Abstract
Drug susceptibility tests (DSTs) for Mycobacterium tuberculosis require at least 7 days of incubation. Drugs that are unstable at 37°C, such as ertapenem, are likely to be degraded before killing or inhibiting slow-growing bacteria. This would alter the MICs of these drugs, including ertapenem, leading to falsely high MICs. Here, we describe a new strategy we developed to perform DSTs and measure MICs for such unstable compounds.
TEXT
Ertapenem, a β-lactam agent of the carbapenem class, has shown promising clinical results and favorable pharmacokinetics against Mycobacterium tuberculosis (1, 2). The scourge of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB), a global problem, has increased the urgency for the use of carbapenems, such as ertapenem, meropenem, and faropenem (2–4). Recently, the first phase 2 study (NCT02349841) to evaluate early bactericidal activity of meropenem and faropenem was completed, and the results are expected soon.
Carbapenems inhibit the peptidase domain of penicillin binding proteins, leading to autolysis and peptidoglycan weakening of the cell wall (5). Degradation of ertapenem on storage following reconstitution and dilution is temperature dependent, and the proposed in-use shelf life is 6 h at room temperature or 24 h at 2°C to 8°C (6). M. tuberculosis has a doubling time of at least 24 h under the best of circumstances (7, 8). M. tuberculosis cell division is particularly slow; FtsZ, a protein responsible for initiating cell division and recruiting proteins for formation of new cell walls, is known to have a polymerization rate that is at least 20 times slower in M. tuberculosis than in Escherichia coli, for example (9). In M. tuberculosis at low pH, the replication rate is up to10 to 20 times slower; kill of such semidormant bacteria is defined as a sterilizing effect (7, 8, 10). Thus, microbial killing and inhibition of growth by the most effective of antibiotics are slow and take place over several days, especially by β-lactams that depend on cell wall turnover.
Drug susceptibility tests (DSTs) for M. tuberculosis using Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) approved methods require at least 7 days of incubation (11). Drugs such as ertapenem, already appearing unstable at 37°C (2), are likely to be degraded before killing or inhibiting slow-growing bacteria, especially semidormant M. tuberculosis. This would be expected to alter the MICs of ertapenem, leading to falsely high MICs and false resistance. Here, we saw the rapid decline of ertapenem during DSTs; therefore, we developed a new strategy to perform DSTs and measure MICs for such unstable compounds.
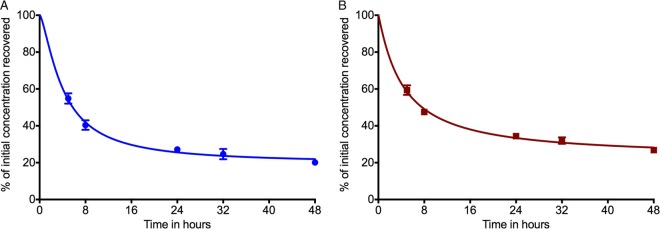
Ertapenem (Sigma) was first dissolved in purified water and subsequently diluted in Middlebrook 7H9 broth to the desired drug concentrations of 5.0 and 50 mg/liter, respectively. The two solutions were incubated at 37°C. After 0, 5, 8, 24, 32, and 48 h, three samples were collected from each solution and immediately stored at −80°C until further analysis. All samples were then fully thawed at room temperature and analyzed in duplicate using fully validated assays (12). The calibration curve of ertapenem was linear over a range of 0.1 to 125 mg/liter, and the correlation coefficient was 0.999. The coefficients of variation between the replicates for each concentration at each time point were 2.7% to 11.2%. Figure 1 shows the decrease in ertapenem concentration in the solution at 37°C. After 5 h of incubation, ertapenem concentration was reduced by 45.3% and 40.7% in comparison with the initial concentrations of 5 and 50 mg/liter, respectively. After 48 h, the concentrations were 20.1% and 26.8% of the time-zero concentrations.
FIG 1.
Liquid chromatography-tandem mass spectrometry analysis of ertapenem to determine the rate of degradation after incubation at 37°C. The percentage of ertapenem measured after incubation at 37°C, after 5 (A) and 50 (B) mg/liter initial concentrations, is shown. M. tuberculosis grows slowly compared to the other bacteria (72 times slower than E. coli when in log-phase growth and 720 times slower for semidormant M. tuberculosis) (5). As shown, the longer the incubation period, the greater the degradation of ertapenem. This could eventually lead to falsely high ertapenem MICs.
M. tuberculosis H37Ra (ATCC 25177) was used in the MIC and dose-response experiments. For each experiment, one stock vial was thawed and bacteria grown to a logarithmic growth phase (log-phase growth) in Middlebrook 7H9 broth enriched with 10% oleic acid-albumin-dextrose-catalase for 4 days at 37°C under shaking conditions and 5% CO2. For semidormant bacteria under acidic conditions, the day 4 culture was inoculated in Middlebrook 7H9 acidified to a pH of 5.8 by means of citric acid, as described previously (7). Sterilizing effect MICs were identified using the broth dilution and the resazurin colorimetric assay (11, 13). M. tuberculosis in acidified Middlebrook 7H9 broth was exposed to the following ertapenem concentrations, in triplicate: 0, 0.075, 0.15, 0.3, 0.6, 1, 1.25, 2, 2.5, 4, 5, 8, 16, 32, and 64 mg/liter. The cultures were incubated at 37°C with 5% CO2 for 7 days. In one set, each replicate received a daily 50% supplementation of ertapenem concentration at volumes of <1%. For the resazurin assay, an aliquot of the day 7 cultures had resazurin (0.001% vol/vol) added to the samples and plates, which were then further incubated for 24 h at 37°C under 5% CO2. The ertapenem MIC without daily ertapenem supplementation was 64 mg/liter, while that with supplementation was 0.6 mg/liter.
Day 7 cultures described above that were not used for resazurin assays were washed twice in normal saline to prevent drug carryover and were subsequently spread on Middlebrook 7H10 agar and incubated for 3 weeks at 37°C for enumeration of CFU counts. Inhibitory sigmoid maximum effect (Emax) curves for concentration versus CFUs per milliliter under sterilizing effect conditions are shown in Fig. 2. Comparison of the two regressions, with the null hypothesis that the maximal kill (Emax) or efficacy and concentration mediating 50% of Emax (EC50) or potency revealed a ratio of probabilities of 7.46 and a difference in corrected Akaike information criteria scores of 4.02, which means that the efficacy and potency differed with the supplementation. The EC50 was 1.41 mg/liter without ertapenem supplementation and 0.19 mg/liter with daily supplementation. The efficacy was 0.751 log10 CFU/ml without daily supplementation versus 2.38 log10 CFU/ml with supplementation. Thus, ertapenem displays potential for a sterilizing effect that would otherwise be masked by not accounting for the degradation.
FIG 2.
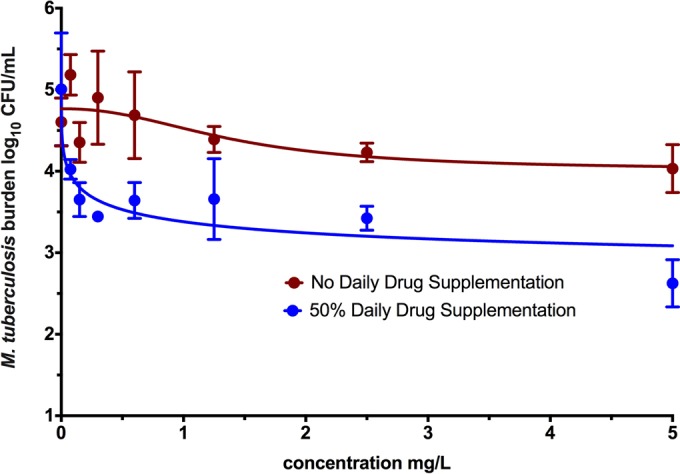
Ertapenem dose response against slowly replicating M. tuberculosis. As shown, when the degradation rate was taken into account by supplementing the drug daily, there was better kill of semidormant M. tuberculosis by ertapenem.
Here, we first show that ertapenem degrades considerably, at rates of >20-fold the doubling times of M. tuberculosis under acidic conditions. This effect is striking when the MICs with and without supplementation are compared. The MICs in the absence of supplementation would be considered in the resistance range by EUCAST breakpoints (14). Second, we show that ertapenem supplementation brings it well within the susceptibility range, suggesting that most of the published MICs for this drug are likely falsely high, and rates of resistance are likely falsely elevated. In addition, since many people have used MICs to choose which of the carbapenems would be better suited for treatment of XDR-TB, good drugs may have been discarded because of the artifactual manner in which current MIC measurements are performed for unstable molecules. Third, we show that ertapenem is likely to have a good sterilizing effect in tuberculosis. Follow-up hollow-fiber studies for a sterilizing effect have been completed in order to identify the ertapenem dose that can be used against both drug-resistant and drug-sensitive M. tuberculosis (our unpublished data).
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) via DP2 OD001886 and R01AI079497 to T.G.
T.G. is a consultant for AstellasPharma USA and LuminaCare solutions and founded Jacaranda Biomed, Inc. J.-W.C.A. received financial support from Merck, Pfizer, Astellas, and Gilead for investigator-initiated studies and/or academic symposia.
Funding Statement
This work was supported by the National Institutes of Health (NIH) via grants DP2 OD001886 and R01AI079497 to Tawanda Gumbo.
REFERENCES
- 1.Tiberi S, D'Ambrosio L, De Lorenzo S, Viggiani P, Centis R, Sotgiu G, Alffenaar JW, Migliori GB. 2016. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 47:333–336. doi: 10.1183/13993003.01278-2015. [DOI] [PubMed] [Google Scholar]
- 2.Van Rijn SP, Altena R, Akkerman OW, van Soolingen D, van der Laan T, de Lange WCM, Kosterink JGW, van der Werf TS, Alffenaar JWC. 7 January 2016. Pharmacokinetics of ertapenem in patients with multidrug-resistant tuberculosis. Eur Respir J. doi: 10.1183/13993003.01654-2015. [DOI] [PubMed] [Google Scholar]
- 3.De Lorenzo S, Alffenaar JW, Sotgiu G, Centis R, D'Ambrosio L, Tiberi S, Bolhuis MS, van Altena R, Viggiani P, Piana A, Spanevello A, Migliori GB. 2013. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 41:1386–1392. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordillot M, Dubee V, Triboulet S, Dubost L, Marie A, Hugonnet JE, Arthur M, Mainardi JL. 2013. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency. 2004. Scientific discussion for the approval of Invanz. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000389/WC500033918.pdf. [Google Scholar]
- 7.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musuka S, Srivastava S, Siyambalapitiyage Dona CW, Meek C, Leff R, Pasipanodya J, Gumbo T. 2013. Thioridazine pharmacokinetic-pharmacodynamic parameters “wobble” during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob Agents Chemother 57:5870–5877. doi: 10.1128/AAC.00829-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hett EC, Rubin EJ. 2008. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72:126–156, table of contents. doi: 10.1128/MMBR.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchison DA. 1979. Basic mechanisms of chemotherapy. Chest 76:771–781. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2003. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic actinomycetes; approved standard. CLSI M24–A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 12.Van Rijn SP, Wessels AM, Greijdanus B, Touw DJ, Alffenaar JW. 2014. Quantification and validation of ertapenem using a liquid chromatography-tandem mass spectrometry method. Antimicrob Agents Chemother 58:3481–3484. doi: 10.1128/AAC.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S, Pasipanodya J, Sherman CM, Meek C, Leff R, Gumbo T. 2015. Rapid drug tolerance and dramatic sterilizing effect of moxifloxacin monotherapy in a novel hollow-fiber model of intracellular Mycobacterium kansasii disease. Antimicrob Agents Chemother 59:2273–2279. doi: 10.1128/AAC.04441-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veziris N, Truffot C, Mainardi JL, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2600. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]