caused the inhibition of L. donovani amastigotes in murine macrophages.
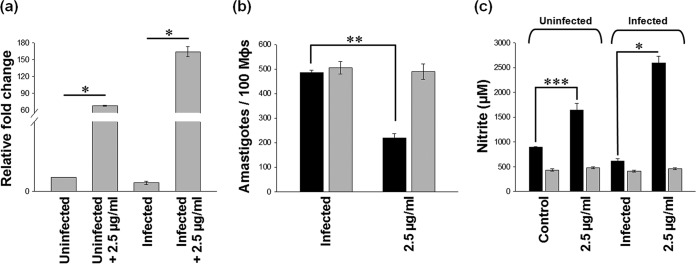
Earlier, we showed that 2.5 μg/ml of astrakurkurone could inhibit the replication of 50% of intracellular amastigotes in L. donovani-infected macrophages (16). The inhibition was associated with upregulation of iNOS2 mRNA expression in infected macrophages (Fig. 1a). It has been also found that the astrakurkurone-induced inhibition of intracellular amastigotes was abrogated in the presence of l-NMMA (an inhibitor of nitric oxide [NO]) in vitro, suggesting the involvement of nitric oxide in amastigote killing (Fig. 1b). Fascinatingly, astrakurkurone could upregulate the level of nitric oxide in infected macrophages compared with in uninfected culture (Fig. 1c). This finding supported the fact that astrakurkurone inhibited the intracellular amastigotes by inducing NO in macrophages, an essential innate arm involved in amastigote killing, in vitro and in vivo (26). The induction of NO extended our interest to explore the effect of astrakurkurone on encouragement of the release of proinflammatory cytokines in vitro.
FIG 1.
Effect of astrakurkurone on NO generation. Astrakurkurone induced the release of NO in uninfected and infected macrophages. (a) Elevation of iNOS2 mRNA was L. donovani infection specific at 6 h, as examined by real-time PCR. (b) Astrakurkurone-induced inhibition of intracellular amastigotes was abrogated in the presence of (gray) versus in the absence of (black) l-NMMA in vitro, suggesting the involvement of NO in amastigote killing. (c) Astrakurkurone induced the release of NO in both uninfected and infected macrophages in the presence (gray) and absence (black) of l-NMMA, as estimated by the nitrite concentration determined by the use of Griess reagent at 48 h. Values are means ± standard errors (n = 5) from 3 independent experiments (*, P < 0.001; **, P < 0.008; ***, P < 0.006).
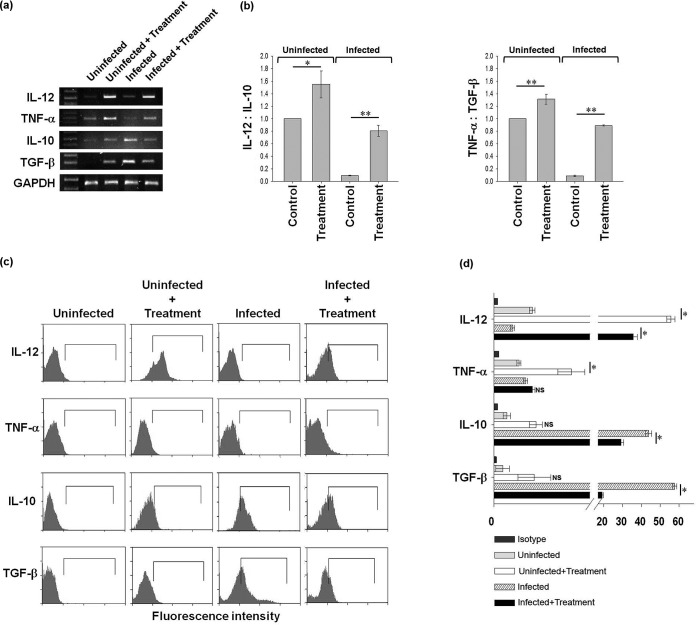
Astrakurkurone encouraged the release of proinflammatory cytokines in L. donovani-infected macrophages.
A therapeutic lead which is able to upregulate the Th1 response by inducing proinflammatory cytokines would actually be effective as a drug against L. donovani infection (27). Astrakurkurone fulfilled that promise, as it encouraged the release of the proinflammatory cytokine IL-12 significantly in infected macrophages in vitro. A small amount of TNF-α was also induced in infected macrophages, with simultaneous downregulation of IL-10 and TGF-β, the anti-inflammatory arms, leading to effective control of Leishmania infection, as estimated by reverse transcriptase semiquantitative PCR (Fig. 2a). However, the in vitro treatment of astrakurkurone also induced the anti-inflammatory cytokines IL-10 and TGF-β in uninfected macrophages, but it has been observed that the ratio of expression of mRNA of proinflammatory cytokines to anti-inflammatory cytokines (IL-12:IL-10 and TNF-α:TGF-β) is higher in both uninfected and infected macrophages than in control macrophages, indicating the bias toward a Th1 response in vitro (Fig. 2b). Specifically it can also be inferred (Fig. 2b) that the ratio of IL-12 to IL-10 and of TNF-α to TGF-β mRNA expression was higher in treated cells (uninfected and infected) than in control macrophages.
FIG 2.
Astrakurkurone could upregulate the release of proinflammatory cytokines in L. donovani-infected macrophages in vitro. (a) Astrakurkurone induced the release of proinflammatory cytokines and prevented the release of anti-inflammatory cytokines in L. donovani-infected macrophages at the mRNA level as measured by semiquantitative reverse transcriptase PCR. Data are representative of results of 5 independent experiments. (b) The densitometry ratio of mRNA expression of proinflammatory to anti-inflammatory cytokines (IL-12:IL-10 and TNF-α:TGF-β) was higher in both uninfected and infected macrophages, which indicated the induction of the Th1 response in vitro (*, P < 0.002; **, P < 0.001). (c) A significant induction of IL-12 in L. donovani-infected macrophages and the simultaneous downregulation of both IL-10 and TGF-β, the anti-inflammatory arms, led to effective control of Leishmania infection in macrophages at 48 h in vitro. Data are representative of results of 5 independent experiments. (d) The statistical significance of the induction of proinflammatory cytokines, as estimated by flow cytometry (*, P < 0.001; NS, nonsignificant).
The upregulation of proinflammation by astrakurkurone has also been evidenced by the flow cytometric analysis (Fig. 2c and d) that the molecule was able to upregulate the IL-12 mRNA significantly and TNF-α mRNA sparingly (Fig. 2d). At the same time, a sharp decline in IL-10 and TGF-β mRNA was observed in infected macrophages in vitro (Fig. 2d).
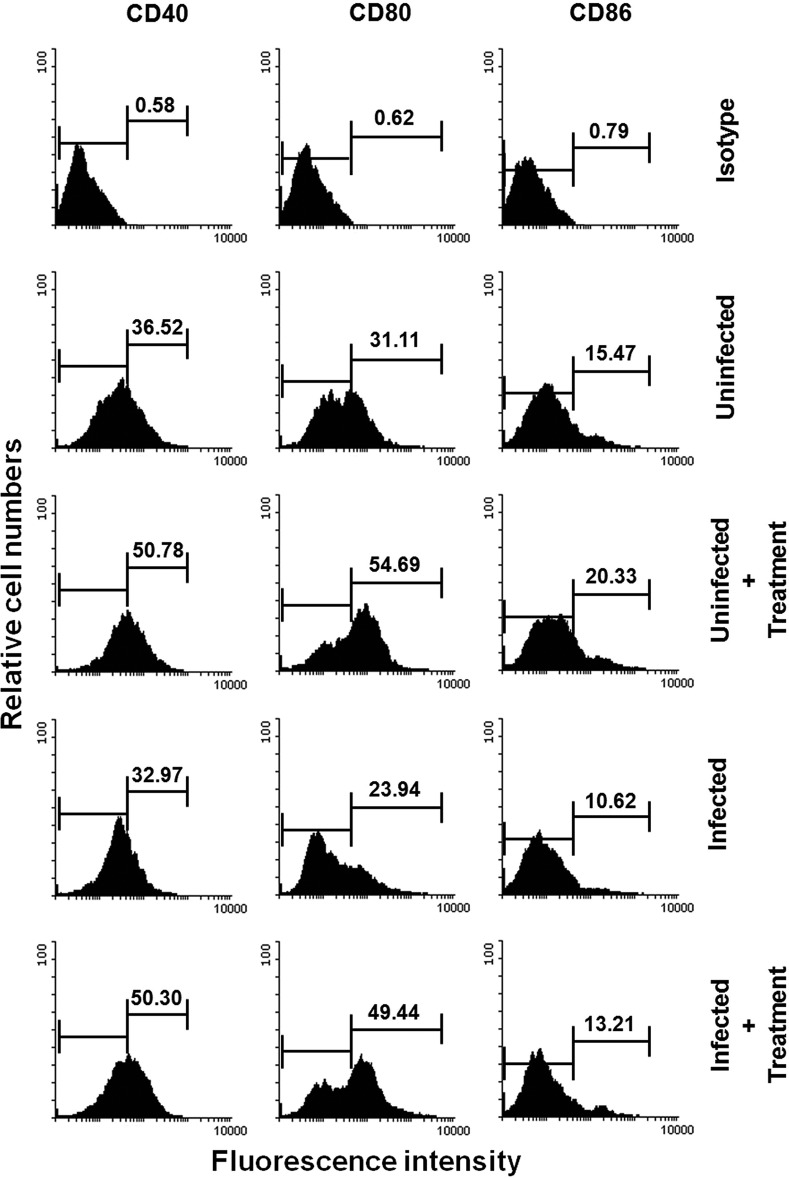
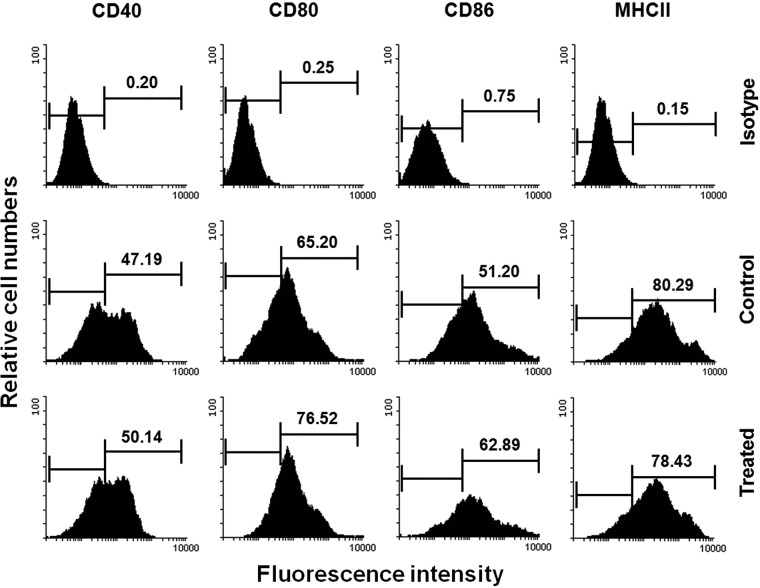
Astrakurkurone induced the expression of costimulatory molecules in infected macrophages and dendritic cells.
Induction of IL-12 is linked with the engagement of CD40 in infected macrophages (21) and dendritic cells (28). The level of costimulatory molecules on the surface of the antigen-presenting cell is also reported to influence both the induction of a T-cell response and the magnitude of cytokine patterns (29). Accordingly, the influence of astrakurkurone on expression of costimulatory phenotypes of uninfected macrophages, infected macrophages, and bone marrow-derived dendritic cells has been explored. Incubation of macrophages with astrakurkurone not only induced the expression of CD40, CD80, and CD86 in uninfected macrophages (Fig. 3) but also rescued the expression of these molecules in infected macrophages after treatment (Fig. 3). Substantial induction in expression of costimulatory molecules in bone marrow-derived dendritic cells has also been documented (Fig. 4). As CD40, IL-12, and TLRs modulate each other in initiation of cellular activation leading to parasite clearance (30–40), we next attempted to explore the involvement of TLRs in astrakurkurone-mediated immunomodulation (Fig. 4).
FIG 3.
Astrakurkurone restored the expression of costimulatory molecules (CD40, CD80, and CD86) in infected macrophages in vitro, as estimated by flow cytometry. Data are representative of results of 5 independent experiments.
FIG 4.
Astrakurkurone enhanced the expression of costimulatory molecules (CD40, CD80, CD86, and major histocompatibility complex class II) in murine bone marrow-derived dendritic cells, as measured by flow cytometry. Data are representative of results of 5 independent experiments.
Astrakurkurone-induced death of intracellular amastigotes involved TLR9.
TLRs are the innate immunity component and intended for the exclusion of various pathogens after recognition of the specific pathogenic patterns. The TLR signaling activates effector functions of macrophages and can lead to either a Th1 or a Th2 response, depending upon the nature of parasitic molecules. Several reports have shown that L. donovani infection causes impairment of TLR-associated functions in macrophages. It has been found that L. donovani suppresses TLR2-mediated p38 mitogen-activated protein kinase (MAPK) activation, which results in decreased IL-12 and upregulated IL-10 production through extracellular signal-regulated kinase 1 and 2 (ERK1/2)-MAPK activation that eventually leads to disease pathogenesis (32). It has been also reported that TLR4 mRNA in the spleen correlates with the parasitic load in Leishmania infantum-infected BALB/c mice (33). The previous reports indicated that the prophylactic and/or therapeutic administration of TLR2 (34, 35), TLR4 (36), or TLR9 agonists (37–39) enhances resistance in L. donovani-infected hamsters or BALB/c mice.
Therefore, we intended to look at the expression of TLR2, TLR4, and TLR9 in the infected marophages in the presence of astrakurkurone in vitro (Fig. 5) and study its cooperation in intracellular amastigote killing. Interestingly, we found that the expression of TLR9 (Fig. 5c), but not of TLR2 (Fig. 5a) or TLR4 (Fig. 5b), was compensated by astrakurkurone treatment in infected macrophages at the mRNA level, as judged by real-time PCR. Astrakurkurone was found to induce the TLR9 expression both in uninfected and infected macrophages.
FIG 5.
Astrakurkurone-mediated killing of intracellular amastigotes involved TLR9. (a to c) Expression of TLR2 (a), TLR4 (b), and TLR9 (c) in treated L. donovani-infected macrophages in vitro. (d) Pretreatment with bafilomycin A1 (TLR9 antagonist) alone and in addition to astrakurkurone curbed the parasite killing, suggesting the involvement of TLR9, a conclusion which is further strengthened by the fact that TLR9 agonist CpG enhances the antiamastigote potential of astrakurkurone. Values are means ± standard errors (n = 5) from 3 independent experiments (*, P < 0.004; **, P < 0.01; ***, P < 0.003; ****, P < 0.001).
This finding inclined us to look at the cooperation of TLR9 in astrakurkurone-induced amastigote inhibition. Infected macrophages were pretreated with a TLR9 antagonist (bafilomycin A1) and an agonist (CpG), followed by treatment with astrakurkurone for 48 h. Interestingly, pretreatment with bafilomycin A1 (the TLR9 antagonist) alone or in combination with astrakurkurone curbed the parasite killing, suggesting the involvement of TLR9, a conclusion which is further strengthened by the fact that CpG enhanced the antiamastigote potential of astrakurkurone (Fig. 5d). Our next approach was to analyze the docking of astrakurkurone with the active site of TLR9 to understand the protein-ligand interaction pattern.
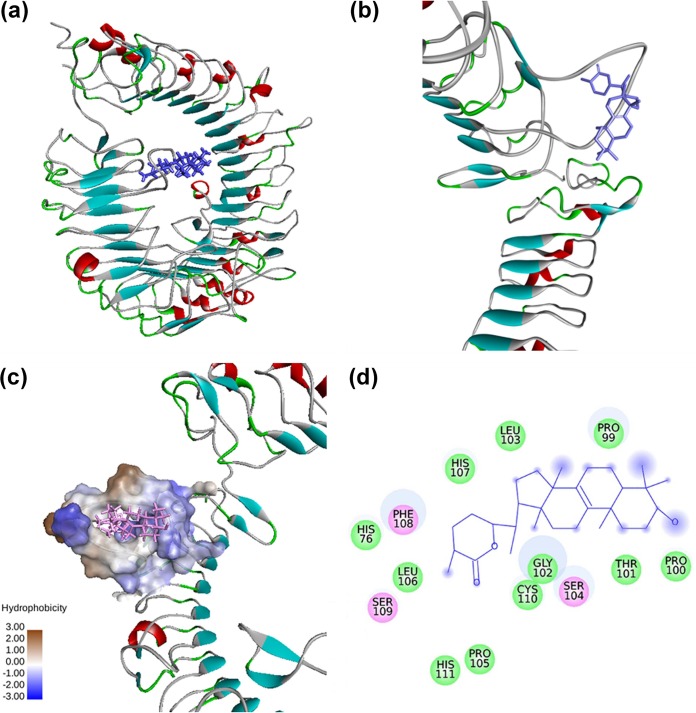
A closer view of receptor-ligand interactions as revealed from docking studies indicated that the best binding energy pose of astrakurkurone is present inside a loop comprising residues 99 to 111 of mouse TRL9. This region of TLR9 is the leucine-rich repeat 2 (LRR2) segment (Fig. 6). Analysis of intermolecular interactions of the generated docked structure of the TLR-astrakurkurone complex showed that residues Pro99, Pro100, Thr101, Gly102, Leu103, Pro105, Leu106, His107, Cys110, and His111 of TLR9 are involved in Van der Waals interactions. Residues Ser104, Phe108, and Ser109 are involved in electrostatic interactions with the agonist astrakurkurone (Fig. 6). Apart from the specified region, His76, which is present in the variable segment of LRR1, also binds with astrakurkurone by a Van der Waals interaction (Fig. 6). It is clear that the binding of TLR9 to the agonist astrakurkurone is predominated by nonbonded interaction, which is supported from interaction energies.
FIG 6.
Interaction between mouse TLR9 and astrakurkurone, as predicted by molecular docking. (a) Mouse TLR9-astrakurkurone docked complex. (b) Closer view of docked complex. (c) The surface of the mouse TLR9 binding site. (d) Intermolecular interaction of TLR9 with astrakurkurone. Residues involved in electrostatic and Van der Waals interactions are represented by pink and green circles, respectively. The solvent-accessible surface of an interacting residue is represented by a blue halo around the residue.
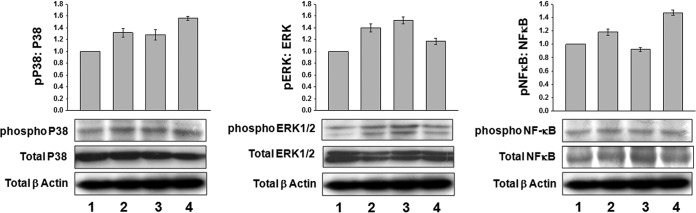
Astrakurkurone differentially regulated phophorylation of p38, ERK1/2, and NF-κB in L. donovani-infected macrophages.
Once the leishmanial signal sets in, it leads to activation of either ERK1/2 or p38. Phosphorylation of p38 is the outcome of descending signal cascades of CD40 activation and engagement and leads to production of IL-12 (21), necessary for disease protection (27), while the phosphorylation of ERK1/2 leads to IL-10 production, a consequence of disease progression (27). In the case of equal protein loading, in uninfected and infected macrophages, there was a considerable increase of phoshporylated p38 at 15 min of treatment with astrakurkurone (Fig. 7), correlating the induction with release of IL-12 (Fig. 2). On the other spectrum, while the phosphorylated ERK1/2 was high in infected macrophages at 15 min, astrakurkurone treatment decreased the phosphorylated ERK1/2 both in uninfected and infected macrophages (Fig. 7), which consequently limited IL-10 production (Fig. 2). A further downstream molecule in the signaling cascade is NF-κB. NF-κB directly binds to DNA and influences the transcription of the Th1-biased cytokine IL-12. It is also instrumental in inducing iNOS. In keeping with the phosphorylation of p38 and ERK1/2, we decided to explore the status of phosphorylation of NF-κB at 15 min of stimulation with astrakurkurone. The total-to-phosphorylated NF-κB expression ratio revealed that, in treated macropahges, phosphorylation is high (Fig. 7), suggesting that the innate arm has been activated to relegate a Th1-biased response, which may be instrumental in clearing the invading parasites from the host macrophages.
FIG 7.
Astrakurkurone differentially regulated phophorylation of p38, ERK1/2, and NF-κB in L. donovani-infected macrophages. A considerable increase of phoshporylated p38 in both uninfected and infected macrophages during the treatment with astrakurkurone has been observed. On the other spectrum, while phosphorylated ERK1/2 was high in infected macrophages, astrakurkurone treatment decreased the phosphorylated ERK1/2 at 15 min. The phosphorylation level of NF-κB after treatment with astrakurkurone also seemed higher than in the untreated infected macrophages. The corresponding histograms show the changes in expression, as measured by densitometry: 1, uninfected; 2, uninfected + treatment; 3, infected; 4, infected + treatment. Data are representative of results of 3 independent experiments.
Astrakurkurone reduced the L. donovani infection in vivo: activation of protective cytokines.
It is very important to see the extension of the in vitro results in the in vivo system, as the host body is a milieu of a number of factors and effectiveness of a potential drug can only be validated if it works within the system. Male BALB/c mice were separated in groups (n = 5) of naive, infected, and treated mice at 1 month postinfection (with astrakurkurone doses of 5 mg/kg body weight and 10 mg/kg body weight) and sacrificed at the 7-week postinfection period. Both doses were found effective, and the increasing doses dramatically reduced the parasite burden in the spleen by 92.37% (P < 0.001) and by 93.71% (P < 0.001), for the 5-mg/kg and 10-mg/kg doses, respectively, at the 7-week postinfected period. They could also inhibit the parasite burden in the liver significantly. However, in the liver, the highest dose (10 mg/kg) inhibited the parasite burden efficiently by 89.38% (P < 0.001) compared with the lower dose (5 mg/kg) inhibition of 65.17% (P < 0.003) (Fig. 8). The spleens from animals of the three groups were taken, and ex vivo cultures were set before harvesting the nonadherent parts for further analysis.
FIG 8.
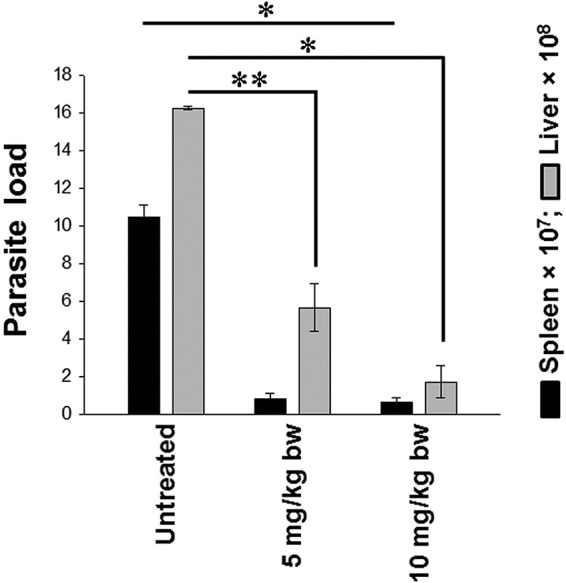
Astrakurkurone effectively reduced the splenic and hepatic parasite loads in L. donovani-infected BALB/c mice in vivo. The different doses of 5 mg/kg and 10 mg/kg of body weight (intramuscularly for 5 consecutive days) reduced the parasite loads by 92.37% and 93.71%, respectively, in the spleen at the 7-week postinfected period. It also inhibited the parasite burden in liver significantly. In the liver, the 5- and 10-mg/kg doses inhibited the parasite replication by 65.17% and 89.38%, respectively. Values are means ± standard errors (n = 5) from 2 independent experiments (*, P < 0.001; **, P < 0.003; infected versus treated animals).
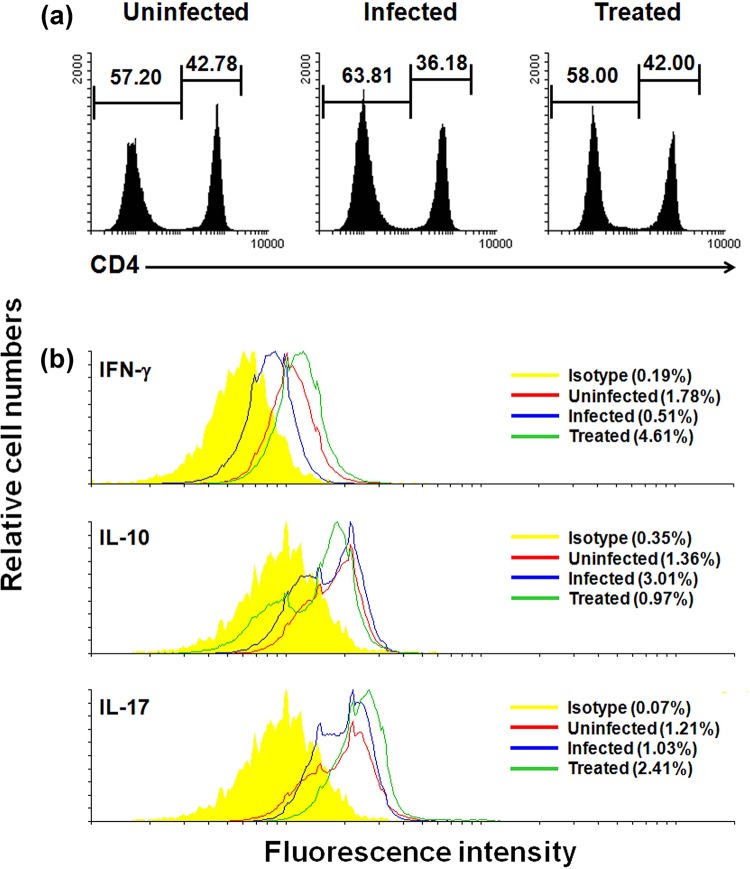
IFN-γ is a determinant, since it affects nitric oxide production. It consequently moves the proinflammatory cytokine arm of downstream signaling by directly affecting NF-κB production via MAPK, which is required to control Leishmania infection. Flowcytometric estimation revealed that there was considerable upregulation of IFN-γ after treatment (Fig. 9), thus establishing astrakurkurone as a proinflammation-inducing lead in vivo, consequently marking its potential as an antileishmanial drug. IFN-γ, though effective in removal of an intracellular pathogen, needs help from other cytokines for all-out clearance. Here is where the interplay of Th1 and Th17 CD4+ T cells are important. Results revealed that, along with IFN-γ, which is a major player activating the proinflammatory wing of the homeostasis, there was also a detectable release of IL-17 by the CD4+ T cells in vivo by astrakurkurone (Fig. 9).
FIG 9.
Astrakurkurone was found to induce CD4+ T cells and protective cytokines in vivo. Splenocytes from uninfected, L. donovani-infected, and treated mice were cultured in the presence of Leishmania-crude soluble antigen (25 μg/ml for 12 h). (a) The in vivo treatment reinstated the percentage of CD4+ T cells, as estimated by flow cytometry. (b) Simultaneously, the CD4+ IFN-γ+ T cells were induced to proliferate more in treated mice (4.61%) than in uninfected (1.78%) and infected (0.51%) mice, with the concomitant reduction of the CD4+ IL-10+ T cells in treated mice (0.97%) compared to that in infected (3.01%) mice. The percentage of CD4+ IL-17+ T cells steadily increased. Data are representative of results of 5 independent experiments.
Astrakurkurone was found to stimulate the CD4+ T cells of active VL patients.
Credibility of a therapeutic lead occurs when it shows comparatively less toxicity toward the host cells, specifically in a diseased condition. The major hurdles of present therapeutic schedules are the depressed immune functions exhibited by the patients with visceral leishmaniasis. Suitable T cell-mediated responses are the bare minimum requirements in effective host resistance in visceral leishmaniasis (41, 42). Correlation between host immune activation and the conquering of parasite replication through IL-12–dependent production of macrophage-activating cytokines (IFN-γ) by Th1-type effector cells is an important factor (42). Interestingly, we found that astrakurkurone exerted no cytotoxic effect on PBMCs of active VL patients; instead, this molecule induced the proliferation of CD4+ cells in PBMCs of active VL patients in vitro (Fig. 10).
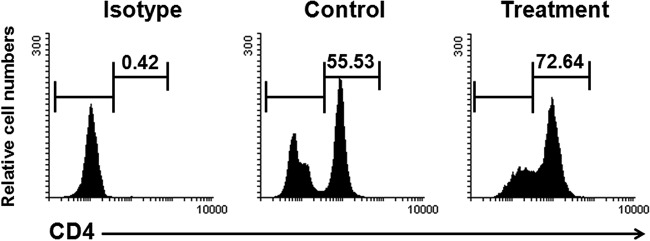
FIG 10.
Astrakurkurone was found to stimulate the CD4+ T cells of active VL patients. PBMCs were isolated from blood of VL patients and treated with 2.5 μg/ml of astrakurkurone. Golgiplug was added 6 h before harvesting the cells to arrest the cytokine secretion. Interestingly, astrakurkurone induced the proliferation of CD4+ T cells of PBMCs of active VL patients in vitro in the presence of Leishmania-crude soluble antigen (25 μg/ml) for 12 h. Data are representative of results of 5 independent experiments.
DISCUSSION
The susceptibility and survivability of Leishmania within macrophages depend on its approach to escape and weaken host defense mechanisms. ROS and reactive nitrogen intermediates (RNIs) produced by the activated macrophages are the initial and essential effector molecules which kill the intracellular amastigotes. In our preceding report, we showed that astrakurkurone could inhibit the intracellular amastigotes, the pathogenic stage in mammalian host, by inducing ROS in macrophages, with a 50% inhibitory concentration of 2.5 μg/ml in vitro (16). Interestingly, astrakurkurone also induced the generation of NO, an essential antiamastigote effector molecule (21, 26, 28–32), considerably in uninfected macrophages and significantly in infected macrophages. The induction of inflammatory response was further reflected by an induction of a proinflammatory cytokine, IL-12, and a fall of the anti-inflammatory cytokines IL-10 and TGF-β by astrakurkurone. However, our observation revealed that astrakurkurone could not induce the release of TNF-α so prominently. It preserves the benefit of this molecule as antileishmanial therapy, because endogenous TNF-α has been reported to induce apoptosis in host macrophages (43). A significant surge in the nitric oxide level in infected macrophages can be justified due to the engagement of CD40 (21) and TLR9 (30) or ultimately because of the activation of NF-κB by astrakurkurone. It has been well documented that all TLRs can enhance CD40 expression, while CD40 augments the expression of only TLR9 (30). As both CD40 and TLR9 can induce expression of IL-12, a cytokine that promotes the IFN-γ–secreting Th1-cell differentiation, the CD40-TLR9 crossregulation implies a positive role in Leishmania infection (31). We found that astrakurkurone could induce IL-12 and CD40 in infected macrophages and bone marrow-derived dendritic cells and also was involved in TLR9-dependent amastigote killing. The in vivo treatment with astrakurkurone could also induce the protective cytokine IFN-γ in infected animals. TLR9 engagement has already been reported to decrease ERK1/2 phosphorylation in L. major-infected macrophages (30). We are considering that astrakurkurone-induced CD40-TLR9 engagement decreased the phosphorylation of ERK1/2, caused the p38MAPK activation, and increased the phosphorylation of NF-κB production following the release of protective mediators IL-12, IFN-γ, and NO, leading to parasite clearance. We have already shown that astrakurkurone could significantly inhibit the clinically important intracellular amastigotes, with a high selective index of 100, in our previous report (16). In this communication, we reported the further promise of this molecule as an antileishmanial agent which remains nontoxic toward the proliferation of immunocompromised PBMCs of active VL patients in vitro. We may claim that, to our knowledge, this is the first conclusive report of its kind to establish an isolated mushroom constituent, astrakurkurone, as an antileishmanial molecule whose administration not only curbs the parasite infection in vivo but also enhances the immune effectiveness of host cells.
ACKNOWLEDGMENTS
We thank the Vice Chancellor, West Bengal State University, and the Vice Chancellor, University of Kolkata, for providing the research infrastructures for this work. We also thank the Director, CU BD Centre of Excellence for Nanobiotechnology, University of Kolkata, for use of the flow cytometry facility. Bafilomycin A1 was given by Dipyaman Ganguly, Senior Scientist, CSIR-Indian Institute of Chemical Biology, Jadavpur, Kolkata. Thanks also to Sanjaya Mallick and Sumanta Basu, CU BD Centre of Excellence for Nanobiotechnology, for their assistance in FACS analysis.
Funding Statement
This work was supported by grants from the Indian Council of Medical Research, Government of India (Tribal/56/2010-ECD-II), and the Department of Biotechnology, Government of India (BT/217/NE/TBP/2011). A.D. is the recipient of a DST-WOS B fellowship from the Government of India (SSD/SS/029/2010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.World Health Organization. 2015. Leishmaniasis fact sheet 375. World Health Organizatio, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs375/en/. [Google Scholar]
- 2.Sundar S, Pandey K, Thakur CP, Jha TK, Das VN, Verma N, Lal CS, Verma D, Alam S, Das P. 2014. Efficacy and safety of amphotericin B emulsion versus liposomal formulation in Indian patients with visceral leishmaniasis: a randomized, open-label study. PLoS Negl Trop Dis 8:e3169. doi: 10.1371/journal.pntd.0003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K. 2009. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg 80:580–582. [PubMed] [Google Scholar]
- 4.World Health Organization. 22 to 26 March 2010. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf. [Google Scholar]
- 5.World Health Organization and Southeast Asia Regional Office. 2015. Regional strategic framework for elimination of Kala-azar from the Southeast Asia Region (2005-2015): WHO project IND CRD 714. World Health Organization, Geneva, Switzerland: http://209.61.208.233/LinkFiles/Kala_azar_VBC-85_Rev_1.pdf. [Google Scholar]
- 6.Alvar J, Ve′lez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, the WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotez PJ, Remme JH, Buss P, Alleyne G, Morel C, Breman JG. 2004. Combating tropical infectious diseases: report of the Disease Control Priorities in Developing Countries project. Clin Infect Dis 38:871–878. doi: 10.1086/382077. [DOI] [PubMed] [Google Scholar]
- 8.Mueller GM, Schmitt JP, Leacock PR, Buyck B, Cifuentes J, Desjardin DE, Halling RE, Hjortstam K, Iturriaga T, Larsson KH, Lodge DJ, May TW, Minter D, Rajchenberg M, Redhead SA, Ryvarden L, Trappe JM, Watling R, Wu Q. 2007. Global diversity and distribution of macrofungi. Biodivers Conserv 16:37–48. doi: 10.1007/s10531-006-9108-8. [DOI] [Google Scholar]
- 9.Swapna S, Syed A, Krishnappa M. 2008. Diversity of macrofungi in semi-evergreen and moist deciduous forest of Shimoga district, Karnataka, India. J Mycol Plant Pathol 38:21–26. [Google Scholar]
- 10.Jin W, Zjawiony JK. 2006. 5-Alkylresorcinols from Merulius incarnatus. J Nat Prod 69:704–706. doi: 10.1021/np050520d. [DOI] [PubMed] [Google Scholar]
- 11.Souza-Fagundes EM, Cota BB, Rosa LH, Romanha AJ, Corrêa-Oliveira R, Rosa CA, Zani CL, Teixeira-Carvalho A, Martins-Filho OA. 2010. In vitro activity of hypnophilin from Lentinus strigosus: a potential prototype for Chagas disease and leishmaniasis chemotherapy. Braz J Med Biol Res 43:1054–1061. doi: 10.1590/S0100-879X2010007500108. [DOI] [PubMed] [Google Scholar]
- 12.Valadares DG, Duarte MC, Oliveira JS, Chávez-Fumagalli MA, Martins VT, Costa LE, Leite JP, Santoro MM, Régis WC, Tavares CA, Coelho EA. 2011. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol Int 60:357–363. doi: 10.1016/j.parint.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Valadares DG, Duarte MC, Ramírez L, Chávez-Fumagalli MA, Martins VT, Costa LE, Lage PS, Ribeiro TG, Castilho RO, Fernandes AP, Régis WC, Soto M, Tavares CA, Coelho EA. 2012. Prophylactic or therapeutic administration of Agaricus blazei Murill is effective in treatment of murine visceral leishmaniasis. Exp Parasitol 132:228–236. doi: 10.1016/j.exppara.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Lai TK, Biswas G, Chatterjee S, Dutta A, Pal C, Banerji J, Bhuvanesh N, Reibenspies JH, Acharya K. 2012. Leishmanicidal and anticandidal activity of constituents of Indian edible mushroom Astraeus hygrometricus. Chem Biodivers 9:1517–1524. doi: 10.1002/cbdv.201100272. [DOI] [PubMed] [Google Scholar]
- 15.Mallick S, Dutta A, Dey S, Ghosh J, Mukherjee D, Sultana SS, Mandal S, Paloi S, Khatua S, Acharya K, Pal C. 2014. Selective inhibition of Leishmania donovani by active extracts of wild mushrooms used by the tribal population of India: an in vitro exploration for new leads against parasitic protozoans. Exp Parasitol 138:9–17. doi: 10.1016/j.exppara.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Mallick S, Dey S, Mandal S, Dutta A, Mukherjee D, Biswas G, Chatterjee S, Mallick S, Lai TK, Acharya K, Pal C. 2015. A novel triterpene from Astraeus hygrometricus induces reactive oxygen species leading to death in Leishmania donovani. Future Microbiol 10:763–789. doi: 10.2217/fmb.14.149. [DOI] [PubMed] [Google Scholar]
- 17.Pal C, Raha M, Basu A, Roy KC, Gupta A, Ghosh M, Sahu NP, Banerjee S, Mandal NB, Bandyopadhyay S. 2002. Combination therapy with indolylquinoline derivative and sodium antimony gluconate cures established visceral leishmaniasis in hamsters. Antimicrob Agents Chemother 46:259–261. doi: 10.1128/AAC.46.1.259-261.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournet A, Gantier JC, Gautheret A, Leysalles L, Munos MH, Mayrarque J, Moskowitz H, Cavé A, Hocquemiller R. 1994. The activity of 2 substituted quinoline alkaloids in BALB/c mice infected with Leishmania donovani. J Antimicrob Chemother 33:537–544. doi: 10.1093/jac/33.3.537. [DOI] [PubMed] [Google Scholar]
- 19.Mallick S, Halder S, Dutta A, Dey S, Maiti S, Bandyopadhyay C, Saha B, Pal C. 2013. Chromone-linked nitrone derivative induces the expression of iNOS2 and Th1 cytokine network but reduces the Th2 cytokine in experimental visceral leishmaniasis. Int Immunopharmacol 15:772–779. doi: 10.1016/j.intimp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh M, Pal C, Ray M, Maitra S, Mandal L, Bandyopadhyay S. 2003. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J Immunol 170:5625–5629. doi: 10.4049/jimmunol.170.11.5625. [DOI] [PubMed] [Google Scholar]
- 21.Awasthi A, Mathur R, Khan A, Joshi BN, Jain N, Sawant S, Boppana R, Mitra D, Saha B. 2003. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J Exp Med 197:1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diller DJ, Merz KM Jr. 2001. High throughput docking for library design and library prioritization. Proteins 43:113–124. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen WL, Chandrasekhar J, Madura JD. 1983. Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 24.Brunger A, Brooks CL, Karplus M. 1984. Stochastic boundary conditions for molecular dynamics simulations of ST2 water. Chem Phys Lett 105:495–500. doi: 10.1016/0009-2614(84)80098-6. [DOI] [Google Scholar]
- 25.Ryckaert JP, Ciccotti G, Berendsen HJC. 1977. Numerical integration of the cartesian equations of motion of a system with constraints. J Comp Phys 23:327–341. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 26.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol 144:4794–4797. [PubMed] [Google Scholar]
- 27.Awasthi A, Mathur RK, Saha B. 2004. Immune response to Leishmania infection. Indian J Med Res 119:238–248. [PubMed] [Google Scholar]
- 28.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. 1996. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev 153:47–83. doi: 10.1111/j.1600-065X.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 30.Chandel HS, Pandey SP, Shukla D, Lalsare K, Selvaraj SK, Jha MK, Saha B. 2014. Toll-like receptors and CD40 modulate each other's expression affecting Leishmania major infection. Clin Exp Immunol 176:283–290. doi: 10.1111/cei.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandel HS, Pandey SP, Roy S, Doyen N, Saha B. 2014. TLR–CD40 cross-talk in anti-leishmanial immune response. Front Immunol 5:220. doi: 10.3389/fimmu.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra D, Naik S. 2008. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol 154:224–234. doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cezario GA, de Oliviera LR, Peresi E, Nicolete VC, Polettini J, de Lima CR, Gatto M, Calvi SA. 2011. Analysis of the expression of Toll-like receptors 2 and 4 and cytokine production during experimental Leishmania chagasi infection. Mem Inst Oswaldo Cruz 106:573–583. doi: 10.1590/S0074-02762011000500010. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharya P, Bhattacharjee S, Gupta G, Majumder S, Adhikari A, Mukherjee A, Majumdar SB, Saha B, Majumdar S. 2010. Arabinosylated lipoarabinomannan-mediated protection in visceral leishmaniasis through up-regulation of Toll-like receptor 2 signaling: an immunoprophylactic approach. J Infect Dis 202:145–155. doi: 10.1086/653210. [DOI] [PubMed] [Google Scholar]
- 35.Shakya N, Sane SA, Shankar S, Gupta S. 2011. Effect of Pam3Cys induced protection on the therapeutic efficacy of miltefosine against experimental visceral leishmaniasis. Peptides 32:2131–2133. doi: 10.1016/j.peptides.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar S, Bhaumik SK, Paul J, De T. 2012. TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog 8:e1002646. doi: 10.1371/journal.ppat.1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta N, Mukherjee S, Das L, Das PK. 2003. Targeting of immunostimulatory DNA cures experimental visceral leishmaniasis through nitric oxide upregulation and T-cell activation. Eur J Immunol 33:1508–1518. doi: 10.1002/eji.200323671. [DOI] [PubMed] [Google Scholar]
- 38.Tewary P, Mehta J, Sukumaran B, Madhubala R. 2004. Vaccination with Leishmania soluble antigen and immunostimulatory oligodeoxynucleotides induces specific immunity and protection against Leishmania donovani infection. FEMS Immunol Med Microbiol 42:241–248. doi: 10.1016/j.femsim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Sane SA, Shakya N, Vishwakarma P, Haq W. 2011. CpG oligodeoxynucleotide 2006 and miltefosine, a potential combination for treatment of experimental visceral leishmaniasis. Antimicrob Agents Chemother 55:3461–3464. doi: 10.1128/AAC.00137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faria MS, Reis FC, Lima AP. 2012. Toll-like receptors in Leishmania infections: guardians or promoters? J Parasitol Res 2012:930257. doi: 10.1155/2012/930257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melby PC, Valencia-Pacheco G, Andrade-Narvaez F. 1996. Induction of macrophage killing of Leishmania donovani by human CD4+ T cell clones. Arch Med Res 27:473–479. [PubMed] [Google Scholar]
- 42.Murray HW, Stern JJ, Welte K, Rubin BY, Carriero SM, Nathan CF. 1987. Experimental visceral leishmaniasis: production of interleukin 2 and interferon gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon gamma. J Immunol 138:2290–2297. [PubMed] [Google Scholar]
- 43.Xaus J, Comalada M, Valledor AF, Lloberas J, López-Soriano F, Argilés JM, Bogdan C, Celada A. 2000. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 95:3823–3831. [PubMed] [Google Scholar]