Abstract
The interindividual and intraindividual variabilities in daptomycin pharmacokinetics were investigated in 23 patients (69 pharmacokinetic profiles) who were treated for several months for bone and joint infections. Population daptomycin clearance was significantly influenced by renal function and was significantly higher in male than in female patients. We observed significant intraindividual changes in daptomycin clearance, which were uncorrelated with changes in renal function, suggesting that therapeutic drug monitoring is important in patients receiving prolonged daptomycin therapy.
TEXT
Daptomycin is a cyclic lipopeptide that has been proposed as an alternative therapeutic option in patients with prosthetic joint infection caused by Staphylococcus or Enterococcus species in the latest Infectious Diseases Society of America (IDSA) guidelines (1).
The population pharmacokinetics (PK) of daptomycin have been described in various groups of patients in previous publications (2–5). However, little information exists on the PK of daptomycin in patients with bone and joint infections (BJI). Also, previous population studies did not investigate daptomycin PK over prolonged therapy, and, to our knowledge, no study has reported the intraindividual PK variability of this drug.
(This work was presented in part at the 54th ICAAC Meeting, Washington, DC, 5 to 9 September 2014, and at the 34th RICAI meeting, Paris, France, 13 to 15 December 2014.)
We performed a retrospective analysis of PK data collected in 23 patients who were treated with daptomycin for BJI in Lyon Center in 2012 and 2013. Therapeutic drug monitoring (TDM) of daptomycin was performed regularly in those patients throughout therapy, roughly every month, to ensure sufficient exposure and to prevent drug accumulation. This project was reviewed by our local institutional review board (CPP Sud-Est III), and a waiver was obtained, as this was a noninterventional study.
On each TDM occasion, a daptomycin PK profile was obtained based on three concentrations usually measured at predose (trough level), 0.5 to 1 h, and 5 to 6 h postdose. A total of 203 daptomycin plasma concentrations and 69 individual PK profiles were determined for the 23 individuals. The exact daptomycin doses, dosing times, dose intervals, blood sampling times, and plasma concentrations were recorded for each subject on each occasion. All patients received daptomycin as a 30-min infusion. The dose interval was 24 h, except for 6 profiles from 4 patients in whom it was 48 h because of renal impairment. Other data available on each occasion included age, sex, height, weight, serum creatinine, estimated glomerular filtration rate (eGFR) provided by the 4-variable modification of diet in renal disease (MDRD) equation (6), and concomitant use of rifampin (10 profiles from 3 patients).
Daptomycin concentrations were determined by using a high-performance liquid chromatography assay with a photodiode array detector. Concentrations were calculated at two wavelengths (260 and 360 nm), and a spectral analysis was performed to ensure chromatographic peaks purity. Accuracy and precision were evaluated at three levels (2.5, 35, and 80 mg/liter). The interday precision was less than 11% with a bias lower than 8%. The lower limit of quantification was 2 mg/liter.
A population approach was used to analyze the PK data. Nonlinear mixed-effects modeling was performed using the stochastic approximation expectation maximization (SAEM) algorithm implemented in the Monolix software (version 4.3.3; Lixoft, Paris, France). Selection of the best structural and covariate model was based on classical criteria, including the likelihood-derived objective function, parameter estimates, predictive performance, and diagnostic plots (7, 8). We assumed log normal distribution of PK parameters. The special feature of this analysis was the inclusion of intraindividual variability, which was implemented by using the interoccasion variability (IOV) routine of Monolix. The interindividual and intraindividual variabilities of PK parameters were coded in Monolix as follows:
| 1 |
where Pik is the parameter value of individual i on occasion k, Cik is the matrix of covariates of individual i on occasion k, μ is the mean population parameter value (fixed effect), and ηi and κik are the interindividual and intraindividual variability terms, respectively. Those random effects, ηi and κik, were assumed to follow normal distributions: ηi ∼ N(0, Ω) and κik ∼ N(0, Γ).
Intraindividual variability was set on clearance and volume of distribution parameters only. As a result, for each subject, a value of daptomycin clearance (CL) and central volume of distribution (V1) were estimated for each TDM occasion. Finally, we studied the correlation between individual, chronological changes in parameter values from one TDM occasion to the next one and the corresponding changes in covariates.
The characteristics of the study population are shown in Table 1. A daptomycin PK profile was obtained on at least two occasions, except for six patients, with a median of three profiles per patient.
TABLE 1.
Characteristics of the study population and pharmacokinetic data set
| Characteristic | Valuea |
|---|---|
| Age (yr) | 68 (19–84) |
| No. of females/males | 9/14 |
| Initial body wt (kg) | 72 (47–140) |
| Initial renal function (eGFR in ml/min/1.73 m2, MDRD equation) | 118 (24–202) |
| Daptomycin dose (mg/kg) | 8 (3–11) |
| Duration of daptomycin TDM (days) | 110 (8–247) |
| No. of TDM occasions per subject | 3 (1–7) |
| No. of measured daptomycin concentrations | 203 |
| Measured daptomycin concn (mg/liter)b | C0 = 13.4 (2.9–50.6) |
| C30 min = 59.7 (21.8–104.1) | |
| C5–6 h = 38.8 (17–70.6) | |
| Estimated daptomycin AUC0–24 (mg · h/liter)c | 683.2 (305.6–1471.1) |
Except where indicated by "No.", data are given as medians (minimum to maximum).
C0, C30 min, and C5–6 h indicate the daptomycin concentrations measured predose (trough concentration), 30 min, and 5 to 6 h postdose, respectively.
AUC0–24 is the individual steady-state AUC calculated over 24 h using the final PK model.
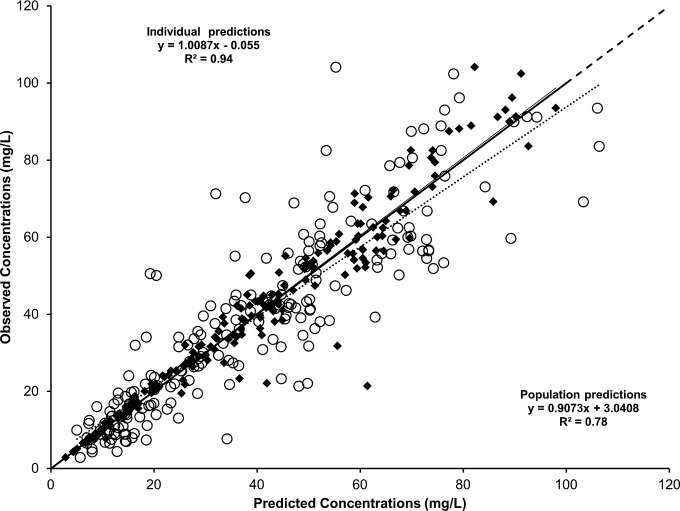
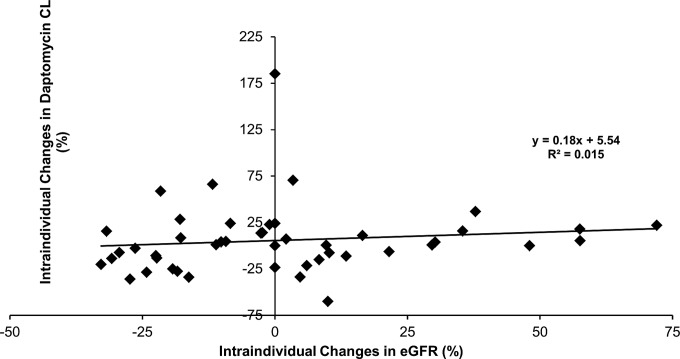
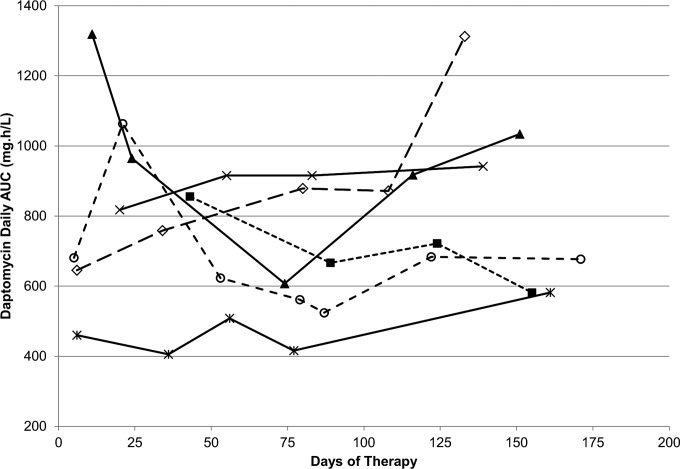
A two-compartment linear PK model, including a proportional residual error, best fit the data. The introduction of intraindividual variability into the model greatly improved the fit (50-point decrease in the Akaike information criterion [AIC]) compared with that of a two-compartment model with interindividual variability only. The final model, including covariates, adequately described the data as shown in Fig. 1. Parameter values of the final model are shown in Table 2. Regarding covariates, sex was found to influence population daptomycin CL and V1, which were 44% (0.81 versus 0.564 liters/h) and 30% (11 versus 8.43 liters) greater in male than in female patients, respectively. Although this needs to be confirmed in a larger study, this finding suggests that male patients may need larger doses of daptomycin in order to achieve a target exposure. Of note, body weight did not appear to significantly influence daptomycin clearance or its volume of distribution in this population. In addition, renal function, as estimated by MDRD-eGFR, was also found to significantly influence population daptomycin CL. A moderate but significant linear correlation was observed between individual estimates of daptomycin CL and eGFR values (R2 = 0.27, data not shown). However, individual chronological changes in daptomycin CL over the TDM period, which ranged from −60% to +185%, were uncorrelated with corresponding changes in renal function as shown in Fig. 2. Figure 3 shows intraindividual changes in daptomycin area under the concentration-time curve (AUC) during therapy. Despite the administration of the same daily dose, the AUC varied substantially in some patients as a result of changes in daptomycin CL.
FIG 1.
Plots of observed daptomycin concentrations (n = 203) versus model-based population (circles and dotted line) and individual (black diamond and solid line) predictions. The dashed line is the line of identity (y = x).
TABLE 2.
Population pharmacokinetic parameters of daptomycin
| Parametera,b | Mean value (relative SE)c | Interindividual variability (%)c | Intraindividual variability (%)c |
|---|---|---|---|
| CL (liter/h) | 0.564 (6%) | 5.3 | 22.5 |
| β1(CL, eGFR) | 0.00307 (24%) | NDd | ND |
| β2(CL, sex) | 0.362 (20%) | ND | ND |
| V1 (liters) | 8.43 (9%) | 4.2 | 24.6 |
| β3(V1, sex) | 0.265 (38%) | ND | ND |
| Q (liters/h) | 0.596 (41%) | 71.2 | ND |
| V2 (liters) | 7.17 (40%) | 59.7 | ND |
CL, daptomycin plasma clearance; Q, intercompartment clearance; V1, central volume of distribution; V2, volume of the peripheral compartment.
Coefficients β quantify the relationships between population parameters (CLpop, V1pop) and covariates as follows: CLpop = 0.564 · exp[β1 · (eGFR/108) + β2 (if male)], where 108 is the median value of eGFR in the study population (ml/min/1.73 m2) and V1pop = 8.43[exp(β3) if male].
Relative standard error and interindividual and intraindividual variability are expressed as coefficient of variation (%).
ND, not determined.
FIG 2.
Plot of individual changes in daptomycin clearance over the therapeutic drug monitoring period versus corresponding changes in renal function (n = 46 pairs).
FIG 3.
Intraindividual changes in daptomycin AUC during therapy. For ease of graphical display, only data from six individuals who had four or more TDM occasions with the same daptomycin daily dose are shown. The AUC values calculated for 24 h were estimated from the final model.
The PK model, population parameter values, and covariates identified in this study are consistent with previous reports (2, 4, 5). In particular, our study and previous works have found that daptomycin clearance correlates with renal function, which is in agreement with the predominant renal excretion of the drug (9). As a result, one might assume that renal function can be used as guidance for initial dosing and dose adjustment during daptomycin therapy.
In that study, we found substantial intraindividual variability in daptomycin PK in a group of patients with prolonged daptomycin administration for BJI. Importantly, such variability was not predictable from corresponding time changes in renal function. Actually, this variability has remained unexplained. These results suggest that renal function has basically no guidance value for adjusting daptomycin dosing during individual therapy.
The main limitation of this study was the limited number of patients included (n = 23). This probably affected the estimation of the interindividual variability, which was surprisingly low for CL and V1, about 5%, while previous studies reported coefficients of variations of 20% to 40% (2, 4). It is noteworthy that these numbers reflect the interindividual variability that is unexplained by covariates, not the overall interindividual variability in CL and V1, which was larger (about 31% for CL and 19% for V1 based on individual estimates on the first TDM occasion). However, this limitation had no influence on the estimation of the mean values and intraindividual variability of PK parameters, and it did not undermine the predictive performance of the model. On the other hand, interindividual variability may well have been overestimated in previous studies, which ignored interoccasion variability as shown in the seminal work of Karlsson and Sheiner (10).
To conclude, this study has shown the significant intraindividual PK variability of daptomycin during long-term use for treatment of BJI, and this variability was unexplained by covariates. Daptomycin TDM appears necessary to control individual exposure and to adjust drug dosage during prolonged therapy. Experimental data have suggested daptomycin maximum concentration of drug in serum (Cmax) and area under the concentration-time curve from 0 to 24 h (AUC0–24) values of 30 to 46 μg/ml and 294 to 375 μg · h/ml, respectively, to achieve a bactericidal activity against Staphylococcus aureus (11). In addition, a clinical study identified that a daptomycin plasma concentration of >24.3 mg/liter was associated with a significant increase in daptomycin muscular toxicity (12). While little information exists on daptomycin PK/pharmacodynamics (PD) in BJI, Traunmüller et al. observed a similar free concentration of daptomycin in bone and plasma (13). Based on these data, and although further research is necessary, a daptomycin peak of >50 μg/ml and trough of <24 μg/ml may be considered target concentrations for TDM of daptomycin in BJI.
ACKNOWLEDGMENTS
All of the members of the Lyon Bone and Joint Infection Study Group are gratefully acknowledged: physicians Tristan Ferry, Thomas Perpoint, André Boibieux, François Biron, Florence Ader, Julien Saison, Florent Valour, Sandrine Roux, Fatiha Daoud, Johanna Lippman, Evelyne Braun, Marie-Paule Vallat, Patrick Miailhes, Christian Chidiac, Yves Gillet, and Laure Hees; surgeons Sébastien Lustig, Philippe Neyret, Olivier Reynaud, Adrien Peltier, Anthony Viste, Jean-Baptiste Bérard, Frédéric Dalat, Olivier Cantin, Romain Desmarchelier, Thibault Vermersch, Michel-Henry Fessy, Cédric Barrey, Francesco Signorelli, Emmanuel Jouanneau, Timothée Jacquesson, Pierre Breton, Ali Mojallal, Fabien Boucher, and Hristo Shipkov; microbiologists Frederic Laurent, François Vandenesch, Jean-Philippe Rasigade, Céline Dupieux, and Sophie Trouillet-Assant; nuclear medicine specialists Isabelle Morelec, Marc Janier, and Francesco Giammarile; PK/PD specialists Michel Tod, Marie-Claude Gagnieu, and Sylvain Goutelle; and clinical research assistant Eugénie Mabrut.
This work was not supported by any academic, company, or sponsor fund.
T.F. received a travel grant (ICAAC 2014) from Novartis. The other authors have no conflicts of interest that are relevant to the content of this study.
REFERENCES
- 1.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 2.Dvorchik B, Arbeit RD, Chung J, Liu S, Knebel W, Kastrissios H. 2004. Population pharmacokinetics of daptomycin. Antimicrob Agents Chemother 48:2799–2807. doi: 10.1128/AAC.48.8.2799-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterfield JM, Mueller BA, Patel N, Cardone KE, Grabe DW, Salama NN, Lodise TP. 2013. Daptomycin pharmacokinetics and pharmacodynamics in a pooled sample of patients receiving thrice-weekly hemodialysis. Antimicrob Agents Chemother 57:864–872. doi: 10.1128/AAC.02000-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Paolo A, Tascini C, Polillo M, Gemignani G, Nielsen EI, Bocci G, Karlsson MO, Menichetti F, Danesi R. 2013. Population pharmacokinetics of daptomycin in patients affected by severe Gram-positive infections. Int J Antimicrob Agents 42:250–255. doi: 10.1016/j.ijantimicag.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Chaves RL, Chakraborty A, Benziger D, Tannenbaum S. 2014. Clinical and pharmacokinetic considerations for the use of daptomycin in patients with Staphylococcus aureus bacteraemia and severe renal impairment. J Antimicrob Chemother 69:200–210. doi: 10.1093/jac/dkt342. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. 2007. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 7.Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2:e38. doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwin CM, Kiang TK, Spigarelli MG, Ensom MH. 2012. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet 51:573–590. doi: 10.1007/BF03261932. [DOI] [PubMed] [Google Scholar]
- 9.Sauermann R, Rothenburger M, Graninger W, Joukhadar C. 2008. Daptomycin: a review 4 years after first approval. Pharmacology 81:79–91. doi: 10.1159/000109868. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson MO, Sheiner LB. 1993. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 11.Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother 48:63–68. doi: 10.1128/AAC.48.1.63-68.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 50:1568–1574. doi: 10.1086/652767. [DOI] [PubMed] [Google Scholar]
- 13.Traunmüller F, Schintler MV, Metzler J, Spendel S, Mauric O, Popovic M, Konz KH, Scharnagl E, Joukhadar C. 2010. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J Antimicrob Chemother 65:1252–1257. doi: 10.1093/jac/dkq109. [DOI] [PubMed] [Google Scholar]