Abstract
Background
Irreversible inhibition of Bruton tyrosine kinase (Btk) by ibrutinib represents a significant therapeutic advance for chronic lymphocytic leukemia (CLL). However, ibrutinib also irreversibly inhibits alternative kinase targets, which potentially compromise its therapeutic index. Acalabrutinib (ACP-196) is a more selective irreversible Btk inhibitor specifically designed to improve upon the safety and efficacy of first generation Btk inhibitors.
Methods
Sixty-one patients with relapsed CLL were treated in a phase 1–2 multicenter study designed to assess the safety, efficacy, pharmacokinetics and pharmacodynamics of oral acalabrutinib. Patients were continuously treated with acalabrutinib 100 to 400 mg once daily in the dose-escalation portion of the study, and 100 mg twice daily in the expansion portion.
Results
Patient demographics include a median age of 62 years; median of 3 prior therapies; 31% del(17)(p13.1) and 75% unmutated immunoglobulin heavy chain variable genes. No dose-limiting toxicities occurred. The most common adverse events observed were headache (43%), diarrhea (39%) and increased weight (26%). Most adverse events were Grade 1–2. At a median follow-up of 14.3 months, the best overall response rate was 95%, including 85% partial response, 10% partial response with lymphocytosis and 5% stable disease. In patients with del(17)(p13.1), the best overall response was 100%. No cases of Richter’s transformation and only 1 CLL progression have occurred.
Conclusions
Acalabrutinib is a highly selective Btk inhibitor that provides effective and well tolerated treatment for patients with relapsed CLL, including those with del(17)(p13.1).
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia. While chemoimmunotherapy prolongs remission duration and overall survival for most CLL patients,1,2 relapse virtually always occurs. This has prompted aggressive discovery efforts for new therapies in CLL. As B-cell receptor signaling is a driving factor for CLL tumor cell survival,3,4 therapeutic targeting of proximal kinases involved in this pathway has occurred. Bruton tyrosine kinase (Btk) is immediately down-stream of the B-cell receptor and is essential for activation of several tumor cell survival pathways relevant to CLL.5 In addition, Btk is involved in chemokine-mediated homing and adhesion of CLL cells to the microenvironment, which contributes to their maintenance and proliferation.6,7 In mice and humans, loss of Btk function results in a B-cell directed phenotype with decreased serum immunoglobulin and increased predisposition to infections. Few other adverse effects have been reported.8–10 The unique structure of this protein, characterized by a cysteine (C481) within the ATP-binding pocket, makes this kinase an attractive therapeutic target. Ibrutinib is a first-in-class, irreversible small molecule inhibitor of Btk with the ability to covalently bind to C481.11 Ibrutinib showed significant monotherapy activity in relapsed and untreated patients with CLL.12–14 Progressive disease on ibrutinib is very uncommon in previously untreated CLL and also in low risk genomic patients.12–14 Among those with high-risk genomic features, progression is more frequent either shortly after the start of ibrutinib due to Richter’s transformation (large cell lymphoma) or later with progressive CLL.15 Ibrutinib also irreversibly binds to other kinases (eg, tyrosine kinase expressed in hepatocellular carcinoma [Tec], epidermal growth factor receptor [EGFR], interleukin-2-inducible T-cell kinase [Itk], and T cell X chromosome kinase [Txk]).11 These pharmacologic features may explain toxicities not typically observed in Btk-deficient patients, such as rash, diarrhea, arthralgias/myalgias, atrial fibrillation, ecchymosis, and major hemorrhage.12–14
Acalabrutinib (ACP-196) is a second-generation, highly selective irreversible inhibitor of Btk with improved pharmacologic features, including rapid oral absorption, a short half-life, and lack of irreversible targeting to alternative kinases, such as EGFR, Itk and Txk. Given the success of ibrutinib in relapsed CLL,12–14 we sought to determine if selective targeting of Btk by acalabrutinib would be clinically effective and differentiated, as measured by response and side effect profile, which represents the most common reason patients discontinue ibrutinib treatment.15,16 Furthermore, we hypothesized it might be possible to administer acalabrutinib twice daily, thus achieving complete and continuous Btk occupancy (greater than 95%), without increased toxicities from inhibition of alternative kinases. We anticipate 24-hour target coverage may reduce drug resistance caused by mutations in the Btk enzyme and may also lower the rate of Richter’s transformations.
Methods
Preclinical studies with CLL cells and normal immune cells were performed according to methods outlined in the Supplementary Appendix after written informed consent as part of an institutional review board-approved protocol at Ohio State University. The phase 1–2 multicenter study was designed to determine the ideal dose, safety, efficacy, pharmacokinetics and pharmacodynamics of acalabrutinib in patients with relapsed CLL. All patients provided written informed consent. An institutional review board approved the protocol at each site. The study was registered at the clinical trials registry of the National Institutes of Health (NCT02029443) and was conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice.
Patients
Eligibility included a diagnosis of relapsed CLL/small lymphocytic lymphoma as defined by the International Workshop on Chronic Lymphocytic Leukemia,17 requiring treatment per the International Workshop on Chronic Lymphocytic Leukemia guidelines; having received at least 1 prior therapy for CLL; adequate performance status (Eastern Cooperative Oncology Group performance status ≤ 2) and organ function including creatinine and bilirubin at least 1.5 times the upper limit of normal and alanine transaminase at least 3 times upper limit of normal; and an absence of active infection. Absolute neutrophil count of at least 750 per microliter and platelet count of at least 50,000 per microliter was required if no bone marrow involvement was present, but no restrictions for cytopenia were applied if CLL bone marrow involvement was present. Exclusion criteria included any malignancy limiting survival to less than 2 years, need for warfarin therapy (other anticoagulation was allowed), active gastrointestinal inflammation or malabsorption, and medicines associated with torsades de points, high grade AV block, or corrected QT interval of 480 ms or greater.
Evaluation and Treatment
All patients had baseline assessment for interphase cytogenetics, immunoglobulin heavy chain variable gene mutational analysis, β2-microglobulin and B symptoms. Patients were successively enrolled to cohorts administering oral acalabrutinib at dosages of 100, 175, 250 and 400 mg once daily as part of the dose-escalation portion of the study or 100 mg twice daily as part of the phase 2 study. Definition of dose-limiting toxicity included Grade 3 non-hematologic toxicity except for alopecia or nausea, vomiting, or diarrhea that resolved with an intervention; Grade 4 neutropenia lasting more than 5 days or febrile neutropenia, Grade 4 thrombocytopenia, Grade 3 thrombocytopenia with bleeding; or dosing delay due to toxicity for more than 7 consecutive days. Escalation to the next cohort was allowed if fewer than 2 dose-limiting toxicities were noted in 6 patients.
Disease Evaluation
Patients were evaluated at screening, weekly for the first month, biweekly for the second month and monthly for 4 months, and every 3 months thereafter with medical history, physical exam and laboratory studies for signs of toxicity. T cell, natural killer cell, and monocyte numbers were measured at baseline and before cycles 3, 10, and 16. Serum immunoglobulins were measured on the same schedule. Adverse events were graded according to the Common Toxicity Criteria of the National Cancer Institute, Version 4.03. Hematologic toxicities were graded per International Workshop on Chronic Lymphocytic Leukemia criteria.17 Study endpoints included overall response rate, progression-free survival, long-term tolerability, pharmacodynamics and pharmacokinetics. Response assessments, including radiologic exam, were performed at the end of cycle 2, 4, 6, 9, 12, 15, 18 and 21 for most patients. Bone marrow biopsy was done in all patients at 12 months or when all other criteria were met for complete response. Response was evaluated based on the International Workshop on Chronic Lymphocytic Leukemia criteria,17 but did not consider isolated lymphocytosis as relapse. A partial response in the setting of lymphocytosis was characterized as a partial response with lymphocytosis.
Pharmacokinetics and Pharmacodynamics
Detailed pharmacokinetic analyses with a validated assay were performed during cycle 1 of therapy. Occupancy of Btk by acalabrutinib was measured in peripheral blood mononuclear cells with the aid of a biotin-tagged analogue probe at baseline, 4 hours post-dose days 1, 8, and 28, and pre-dose days 2, 8, and 28. Phosphorylation of Btk was measured using an intracellular flow cytometry assay. Immunoblot analysis was done using methods previously described.18 The murine thrombosis model has been previously described elsewhere.19 Additional experimental details for laboratory studies are provided in the Supplementary Appendix.
Study Responsibilities
This study was designed by the first, third and last author together with the sponsor, Acerta Pharma. The clinical investigators and their research teams collected and evaluated all the data and assessed all the patients. The sponsor was responsible for analyzing the data. Investigators had open access to the data and analyses. The first author wrote the first draft of the manuscript; all authors reviewed the manuscript and approved the final version.
Statistics
Results are presented through 01 October 2015. All safety and efficacy analyses included patients who received acalabrutinib. One patient who discontinued treatment after 8 days was not evaluable for response. Descriptive statistics were used to summarize the findings. Wilcoxon Signed-Rank test was performed to assess change from baseline for immune cell counts, cytokine levels and immunoglobulin levels. Only patients with values at baseline and for each follow-up visit were included, and multiplicity was not adjusted. Progression-free survival was defined as time from first dose to documented disease progression or death and was estimated using Kaplan-Meier methodology.20 Data on progression-free survival were censored at the last clinical assessment for patients who discontinued treatment without documented disease progression. Non-compartmental pharmacokinetic analyses were done using validated WinNonlin® software (Certara USA, Princeton NJ).
Results
Pharmacology
The chemical structures of acalabrutinib and ibrutinib are shown in supplemental Figure S1. Acalabrutinib shows dose-dependent inhibition of B-cell receptor signaling in primary CLL cells (supplemental Figure S2a). In kinase inhibition assays, acalabrutinib is a more selective Btk inhibitor than ibrutinib (supplemental Table S1). These biochemical findings are physiologically relevant as acalabrutinib does not inhibit EGFR signaling (supplemental Figure S2b). Also, the lack of Itk inhibition (supplemental Figure S2c) likely explains why acalabrutinib does not inhibit antibody-mediated killing by natural killer cells of obinutuzumab-coated CLL cells (supplemental Figure S2d). These findings provide structural, biochemical and in vitro differentiation of acalabrutinib from ibrutinib. These data, combined with objective clinical responses in a study of naturally occurring canine B-cell lymphomas, provided justification for clinical development of acalabrutinib in CLL.21
Patient Demographics
This study includes 61 patients who were sequentially enrolled across 6 global sites and received at least 1 dose of acalabrutinib. The baseline characteristics are provided in Table 1. At a median follow-up of 14.3 (range 0.5 to 20) months, 87% (53 of 61 patients) are still receiving treatment. The primary reasons for discontinuation were 2 investigator/patient decisions; 1 patient with active autoimmune hemolytic anemia that required additional therapy; 1 fatal pneumonia; 1 adverse event each of diarrhea, gastritis and dyspnea; and 1 CLL progression.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | Total (N=61) |
|---|---|
| Median age (range), years | 62 (44–84) |
| ≥65 years, no. (%) | 27 (44) |
| ≥70 years, no. (%) | 15 (25) |
| Diagnosis, no. (%) | |
| Chronic lymphocytic leukemia | 61 (100) |
| Performance status, no. (%) | |
| 0 | 22 (36) |
| 1 | 36 (59) |
| 2 | 3 (5) |
| Bulky lymph node disease, no. (%) | |
| ≥5 cm in diameter | 28 (46) |
| ≥10 cm in diameter | 3 (5) |
| Rai risk classification,* no. (%) | |
| Low | 1 (2) |
| Intermediate | 19 (31) |
| High | 41 (67) |
| Median (range) no. of prior therapies | 3 (1–13) |
| Cytopenia at baseline, no. (%) | |
| Absolute neutrophil count ≤1,500 μL | 15 (25) |
| Hemoglobin ≤11.0 g/dL | 21 (34) |
| Platelets ≤100,000/μL | 32 (53) |
| Prognostic factors, no. (%) | |
| Unmutated immunoglobulin variable-region heavy-chain gene | 38/51 (75) |
| (17)(p13.1) deletion† | 18/59 (31) |
| (11)(q22.3) deletion† | 17/59 (29) |
| β2-microglobulin >3.5 mg/L | 47/58 (81) |
Rai stage is derived at time of screening for this trial.
Determined by local laboratory or medical history.
Pharmacokinetics
ACP-196 was rapidly absorbed and eliminated after oral administration (Figure 1a). Pharmacokinetic results showed exposure of ACP-196 was dose proportional with no drug accumulation. Mean half-life was approximately 1 hour across all cohorts. Additional pharmacokinetic parameters are summarized in supplemental Table S2.
Figure 1. Acalabrutinib Pharmacokinetics and Pharmacodynamics.
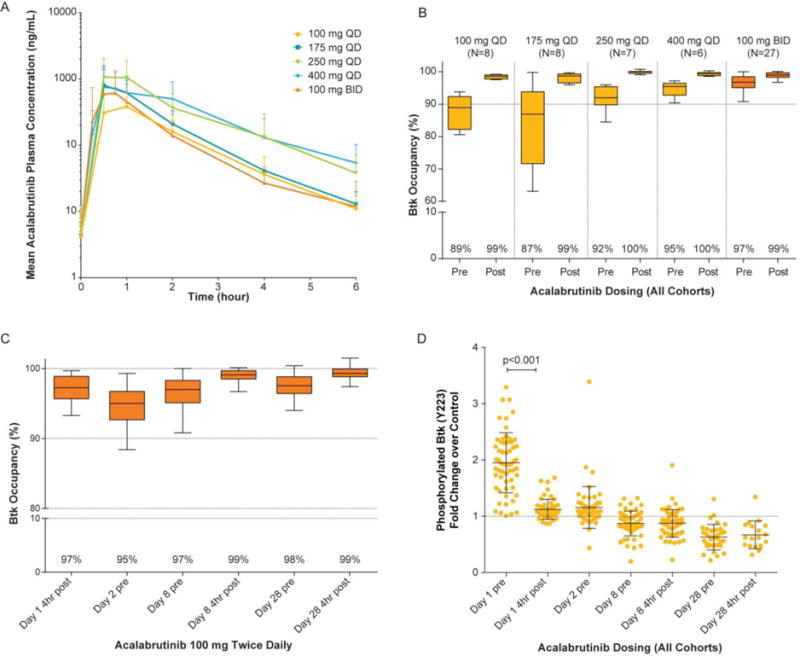
Panel A shows mean acalabrutinib plasma concentrations over time for the once daily (QD) and twice daily (BID) cohorts. Panel B shows Btk occupancy for each cohort before (pre) and 4 hours after (post) dosing on Day 8 (steady-state). For the BID cohort, Btk occupancy was evaluated for the morning dose only. Box extends from the 25th to 75th percentiles and whiskers go 1.5 times the interquartile distance per Tukey method. Panel C shows Btk occupancy over time for the 100-mg BID cohort (n=28). Panel D shows change in phosphorylated Btk levels over time for all patients.
Btk denotes Bruton tyrosine kinase.
Pharmacodynamics
Acalabrutinib binding to the C481 residue was assessed in all treatment cohorts with data summarized in Figure 1b. Notably, starting with the 100-mg once daily dose, Btk occupancy was complete 4 hours after dosing (99% to 100%) and ranged from 87% to 96% at predose with once daily dosing. Since acalabrutinib has no plasma accumulation, we explored the feasibility and safety of 100 mg twice daily dosing. Figure 1c demonstrates improved Btk occupancy of 99% four hours post-dose and 97% predose on days 8 and 28. The interruption of B-cell receptor signaling was also assessed by measuring phosphorylated Btk shown in Figure 1d. After treatment with acalabrutinib, complete loss of phosphorylated Btk was observed at the respective time points across all cohorts. Assessment of platelet aggregation derived from blood of patients receiving acalabrutinib or ibrutinib (as positive control) shows inhibition with the latter, but not the former (supplemental Figure S3). Pro-inflammatory cytokines decreased significantly from baseline to day 28 of treatment (supplemental Figure S4).
Safety
Long-term therapy with acalabrutinib has been well tolerated. The most common adverse events were headache (43%), diarrhea (39%), increased weight (26%), pyrexia (23%) and upper respiratory tract infection (23%); most events were Grade 1 or 2 and resolved over time (Table 2). Severe diarrhea, rash, arthralgia/myalgia, bruising and bleeding events were rare. No major hemorrhage or atrial fibrillation was noted. Serious adverse events are listed in supplemental Table S3. Serum IgG, IgA and IgM were measured over time and did not demonstrate significant change among patients, excluding those receiving intravenous immunoglobulin (supplemental Figure S5). T cell (CD4+, CD8+), natural killer cell and monocyte numbers similarly demonstrated no clinically significant change over time (supplemental Figure S6).
Table 2.
Adverse Events
| Adverse Event* | All Grades† | Grades 1–2 | Grades 3–4 |
|---|---|---|---|
| number of patients (percent) | |||
| Headache | 26 (43) | 26 (43) | 0 (0) |
| Diarrhea | 24 (39) | 23 (38) | 1 (2) |
| Increased weight | 16 (26) | 15 (25) | 1 (2) |
| Pyrexia | 14 (23) | 12 (20) | 2 (3) |
| Upper respiratory tract infection | 14 (23) | 14 (23) | 0 (0) |
| Fatigue | 13 (21) | 11 (18) | 2 (3) |
| Peripheral edema | 13 (21) | 13 (21) | 0 (0) |
| Hypertension | 12 (20) | 8 (13) | 4 (7) |
| Nausea | 12 (20) | 12 (20) | 0 (0) |
| Contusion | 11 (18) | 11 (18) | 0 (0) |
| Arthralgia | 10 (16) | 9 (15) | 1 (2) |
| Petechiae | 10 (16) | 10 (16) | 0 (0) |
| Decreased weight | 10 (16) | 10 (16) | 0 (0) |
Listed adverse events were reported in at least 15% of patients, on or before the data cutoff date of October 1, 2015, regardless of cause.
The all-grade column includes one patient with a Grade 5 event of pneumonia.
Clinical Response
The clinical activity of acalabrutinib was rapid and robust, as shown in Figure 2a and 2b, with 98% of patients (59 of 60) experiencing reduction in lymphadenopathy and 61% (37 of 61) experiencing concomitant treatment-related lymphocytosis (defined as at least a 50% increase from baseline and above absolute lymphocyte count of 5,000 cells/μL). Despite substantial reductions in lymphadenopathy, treatment-related lymphocytosis was not as significant or persistent as reported with ibrutinib12–14 and other B-cell receptor inhibitors22. Also, in patients with baseline cytopenia, improvements were noted in hemoglobin levels, platelet count and absolute neutrophil count were noted in the great majority of patients (supplemental Table S4). In the 16 patients with B symptoms at baseline, resolution occurred in 88% of patients by cycle 4 and 100% of patients by cycle 9 (supplemental Table S5).
Figure 2.

Response to Acalabrutinib. Panel A shows the median percent change from baseline in absolute lymphocyte count (ALC) and the sum of products of lymph node diameters (SPD) in all patients. The bars represent 95% confidence intervals of the median change from baseline. Panel B shows greatest change from baseline in lymphadenopathy for patients with baseline lymphadenopathy and at least one on treatment measurement. All measurements were based on radiologic assessments. Panel C and D provide investigator-assessed best response by cohort and over time, respectively. In Panel D responses are depicted for all available patients at each timepoint.
BID denotes twice daily; QD denotes once daily
With a median follow up of 14.3 months, the best overall response rate including partial response and partial response with lymphocytosis was 95% (partial response, 85%; partial response with lymphocytosis, 10%, and stable disease, 5%). Responses were observed across all cohorts (Figure 2c) and continued to improve over time (Figure 2d). Of the 18 patients with del(17)(p13.1), the response rate was 100% (partial response, 89%; partial response with lymphocytosis, 11%). In the 4 patients with prior idelalisib therapy, the response rate was 100% (partial response, 75%, partial response with lymphocytosis, 25%). At the time of analysis, only 1 patient with del(17p)(p13.1) progressed on therapy with a newC481S BTK (major clone) and L845F PLCG2 (minor clone) mutation as reported with ibrutinib treatment23 No cases of Richter’s transformation have been reported. A Kaplan-Meier plot of progression-free survival is shown in Figure 3. Only 2 events have been noted, thus far, a death from pneumonia (13 months) and a single progression (16 months).
Figure 3.

Kaplan-Meier Curve for Progression-free Survival
Discussion
The introduction of irreversible Btk inhibitors such as ibrutinib for treatment of CLL and other related B-cell lymphoproliferative disorders represents a major therapeutic advance.12–14 Concurrent with ibrutinib clinical development, another irreversible Btk inhibitor, CC-292, was studied in CLL. CC-292 was well tolerated, but demonstrated inferior clinical results compared with ibrutinib.24,25 At the time, it was suggested CC-292 maybe a more selective inhibitor of Btk than ibrutinib. This introduced the question do irreversible Btk inhibitors need to inhibit alternative targets, as ibrutinib does, to ensure efficacy? Herein, we demonstrate acalabrutinib has more selective and different biochemical, in vitro, in vivo and pharmacokinetic properties as compared with ibrutinib. These preclinical findings prompted initiation of this first-in-human study in relapsed patients with CLL, wherein a high response rate and durable remissions have been demonstrated. Notably, no early Richter’s transformation and only one late CLL progression have occurred even though this trial included a high-risk relapsed CLL population. Thus, these results support the conclusion that only Btk inhibition is needed to ensure marked efficacy in CLL.
Along with this clinical activity, the safety of acalabrutinib was also favorable, despite prolonged continuous administration. In ibrutinib clinical studies, side effects, such as rash, diarrhea, blurred vision, atrial fibrillation, arthralgias/myalgias, major hemorrhage (including subdural hematomas) and bruising/ecchymosis, have been reported.12–14 For some patients these toxicities have resulted in discontinuation of therapy. Our preclinical work with acalabrutinib demonstrates many of the alternative kinase targets of ibrutinib, including EGFR, Itk, Txk, are not influenced at pharmacologic concentrations. Furthermore, in patients receiving ibrutinib, we demonstrate diminished platelet aggregation, whereas with acalabrutinib we do not observe this. This finding may reflect why major bleeding events with acalabrutinib have, thus far, not occurred. In addition, no cases of atrial fibrillation have been reported to date despite 14 months of follow up at which time many such events were noted on ibrutinib.13 In contrast, transient headaches appear to be more common with acalabrutinib than historical experience with ibrutinib, occurring early in treatment and generally resolving with time. Randomized studies will be required to fully appreciate differences in adverse events between acalabrutinib and ibrutinib.
Having an alternative Btk inhibitor with more selective pharmacologic features for clinical use is attractive and offers the opportunity to improve on efficacy observed with ibrutinib. In particular, genetic studies using 2 CLL mouse models26,27 and several types of lymphoma23,28,29 suggest that a selective and potent pharmacologic inhibitor of Btk offers a potential advance for treating this disease. The short half-life and selective properties of acalabrutinib allow twice daily dosing with virtually complete and continuous Btk inhibition without increased toxicities. Twice daily dosing of ibrutinib has not been pursued. For highly proliferative CLL and other more aggressive B-cell malignancies, twice daily dosing may improve patient outcomes compared with ibrutinib. Indeed in this study, no cases of de novo Richter’s transformation have occurred as reported early in treatment with ibrutinib.15 Exploration of acalabrutinib in the treatment of Richter’s transformation is ongoing based upon this observation.
In summary, data from a highly selective Btk inhibitor, acalabrutinib, provide strong justification for further clinical investigation examining efficacy and safety compared with ibrutinib and other CLL therapies. Based on these data and to meet these goals, a comparative phase 3 study (NCT02477696) of acalabrutinib versus ibrutinib has commenced in patients with high-risk, relapsed CLL.
Supplementary Material
Acknowledgments
The authors wish to thank the investigators and coordinators at each of the clinical sites; patients who participated in this trial and their families; and members at Acerta Pharma who contributed to the results of this trial including Tasheda Navarro, Fanny Krantz, Greg Slatter, and Michael Gulrajani. Support was provided by the National Cancer Institute (P01 CA095426, R35 CA197734, and R01 CA177292), Leukemia and Lymphoma Society, Four Winds Foundation, and the D. Warren Brown Foundation.
References
- 1.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 3.Quiroga MP, Balakrishnan K, Kurtova AV, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–37. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponader S, Chen S-S, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rooij MFM, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 8.Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science (New York, N Y) 1993;261:358–61. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, Zhang M, Winkelstein J, Chen SH, Ochs HD. Unique mutations of Bruton’s tyrosine kinase in fourteen unrelated X-linked agammaglobulinemia families. Human molecular genetics. 1994;3:1899–900. doi: 10.1093/hmg/3.10.1899. [DOI] [PubMed] [Google Scholar]
- 10.Saffran DC, Parolini O, Fitch-Hilgenberg ME, et al. Brief report: a point mutation in the SH2 domain of Bruton’s tyrosine kinase in atypical X-linked agammaglobulinemia. The New England journal of medicine. 1994;330:1488–91. doi: 10.1056/NEJM199405263302104. [DOI] [PubMed] [Google Scholar]
- 11.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:1278–9. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 13.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. The lancet oncology. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1:80–7. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–7. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JTK, Zhou H, et al. Modifying murine von Willebrand factor A1 domain for in vivo assessment of human platelet therapies. Nat Biotechnol. 2008;26:114–9. doi: 10.1038/nbt1373. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, M P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1957;53:457–81. [Google Scholar]
- 21.Gardner HL, H B, Izumi R, et al. ACP-196: A second generation Btk inhibitor demonstrates biologic activity in a canine model of B-cell non-Hodgkin lymphoma. AACR 2014 Annual Meeting. 2014 Abstract 1744. [Google Scholar]
- 22.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woyach JA, Furman RR, Liu TM, et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans EK, Tester R, Aslanian S, et al. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. The Journal of pharmacology and experimental therapeutics. 2013;346:219–28. doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- 25.Brown J, Harb WA, Hill BT, Gabrilove G, Sharman JP, Schreeder MT, Barr P, Foran JM, Miller TP, Kelly KR, Mahadevan DR, Ma S, Barnett E, Marine J, Nava-Parada1 P, Azaryan A, Mei J, Kipps TJ. Phase 1 Study Of Single Agent CC-292, a Highly Selective Bruton’s Tyrosine Kinase (BTK) Inhibitor. Relapsed/Refractory Chronic Lymphocytic Leukemia Blood (sup) 2013 doi: 10.3324/haematol.2015.140806. Abstr 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kil LP, de Bruijn MJ, van Hulst JA, Langerak AW, Yuvaraj S, Hendriks RW. Bruton’s tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. American journal of blood research. 2013;3:71–83. [PMC free article] [PubMed] [Google Scholar]
- 27.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL) Blood. 2014;123:1207–13. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nature medicine. 2015;21:922–6. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. The New England journal of medicine. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


