Introduction
Hormonal contraceptives are the most widely used form of contraception in sub-Saharan Africa with 12 million women using an injectable form (‘injectables’ or IHC),[1] and uptake of IHC has been rapidly increasing over the last two decades in many parts of the world.[2]
Some high-quality prospective observational studies have reported a statistically significant increase in risk of HIV acquisition associated with IHC use,[3–6] but others have not.[7–11] Among the former, the largest risk estimate detected was a two-fold increase in HIV acquisition (odds ratio (OR) = 2.19 (95% confidence interval (CI) 1.01-4.74) in a recent analysis of women using IHC in heterosexual serodiscordant couples [5]; the other studies detected point estimates of 1.48 (1.02-2.15),[4] 1.72 (1.19-2.49)[6] and 1.73 (1.28-2.34).[3] Although there is no scientific consensus that IHC is causally related to HIV risk in women, any factor found to increase HIV acquisition is potentially important and could have significant public health implications. In countries with high levels of IHC use, increased HIV risk could have a substantial effect if the underlying risk for HIV acquisition is high. If there is a causal relationship between the two, reductions in IHC usage in such areas may lead to a decrease in HIV infections, but there may also be other significant consequences of the reduced availability of effective contraceptive options for women. For example, increases in unintended pregnancies would lead to a number of adverse outcomes including maternal and infant deaths, particularly in regions with a high birth rate and maternal mortality. Since the data on the impact of pregnancy on risk of HIV acquisition is also conflicting, increases in unintended pregnancy may also impact the HIV risk.[8, 12–14] Conversely, there may be some countries with high IHC but low HIV incidence and maternal mortality where the potential interaction with HIV acquisition will have little public health impact.
A technical consultation was convened by the WHO in January 2012 in light of these concerns. After considering the totality of evidence, the panel concluded that the medical eligibility criteria (MEC) for IHC use should remain unchanged from the current rating, and added a strong clarification to note that women using progestogen-only injectables who are at high risk of HIV should be strongly advised to also always use male or female condoms and other preventive measures.[15] Increased uptake of interventions such as ART for an HIV-infected partner of IHC users may also partially offset any increase in HIV risk for these women.
In this analysis we model the global public health implications of an interaction between IHC use and HIV risk under different assumptions about the true effect size. We use country-level outcomes on HIV infections, live births, maternal deaths, and HIV-related deaths to explore the potential contribution of IHC use to HIV spread and the overall consequences of its removal.
Methods
Distribution of injectable hormonal contraception and HIV
First, we assessed HIV prevalence[16–20] and IHC use[2] for each country. We defined IHC use as common if the most recent estimate of current IHC use is within the top quartile of all countries with available data[2] and we defined HIV prevalence above 1% as high. All analyses and parameters refer to 15-49 year-old women.
Effects of an IHC–HIV interaction on HIV infections per year
We conducted a modelling exercise to estimate the number of female HIV acquisitions per year that could be attributed to IHC use under two hypothetical scenarios. First, we used one of the largest effect sizes estimated for the IHC-HIV interaction to date (OR=2.19[5]); this is the higher of the two estimates from the Partners in Prevention HSV/HIV Transmission Study[5] and was calculated using marginal structural modelling. We equated the OR to a relative risk (RR) for this analysis as the estimated HIV incidence is sufficiently low. Then we used a hypothetical weak estimate of interaction strength (RR=1.2) as a comparison. The additional HIV infections per year attributable to a possible IHC-HIV interaction are estimated as:
| (1) |
Parameter definitions for all equations are detailed in Box 1. This model considers HIV acquisition in women only, and does not account for transmission from HIV-positive women to uninfected men. HIV incidence is estimated by assuming equilibrium prevalence and a mean survival time with HIV infection of ten years Full derivations for all equations are given in the technical appendix.
Effects of reduced IHC use on live births and maternal deaths per year
We then explore the impact of reducing IHC use on the number of live births per year. This statistic is a measure of the potential increase in unintended pregnancies, although it is likely to be an underestimation as it does not capture still-births, spontaneous, elective or therapeutic abortions. We simulate a scenario whereby all IHC use is stopped and IHC replaced for some women with a hypothetical alternative contraceptive that has no association with HIV risk. The proportion of women transitioned to the alternative is assumed to be equal to the proportion of non-IHC-using women who report use of other contraceptive methods to reflect the current availability and uptake of other contraceptive methods for that country[2]; the remainder are assumed to stop using any form of contraception. For all calculations we assume that IHC has a failure rate of 6% per year and 85% of women not using contraception become pregnant within one year.[22] The alternative contraception has a hypothetical typical failure rate of 12% per year, which is the mean of four common non-IHC contraceptive methods (both modern and traditional): male condom, 18%; combined oral pill (COC), 9%; copper intra-uterine device (IUD), 0.8%; and withdrawal, 22% [22]. Sensitivity analyses indicate that variations in this assumption have a limited impact on the qualitative results (Supplementary table 1). All pregnancies are assumed to result in live births, which may be an overestimate as described above.
Under these conditions, the change in the number of live births per year on removal of IHC is given by:
| (2) |
The change in maternal deaths on cessation of IHC use is estimated from the change in live births, ΔB (eq. 2), using the per-country maternal mortality ratio (MMR)[24]:
| (3) |
The MMR represents mortality during or related to pregnancy, child birth or within 42 days of termination of pregnancy[25] so will capture the excess maternal mortality related to unintended pregnancies that do not reach full term as well as those that result in live births.
Net consequences on maternal and HIV-related deaths following reduced IHC use
To explore the net consequence of the removal of IHC from family planning services, we calculated the net change in the total number of resulting maternal deaths and HIV-related deaths. An excess infection was counted as an additional HIV-related death if the expected timing of death, after allowing for the effects of antiretroviral therapy (ART), was before the estimate of life-expectancy.[26] ART coverage is defined by country for low and middle income countries where data is available, else a regional mean is assigned.[27, 28] For high income countries, where ART coverage information is not routinely reported, we assume a high coverage of 80%. The mean age at infection is assumed to be 25 years for all countries; women with HIV are assumed to have a survival time of 10 years if untreated,[29] or 20 years if treated.[30, 31] A five per-cent discount rate per year of survival post-infection was applied to all HIV-related deaths. The excess number of HIV-related deaths attributable to a possible IHC-HIV interaction is given by:
| (4) |
The net change in HIV-related and maternal deaths resulting from cessation of IHC use is calculated by subtracting equation (eq.) 4 from eq. 3. In addition to the two estimates for the strength of the interaction between IHC and HIV risk defined above, for this statistic we include a third ‘null’ estimate of the IHC-HIV association, where we assume that no true causal relationship exists between IHC and risk of HIV acquisition (RR=1.0). This illustrates the public health outcomes of reduced IHC use if there is no true biological association.
Results
Distribution of injectable hormonal contraception and HIV
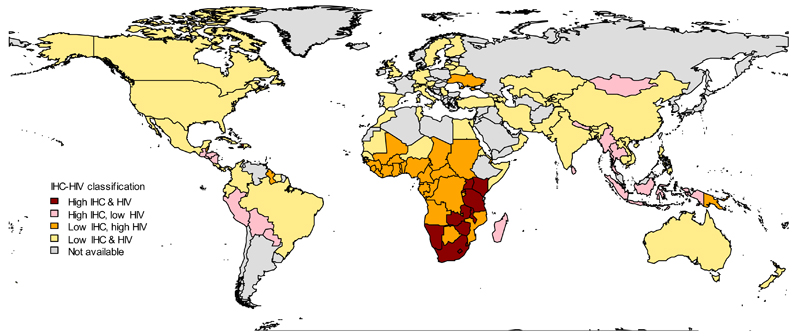
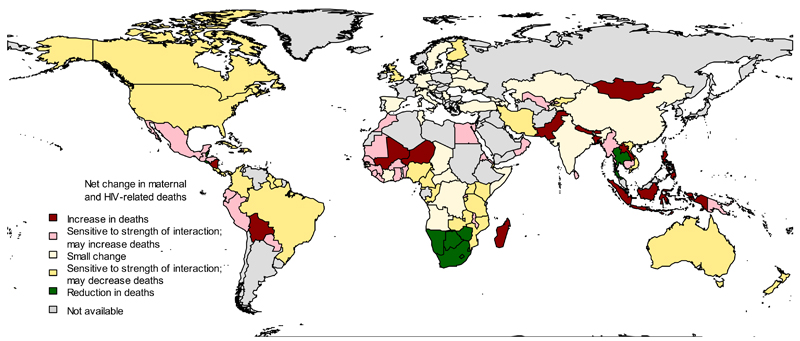
Figure 1 charts the global pattern of IHC use and HIV prevalence, and highlights that high IHC use coincides with high HIV prevalence predominantly in southern and eastern Africa. The generalised epidemic in this region means that many women, including those who are using IHC, may be exposed to HIV. Haiti also has both a high prevalence of IHC use and a high prevalence of HIV.
Figure 1. Prevalence of injectable hormonal contraceptive (IHC) use and HIV prevalence by country.
Countries are coloured according to HIV prevalence[16–20] and IHC use[2] among 15-49 year-old women: red = high IHC and high HIV; pink = high IHC and low HIV; orange = low IHC and high HIV; yellow = low IHC and low HIV. HIV prevalence above 1% and IHC prevalence in the top quartile globally are defined as high and all values below these thresholds are low. HIV prevalence data was unavailable for 15-49 year-old women in Brazil, China, the Democratic Republic of the Congo (DRC) and Thailand therefore we use reported HIV prevalence from antenatal clinics for Brazil,[17] DRC,[19] and Thailand[20] and adult HIV prevalence for China.[18]
Effects of an IHC–HIV interaction on HIV infections per year
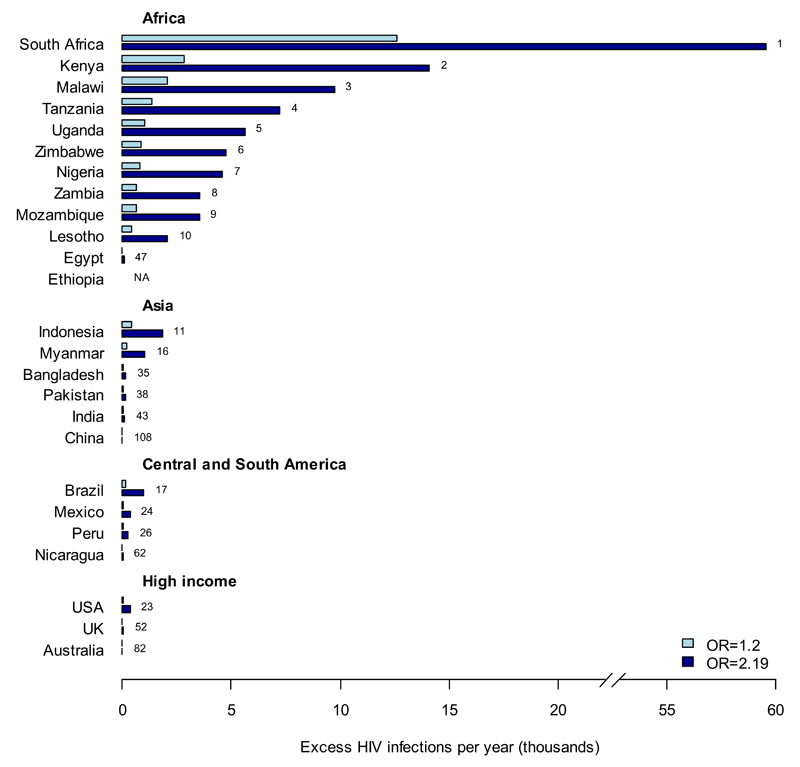
The estimated numbers of HIV infections in a country that could be attributed to IHC use under two different assumptions about the IHC-HIV interaction are shown in Figure 2A (see supplementary table 2 for all countries). Globally, a total of 27,000-130,000 infections per year (for RR=1.2-2.19) would be attributable to IHC, 87-88% of which occur in southern and eastern Africa. For example, 13,000-59,000 HIV infections per year (5.4-25% of current annual infections in women for RR=1.2-2.19, data not shown) in South Africa may be attributable to IHC use and 2,900-14,000 (4.1-20%) in Kenya.
Figure 2. A. Excess HIV infections per year attributable to a hypothesised IHC-HIV interaction.
Countries are ranked according to the total number of HIV infections per year that would be attributed to a putative IHC-HIV interaction with effect size (1) RR=1.2 and (2) RR=2.19.[5] The ten countries with the highest excess infections are shown together with selected examples from different regions. Country rankings out of 116 countries with available data are annotated next to the respective bars. Data for all other countries can be found in supplementary table 2 (online).
There are also high levels of excess infections relative to the current total infections in Southeast Asia (e.g. Indonesia: 400-1,900 (6.0-27%); Myanmar: 210-1,000 (3.8-19%)) and some Central and South American countries (Brazil: 170-1000 (0.79-4.5%); Peru: 54-270 (3.5-18%)), although the absolute number of excess infections is low everywhere outside of Africa. Countries where very few infections are potentially attributable to IHC either have very low IHC use (India: 19-110 (0.02-0.12%)) or low HIV incidence among women (USA: 61-360 (0.28-1.6%)). These excess infections would be offset by a 17-54% reduction in HIV incidence among IHC users for RR=1.2-2.19, which could be mediated by increased condom use or ART uptake among HIV-positive partners of IHC users.
Effects of reduced IHC use on live births and maternal deaths per year
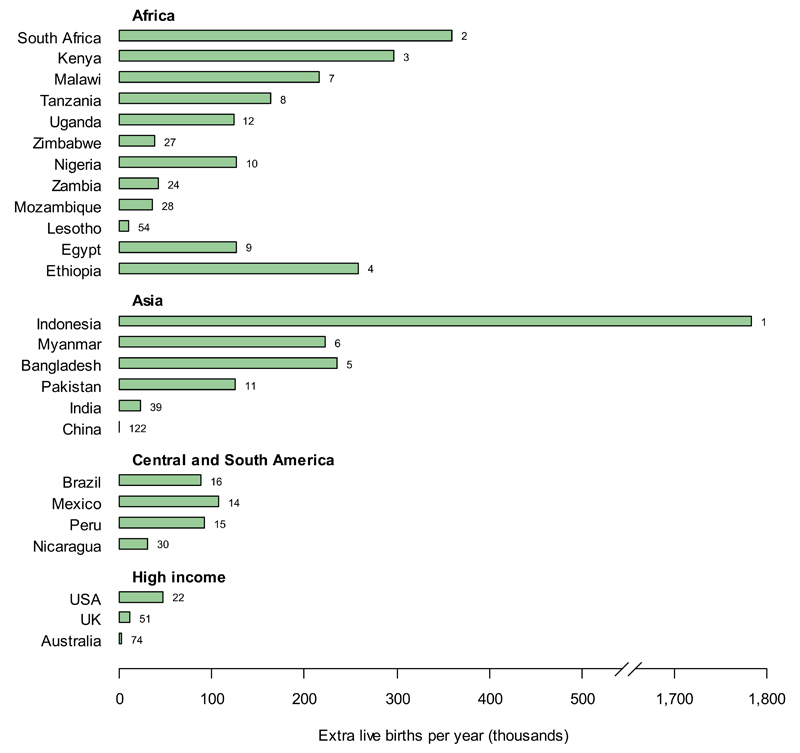
If IHC was removed and only partially replaced with an alternative contraceptive, there would be an increase in the number of live births, due to increased numbers of unintended pregnancies (fig. 2B). These would be substantial in countries with high IHC use and high birth rates; primarily in southern and eastern Africa (South Africa: 360,000 extra live births per year (34% of current annual live births); Kenya: 300,000 (24%)), South and South-East Asia (Bangladesh: 235,000 (6.5%); Indonesia: 1.8 million per year (39%)), and Central and South America (Nicaragua: 30,000 (25%); Peru: 92,000 (18%)). The absolute change in the number of live births for each country is dependent on current IHC use,[2] population size,[21] birth rate,[23] and the reported use of alternative contraceptive methods,[2] which combine to give the largest increase in live births for Indonesia. The scale of the increased numbers of live births following reduced IHC use is much greater than the level of excess HIV infections if IHC use continued.
B. Excess live births per year resulting from cessation of all IHC use;
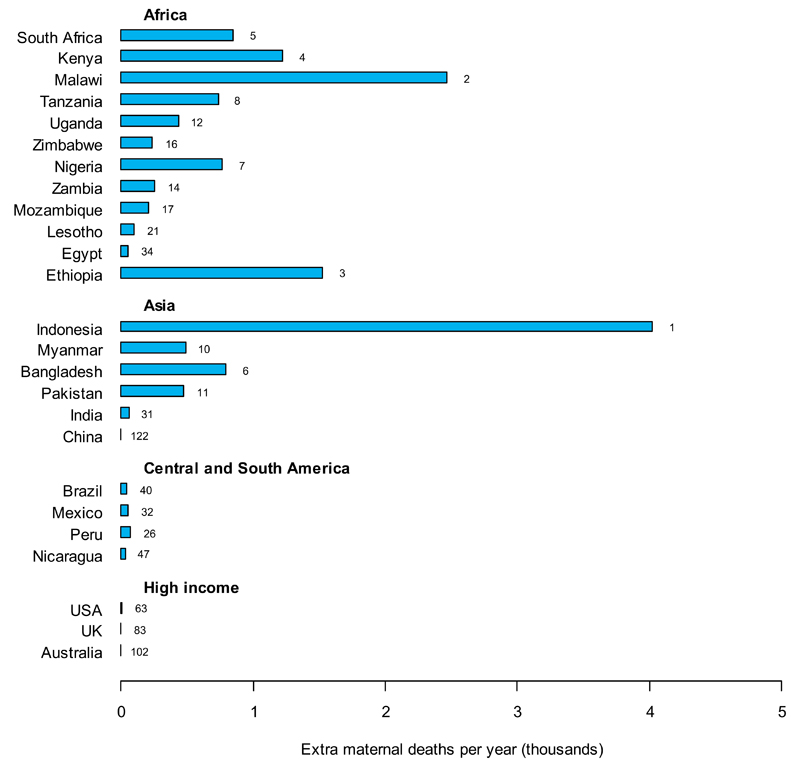
In the same scenario, these unintended pregnancies are accompanied by increases in maternal deaths (fig. 2C), particularly in countries where high levels of IHC use coincide with a high MMR (Malawi: 2,500 extra maternal deaths per year (36% of current annual maternal deaths); Ethiopia: 1,500 (10%)) or high birth rate (Indonesia: 4,000 (39%); Bangladesh: 780 (6.5%)).
C. Excess maternal deaths per year resulting from cessation of all IHC use.
It is assumed that all women previously using IHC are transitioned to either no contraception or a hypothetical alternative, in proportion to the method mix reported by the non-IHC-using population for each country.[2] Countries are ranked according to the excess number of live births per year that can be attributed to cessation of all IHC use. The ten countries with the highest numbers of excess births are shown together with the examples from fig. 2A. Country rankings out of 134 countries with available data are annotated next to the relevant bars. Data for all other countries can be found in supplementary table 2 (online).
Net consequences on maternal and HIV-related deaths following reduced IHC use
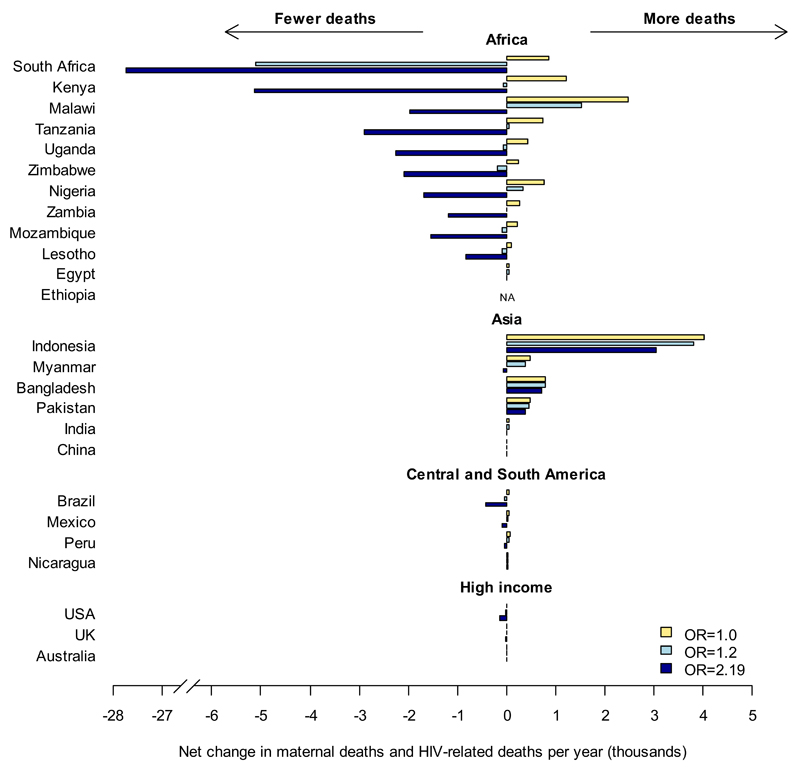
Figs. 3A and 3B show the direction and magnitude of the net change in HIV-related and maternal deaths if IHC use were reduced, under different assumptions about the effect size of an IHC-HIV interaction.
Figure 3. Change in the number of net maternal and HIV-related deaths resulting from cessation of IHC use.
A. Direction of change. Countries are coloured according to the direction of change in the net number of maternal and HIV-related deaths that result directly from stopping IHC use assuming (1) RR=1.2 and (2) RR=2.19: red = expected increase in net maternal and HIV-related deaths (>0.5% under both RR assumptions); pink = change in net deaths is dependent on the effect size (>0.5% increase only when RR=1.2); cream = reductions in IHC use unlikely to provide public health benefit in terms of deaths prevented (<0.5% change with both estimates); yellow = change in net deaths is dependent on the effect size (>0.5% decrease only when RR = 2.19); green = expected decrease in net deaths (>0.5% under both RR assumptions); grey = data not available.
B. Absolute change. The change in net maternal and HIV-related deaths on cessation of all IHC use, assuming (1) RR=1.0 (yellow), (2) RR=1.2 (light blue) and (3) RR=2.19 (dark blue), shown for the countries presented in fig. 2. Data for all other countries can be found in supplementary table 2 (online).
In countries where the decrease in HIV incidence outweighs the increase in maternal deaths, irrespective of the assumed strength of the IHC-HIV association (RR=1.2 or 2.19), we may see a reduction in total deaths on cessation of IHC use (fig. 3A, green). These are mainly limited to southern Africa, with the largest decrease in South Africa (5,100-27,000 fewer deaths per year (4.5-24% decrease compared to total expected HIV-related and maternal deaths for RR=1.2-2.19, fig. 3B).
There is a wider range of countries where the net effects of reducing IHC depends strongly on the assumed association between IHC and HIV risk (fig. 3A, yellow): if there is a strong interaction (RR=2.19) then there could be fewer overall deaths, but with a weaker association (RR=1.2) there would be little net change relative to the current number of HIV-related and maternal deaths. This includes low and middle income countries with moderate or high HIV incidence rates (e.g. Kenya: 64– 5100 fewer deaths (-0.18 to -14%); Tanzania: 61 extra – 2900 fewer deaths (+0.16 to -7.4%)) and high income countries with low maternal mortality where any decrease in HIV-related deaths outweighs increases in maternal deaths (USA: 17 – 140 fewer deaths (-0.18 to -1.4%)).
In countries where maternal mortality is high compared to HIV incidence, reducing IHC use would be likely to increase the overall deaths, regardless of the RR estimate used (fig. 3A, red). This includes areas of Central and South America (Nicaragua: 28 – 16 extra deaths (+14 to +7.9%)), West and North Africa (Egypt: 46 – 6 extra deaths (+3.3 to +0.46%)), and parts of Asia (Indonesia: 3,000-3,800 extra deaths (+28 to +22%); Bangladesh: 780 – 714 extra deaths (+5.9 to +5.4%)), with the maximum increase in Indonesia. In other countries, there could be a net increase in deaths following reduction in IHC use, depending on the strength of the IHC-HIV association (fig. 3A, pink), for example Malawi: 1500 extra - 2000 fewer deaths (+6.3 to -8.2%); Myanmar: 380 extra – 75 fewer deaths (+7.2 to -1.5%).
The areas where there is little change in net maternal and AIDS-related deaths on cessation of IHC (fig. 3A, cream) are those where injectables are not commonly used (India: 49-1 extra deaths (+0.090 to +0.00086%)).
Fig. 3B illustrates the potential scale of the changes in net deaths and its sensitivity to the assumed IHC-HIV interaction strength. Whilst the potential reduction in deaths resulting from reductions in IHC use is greatest in southern Africa with the higher effect estimate, these countries would also see some of the largest increases in maternal mortality if there was no true association between IHC use and HIV risk (RR = 1). Overall, stopping all IHC use would reduce the total deaths per year globally by 47,000 with an odds ratio of 2.19, but would result in an increase of 3,400 deaths with the more modest estimate of 1.2. If there is no real effect of IHC on HIV acquisition then stopping IHC would cause at least 16,000 more maternal deaths per year worldwide, spread primarily through Africa and South and South-East Asia (fig. 3B).
Sensitivity analyses show that varying the proportion of women stopping IHC use who are transitioned onto the background established mix of non-IHC contraceptives or a specified replacement can have a large impact on the change in net deaths in countries where the baseline level of IHC replacement is very low (e.g. Nigeria: 12% transitioned to background mix, Supplementary Table 1) or very high (Brazil: 79%). Reducing HIV incidence among IHC users also has a major impact on net deaths by reducing HIV-related deaths without increasing maternal deaths. Varying the assumed background or replacement contraceptive efficacies at baseline uptake levels (which are generally low) has a limited impact on model outputs.
Discussion
High IHC use and high HIV prevalence coincide primarily in southern and eastern Africa. There are over ten million women using IHC in these countries who are therefore at a potentially elevated risk of HIV acquisition, or if IHC use was reduced, increased unintended pregnancy and maternal death.
If the association between use of injectables and HIV acquisition is real, a reduction in IHC use is likely to reduce HIV infections in regions of high incidence. However, where MMR is also high, and in the absence of an effective alternative contraceptive, reductions in IHC use would increase unintended pregnancies and maternal deaths. The direction of change in the overall number of deaths resulting from cessation of IHC therefore depends on the relative levels of HIV incidence, birth rate and MMR, as well as the true effect size of the interaction, if any. In countries with low HIV incidence, high birth rate and high MMR, such as Indonesia, reducing IHC use would be expected to have a harmful overall effect; whereas for countries with high HIV incidence and IHC use, such as South Africa, reducing IHC could lead to fewer deaths overall, if there is a real association between IHC and HIV acquisition. For countries in between these extremes, the overall outcome is heavily dependent on the magnitude of the assumed effect size. If there is no causal link between IHC and HIV risk (RR=1) then withdrawal of IHC would lead to an increase in net deaths in all countries (fig. 3B).
The simple metric we have used for this exploratory analysis is based on only two outcomes of reducing IHC use: the potential decrease in HIV-related deaths due to a reduction in infections, and the increase in maternal deaths due to additional unintended pregnancies. It does not account for infant deaths or the broad range of consequences of unintended pregnancies and HIV infection for women and their families. The wider effects of HIV infection include the health burden of HIV, reduced ability to work and care for children plus the associated economic costs, possible vertical transmission and social stigma. For unintended pregnancies, as well as the pregnancy itself, other possible outcomes are any elective or spontaneous abortions – which have important implications for morbidity and mortality particularly in countries where safe and legal abortion services are not available,[32, 33] complications during pregnancy or birth, a reduction in years of schooling and consequent loss of earning potential, the economic cost of interruption to employment and childcare costs.[34]
We have modelled a hypothetical scenario where there is no replacement contraceptive as effective as IHC. This may result in overestimation of the increase in live births and maternal deaths that would occur if IHC use was reduced. If a practical, effective and acceptable alternative contraception could be provided, many of the adverse events associated with unintended pregnancies would be mitigated and, in our analysis, the relative benefit of the reduced IHC use would increase. However, in many developing countries, in practice there are very few alternative forms of contraception available to women.[35] Conversely, future increases in ART coverage would reduce the effective mortality rate of HIV by extending the expected survival time, as defined in equation (4). However, this model does not capture the other costs and morbidity associated with HIV infection or any reduction in overall HIV incidence due to the community-level effects of increased ART coverage on transmission,[36] which may occur to some extent even when ART is used along current international guidelines.[37, 38] Our method also overestimates the impact of reducing IHC use on HIV spread in countries where HIV risk is limited to specific sub-populations who may not be the main recipients of IHC.
Furthermore, cessation of IHC use has been applied to all women irrespective of HIV status, which may increase levels of perinatal HIV transmission among HIV-positive women; and HIV risk has been applied to all women homogeneously, where in reality graduations in risk behaviour will affect the apparent overlap between use of injectables and risk of HIV acquisition. Further analyses are needed to incorporate this range of issues in a full exploration of the aggregated effects and the wider social impact of unintended pregnancies and HIV transmission. There are also potential interactions between the two outcomes. There is mixed evidence with respect to the effect of pregnancy on HIV acquisition[8, 12–14] and one study that indicates that pregnancy increases the risk of transmission[14]; and reducing IHC-related HIV infections may also reduce the overall MMR in some countries,[24] which could partially offset increases in maternal deaths resulting from withdrawal of IHC. These considerations have not been included in this analysis but will be important to evaluate in future modelling studies.
We have limited this analysis to the key variables affecting HIV acquisition for women using IHC at present. These should also be considered in the context of future changes in other factors affecting HIV incidence among women using IHC, for example increasing dual IHC plus condom use following the recent WHO technical statement [15] or increasing access to ART among HIV-positive partners. The decrease in incidence required to offset any excess risk attached to IHC use increases with the strength of the interaction but may be achievable through combination of multiple prevention strategies.
If use of injectable contraceptives increases HIV acquisition risk, reducing IHC could reduce new HIV infections, but this must be balanced against important other consequences, in particular increased unintended pregnancies and maternal deaths. It will be crucial to gain a clearer understanding of the actual interaction between IHC and HIV but unless the true effect size approaches the higher reported estimates, it is unlikely that reductions in IHC could result in public health benefit, with the possible exception of those countries in southern Africa with the largest HIV epidemics.
Supplementary Material
Acknowledgements
This work was supported by grants from the Wellcome Trust (090285/Z/09/Z) and the Qatar National Research Foundation (NPRP 08-068-3-024). The authors thank Geoff Garnett (Bill and Melinda Gates Foundation and Imperial College London).
Footnotes
Competing interests: None declared
References
- 1.Darroch J, Sedgh G, Ball H. Contraceptive Technologies: Responding to Women’s Needs. New York: Guttmacher Institute; 2011. [Google Scholar]
- 2.United Nations Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2010 (POP/DB/CP/Rev2010) 2011 [Google Scholar]
- 3.Baeten JM, Benki S, Chohan V, Lavreys L, McClelland RS, Mandaliya K, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 4.Morrison CS, Chen P-L, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infectious Diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012;26:375–380. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 7.Morrison CS, Skoler-Karpoff S, Kwok C, Chen P-L, van de Wijgert J, Gehret-Plagianos M, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS. 2012;26:497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 8.Reid S, Dai J, Wang, Sichalwe B, Akpomiemie G, Cowan F, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. Journal of Acquired Immune Deficiency Syndromes. 2010;53:606–613. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinschmidt I, Rees H, Delany S, Smith D, Dinat N, Nkala B, et al. Injectable progestin contraceptive use and risk of HIV infection in a South African family planning cohort. Contraception. 2007;75:461–467. doi: 10.1016/j.contraception.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Myer L, Denny L, Wright TC, Kuhn L. Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. International Journal of Epidemiology. 2007;36:166–174. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 11.Kiddugavu M, Makumbi F, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, et al. Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. International Journal of Epidemiology. 2003;17:233–240. doi: 10.1097/00002030-200301240-00014. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. The Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 13.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–1034. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 14.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. World Health Organisation; 2012. Hormonal contraception and HIV: Technical statement. [PubMed] [Google Scholar]
- 16.Population Reference Bureau. The World’s Women and Girls 2011 Data Sheet. 2009 [Google Scholar]
- 17.Szwarcwald CL, Barbosa Júnior A, de Souza-Júnior PRB, de Lemos KRV, de Frias PG, Luhm KR, et al. HIV testing during pregnancy: use of secondary data to estimate 2006 test coverage and prevalence in Brazil. Brazilian Journal of Infectious Diseases. 2008;12:167–172. doi: 10.1590/s1413-86702008000300002. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS. AIDSinfo country factsheets. 2009 [Google Scholar]
- 19.Behets F, Edmonds A, Kitenge F, Crabbé F, Laga M. Heterogeneous and decreasing HIV prevalence among women seeking antenatal care in Kinshasa, Democratic Republic of Congo. International Journal of Epidemiology. 2010;39:1066–1073. doi: 10.1093/ije/dyq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UNGASS Country Progress Report Thailand. National AIDS Prevention and Alleviation Committee; 2010. [Google Scholar]
- 21.United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2010 Revision (comprehensive Excel tables) New York: 2011. [Google Scholar]
- 22.Trussell J. Contraceptive efficacy. In: Hatcher R, Trussell J, Nelson A, Cates W Jr, Kowal D, Policar M, editors. Contraceptive Technology: Twentieth Revised Edition. New York: Ardent Media; 2011. [Google Scholar]
- 23.United Nations Department of Economic and Social Affairs, Population Division. World Fertility Data 2008 (POP/DB/Fert/Rev2008) 2009 [Google Scholar]
- 24.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. The Lancet. 2010;375:1609–1623. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Maternal Mortality Ratio (per 100,000 live births) In. [Google Scholar]
- 26.Population Reference Bureau. World Population Data Sheet. 2011 [Google Scholar]
- 27.WHO, UNAIDS, UNICEF. Global HIV/AIDS Response: Epidemic update and health sector response progress towards Universal Access, Progress Report 2011. 2011 [Google Scholar]
- 28.WHO, UNICEF, UNAIDS. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 [Google Scholar]
- 29.Todd J, Glynn J, Marston M, Lutalo T, Biraro S, Mwita W, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS. 2007;21:S55–63. doi: 10.1097/01.aids.0000299411.75269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sexually Transmitted Infections. 2008;84:i31–i36. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahy M, Lewden C, Brinkhof MWG, Dabis F, Tassie J-M, Souteyrand Y, et al. Derivation of parameters used in Spectrum for eligibility for antiretroviral therapy and survival on antiretroviral therapy. Sexually Transmitted Infections. 2010;86:ii28–ii34. doi: 10.1136/sti.2010.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler AJ, Filippi V, Thomas SL, Ronsmans C. Incidence of severe acute maternal morbidity associated with abortion: a systematic review. Tropical Medicine & International Health. 2012;17:177–190. doi: 10.1111/j.1365-3156.2011.02896.x. [DOI] [PubMed] [Google Scholar]
- 33.Ǻhman E, Shah IH. New estimates and trends regarding unsafe abortion mortality. International Journal of Gynecology and Obstetrics. 2011;115:121–126. doi: 10.1016/j.ijgo.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Darroch J, Ashford L, Vlassoff M. New York: Guttmacher Institute and United Nations Population Fund; 2009. Adding It Up: The Costs and Benefits of Investing in Family Planning and Maternal and Newborn Health. [Google Scholar]
- 35.Ross J, Hardee K, Mumford E, Eid S. Contraceptive Method Choice in Developing Countries. International Family Planning Perspectives. 2002;28:32–40. [Google Scholar]
- 36.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, et al. HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group. HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making. PLoS Med. 2012;9:e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.