Abstract
Objective
The cytokine/chemokine network is the language used by the innate and adaptive immune system to orchestrate effective immune responses. Here, we describe the cross-sectional association between cytokine levels and stage of HIV infection to gain novel insights into HIV-1 immunopathogenesis and identify novel therapeutic targets.
Design
Concentrations of 31 cytokine/chemokines were retrospectively measured in blood and semen collected from 252 individuals enrolled in 4 well-characterized cohorts: HIV-uninfected, HIV-infected in early phase of infection, HIV-infected in late phase of infection, and HIV-infected on ART.
Methods
Cytokine/chemokine levels were measured by multiplex-bead-array. Comparisons between groups were performed by Mann-Whitney U-test and p-values were adjusted for multiple comparisons using the Benjamini-Hochberg method.
Results
HIV-infection skewed the cytokine/chemokine network towards a pro-inflammatory response in both blood and semen. Such changes emerged within the first weeks of infection and were maintained thereafter: among untreated HIV-infected individuals, none of the 31 measured cytokines were significantly different between early and later stages of infection. Suppression of plasma HIV-1-RNA with ART did not result in normalization of the levels of pro-inflammatory cytokines in blood. In contrast, in semen, several pro-inflammatory cytokines were even further up regulated in ART-treated compared to HIV-uninfected and HIV-untreated individuals.
Conclusions
The profound disruption in the cytokine network is evident in blood and semen from the earliest stage of HIV-infection shortly after the first detection of systemic viremia. These changes are maintained throughout the chronic phase of the infection and do not normalize despite ART and suppression of plasma HIV-1-RNA.
Keywords: cytokine, chemokine, HIV-1, ART, semen, blood
INTRODUCTION
Persistent inflammation during HIV infection has been associated with accelerated disease progression, end-organ diseases and increased mortality[1–3] and is reflected by increased levels of cytokines/chemokines in blood plasma[4–6]. Similarly, the cytokine/chemokine network in genital secretion reflects the functional status of immune cells in the genital tract, where the key events related to genital HIV shedding and sexual transmission occur[7–10]. In comparison with other body fluids, semen is enriched with several cytokines/chemokines (in particular interleukin (IL)-7 and transforming growth factor (TGF)-β), reflecting the unique immunological features of the male genital tract of immunocompetent individuals[7–9, 11, 12]. In HIV-infected individuals, several cytokines/chemokines are upregulated in genital secretions[8, 9, 13, 14], which in turn, can favor HIV-1 transmission by promoting local HIV-1 replication and dissemination[15].
HIV-1 infection does not only affect the absolute levels of individual cytokine/chemokine, but also reorganizes the cytokine/chemokine networks, establishing new strong correlations between various cytokines and thus imposing a high level of rigidity to the cytokine/chemokine network. This phenomenon, which has been described both in blood and semen is likely to reflect a reduced ability of the immune system to respond to microbial challenges and might affect the transmission of different sexually transmitted infections (STIs), including HIV-1 itself[16].
Although changes in levels of individual cytokine/chemokine during HIV-1 infection have been previously described[8, 9, 12], we sought to assess for the first time the cytokine network in the blood and semen for men with different stages of HIV-1 infection and the effect that antiretroviral therapy (ART) might have on these cytokine levels.
To address this question, we characterized the cytokine/chemokine network in 4 groups of men: (i)HIV-uninfected individuals; (ii)untreated early HIV-infected individuals (sampled within 90 days from estimated date of infection [EDI]), (iii)untreated late HIV-infected individuals (sampled more than 90 days after EDI) and (iv)ART treated HIV-infected individuals with undetectable HIV RNA levels in blood (<50 copies/ml).
METHODS
Study Population
A total of 252 paired semen and blood samples were included in this study from 40 HIV uninfected men (Group 1), and 116 HIV-infected ART-naive participants from the San Diego Primary Infection Cohort (SD-PIC). In the SD-PIC cohort, the estimated date of infection (EDI) was characterized using well-defined stepwise rules on the basis of serologic and virologic criteria[17] (Supplemental Table 1, http://links.lww.com/QAD/A830). Based on such criteria, we identified 42 untreated early HIV-infected individuals (sampled <90 days from EDI; Fiebig stages 1–5, Group 2) and 74 untreated late HIV-infected individuals (sampled >90 days from EDI; Fiebig stage 6, Group 3). Additionally, we included 96 ART-treated chronically HIV-infected subjects from the California Collaborative Treatment Group (CCTG)-592 Study with completely suppressed HIV RNA in blood plasma (<50 copies/ml) (Group 4). Paired blood and semen samples were collected at the time of study enrollment. Potential infection from Neisseria gonorrhoeae and Chlamydia trachomatis were tested from urine, using transcription mediated amplification (Aptima, Gen-Probe, San Diego, California). The studies were conducted with appropriate written subject consent and were approved by the Human Research Protections Program at the University of California San Diego, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, and the University of Southern California.
HIV-1 RNA and cytokines/chemokines quantification
HIV-1 RNA levels were measured in seminal plasma as previously described[18]. Levels of 31 cytokines/chemokines were measured in blood and seminal plasma using a multiplex bead-array-assay as previously described[8] (See supplementary data, http://links.lww.com/QAD/A830). The NIH laboratory that performed luminex measurements is part of the Microbicide Quality Assurance Program (MQAP)[19].
Statistical Analyses
Statistical analysis was performed in SAS v9.2. Cytokine levels were reported as continuous variables by median and interquartile ranges. Comparisons between all groups for difference of medians were done by the Kruskal-Wallis test. Comparisons between pairwise groups were done using the Mann Whitney U test. P values for pairwise comparisons were adjusted for multiple comparisons using the Benjamini-Hochberg method (statistical significance with raw p<=0.0002 as a cutoff).
RESULTS
Study Participants’ Demographics and Clinical Data
Detailed characteristics of each cohort are described elsewhere[18, 20, 21] and summarized in Table 1. All HIV-infected men and 67% of the HIV-uninfected men had sex with other men as their main HIV risk factor. Since we did not find any significant differences in general demographics and cytokine levels between MSM and heterosexual men in the HIV-uninfected group, we combined them into one group (Group 1) for the purpose of this analysis.
Table 1.
Participants’ characteristics
| HIV− | Early HIV+ART− | Late HIV+ART− | Chronic HIV+ART− | |
|---|---|---|---|---|
| N | 40 | 42 | 74 | 96 |
| Age (years); median (IQR) | 34 (30–43) | 33 (27–41) | 33 (27–39) | 46 (38–50) |
| Men who have sex with men; N (%) | 27 (67) | 42 (100) | 74 (100) | 96 (100) |
| Estimated Duration of Infection (days); | N/A | 75 (33–82) | 147 (104–194) | N/A |
| Time on ART (years); median (IQR) | N/A | N/A | N/A | 2.4 (1.1–4.7) |
| log10 HIV RNA in blood; median (IQR) | N/A | 5.16 (4.83–5.58) | 4.44 (3.84–4.95) | < 50 copies/ml |
| Detectable HIV RNA in semen; N (%) | N/A | 31 (73.8) | 52 (70.3) | 6 (6.3) |
| log10 HIV RNA in semen; median (IQR) | N/A | 2.35 (0.54–3.40) | 2.13 (1.04–2.99) | 2.19 (2.00–2.30) |
| CD4+ T-cell counts/μl; median (IQR) | N/A | 520 (435–614) | 529 (412–744) | 577 (385–745) |
Five of our participants were positive by nucleic acid testing at the time of sample collection. This includes one case of Chlamydia in the HIV-negative cohort and in the ART-treated group, and 3 cases of Chlamydia/Gonorrhea in the HIV-infected untreated group (SD-PIC).
Cytokines/chemokine network in blood plasma of the studied groups
First, we compared median levels of each cytokine/chemokine in blood plasma between the four groups described above. We found that five of the 31 measured cytokines (MCP-1, MIP-1α, MIG, IP-10, and I-TAC) were significantly up regulated in blood plasma of Group 2 compared to Group 1 (Figure 1A; Supplemental Table 2, http://links.lww.com/QAD/A830). These cytokines were all mediators of innate immunity, inflammation and chemotaxis. The extent of the upregulation ranged between 2.4 and 23.4 times with I-TAC, MIP-1α, and IP-10 being the most up regulated (respectively 9.3, 22.3, and 23.4 times). In Group 3, IL-18, in addition to the 5 previously mentioned cytokines, was up regulated in blood plasma compared to Group 1 (Figure 1B; Supplemental Table 2, http://links.lww.com/QAD/A830). The extent of the upregulation of these 6 cytokines ranged between 2.3 and 16.9 with MIP-1α, IL-18, I-TAC, and IP-10 being the most up regulated (respectively 4.6, 4.6, 8.5, and 16.9 times). None of the 31 measured cytokines was significantly different in blood plasma between Group 2 and Group 3 (Supplemental Table 2, http://links.lww.com/QAD/A830).
Figure 1.
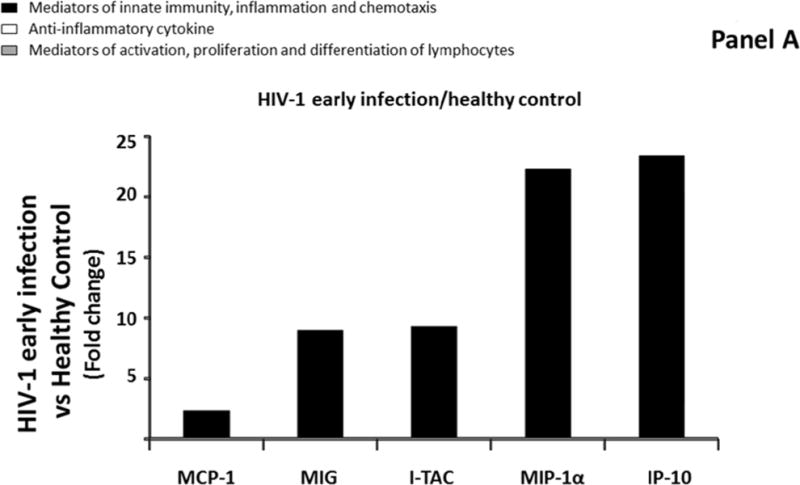
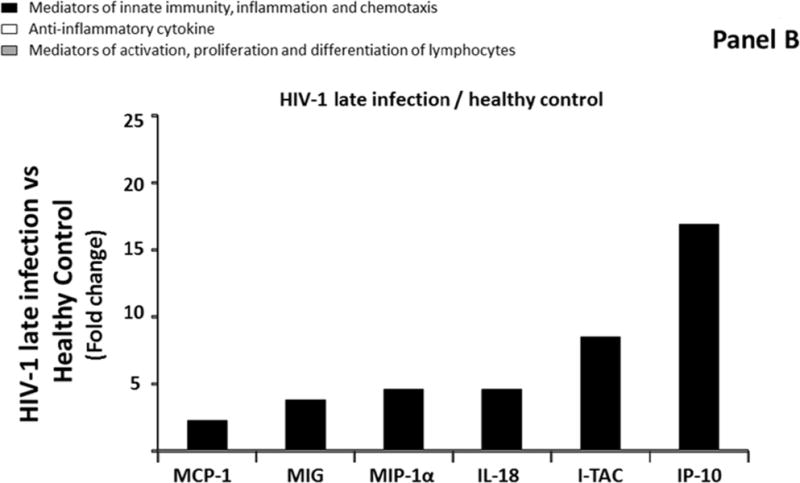
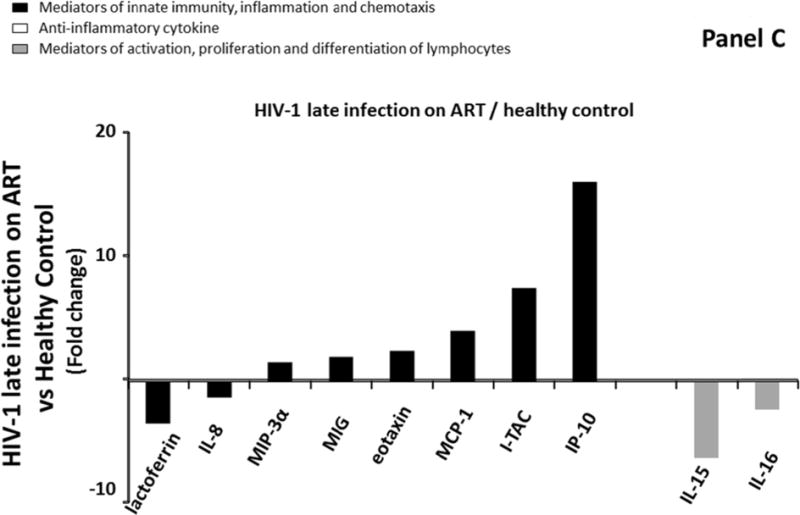
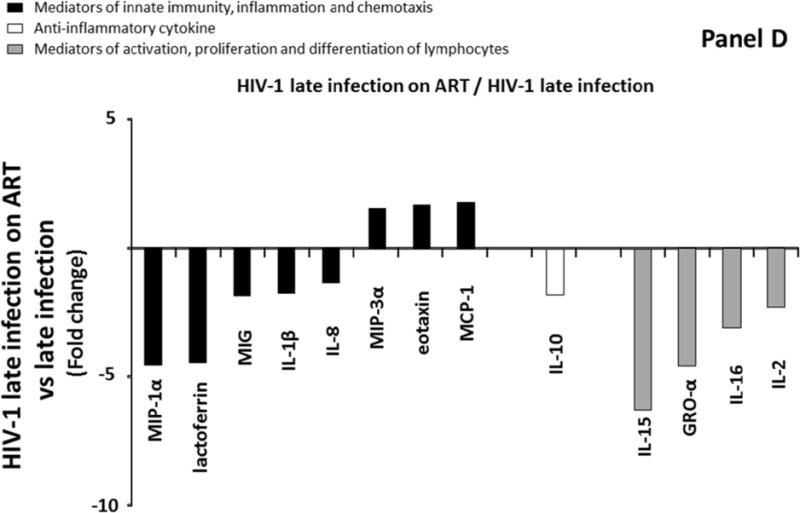
Comparison of cytokine/chemokine median levels in blood plasma from HIV-negative controls (group 1), early HIV-infected (< 90 days from estimated date of infection) antiretroviral therapy (ART)-naïve individuals (group 2), late HIV-infected ART-naïve individuals (group 3), and chronically HIV-infected individuals on ART with suppressed HIV RNA < 50 copies/ml (group 4). Represented are the cytokine/chemokine fold increase or decrease (ratio of medians) statistically significant between early HIV-1-infected (ART)-naïve individuals and HIV-1 uninfected controls (A), late HIV-infected ART-naïve individuals and HIV-1 uninfected controls (B), chronically HIV-infected individuals on ART and HIV-1 uninfected controls (C), and chronically HIV-infected individuals on ART or not (D). Comparisons between pairwise groups were done using the Mann Whitney U test. P values for pairwise comparisons were adjusted for multiple comparisons using the Benjamini-Hochberg method (resulting in a raw p<=0.0002 as a cutoff for statistical significance).
Despite complete suppression of HIV-1 viral replication (<50 copies/ml), ART initiation did not normalize the cytokine network in blood plasma but rather expanded the panel of cytokines that were modulated compared to HIV-1 uninfected individuals: the levels of ten cytokines (IL-8, IL-15, IL-16, MCP-1, eotaxin, MIP-3α, MIG, IP-10, ITAC, and lactoferrin) were different in blood plasma of Group 4 compared to Group 1. Among these ten cytokines, 2 mediators of activation, proliferation and differentiation of lymphocytes (IL-15 and IL-16) were downregulated 6.3 and 2.4 times, respectively compared to uninfected controls. The remaining eight cytokines were mediators of innate immunity, inflammation and chemotaxis and six of them were up regulated, ranging from 1.3 to 16.2 times with MCP-1, I-TAC and IP-10 being the most up regulated (respectively 4.1, 7.6, and 16.2 times) (Figure 1C; Supplemental Table 2, http://links.lww.com/QAD/A830).
To further evaluate the effect of ART initiation on the cytokine network, we also compared cytokines levels between group 3 and group 4, and we observed that 13 cytokines belonging to three distinct groups of cytokines were significantly different between these two groups (Figure 1D). A total of 10 out of these 13 cytokines were down regulated in ART-treated (Group 4) compared to ART-naïve subjects (Group 3) (MIP-1α, lactoferrin, MIG, IL-1β, IL-8, IL-10, IL-15, GRO-α, IL-16, and IL-2) (Figure 1D; Supplemental Table 2, http://links.lww.com/QAD/A830). The extent of downregulations ranged between 1.4 and 6.3 times, with GRO-α, MIP-1α, and IL-15 being the most downregulated cytokines (respectively 4.6, 4.6, 6.3 fold). On the other hand, three out of these thirteen cytokines (MIP-3α, MCP-1, and eotaxin) were significantly upregulated in participants with ART-mediated suppression of plasma HIV-RNA (Group 4).
Cytokines/chemokine network in seminal plasma of the studied groups
Similarly to blood, we compared median levels of each cytokine/chemokine in seminal plasma in the four groups described above. Only four of the 31 measured cytokines were significantly modulated (IL-1β, IL-1α, TGF-β, IL-15,) in seminal plasma of Group 2 compared to Group 1 (downregulation ranging from 2.2 to 3.4 times) (Figure 2A; Supplemental Table 3, http://links.lww.com/QAD/A830). In Group 3, IL-15 was downregulated 2.1 times compared to Group 1, while IL-12 and IFN-γ were significantly upregulated by 9, and 33.3 times respectively (Figure 2B; Supplemental Table 3, http://links.lww.com/QAD/A830). Similarly to blood, none of the 31 measured cytokines were significantly different between Group 2 and Group 3 (Supplemental Table 3, http://links.lww.com/QAD/A830).
Figure 2.
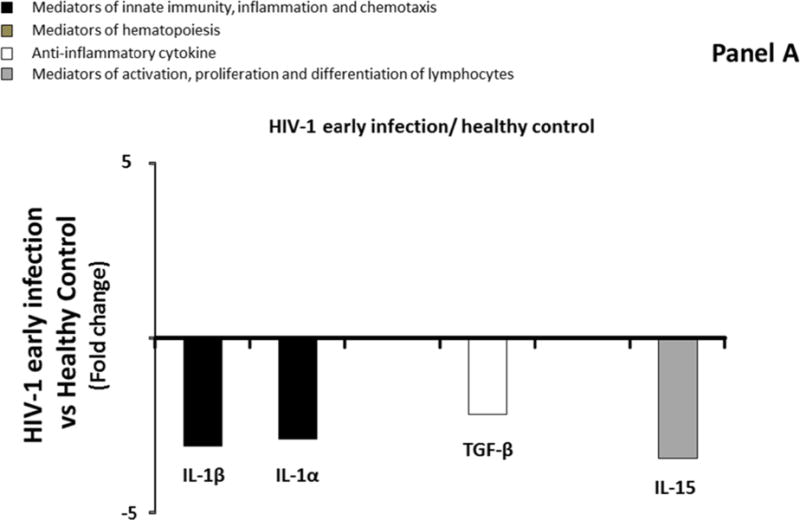
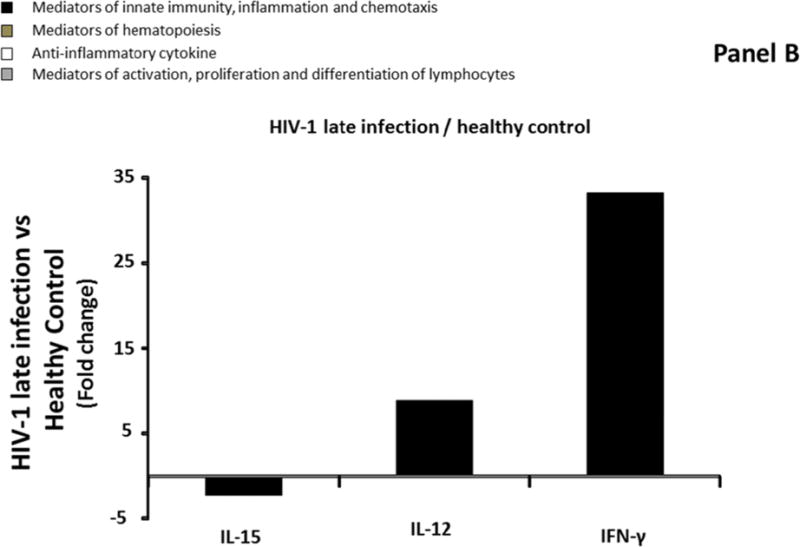
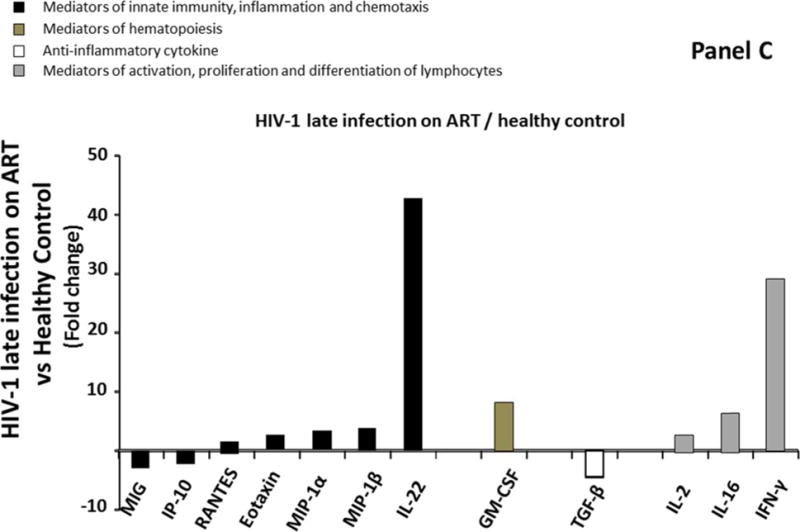
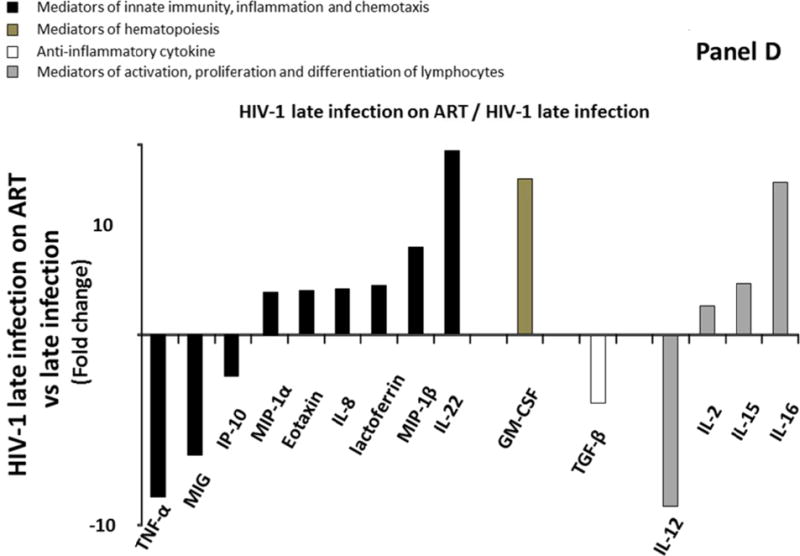
Comparison of cytokine/chemokine median levels in seminal plasma from HIV-negative controls (group 1), early HIV-infected (< 90 days from estimated date of infection) antiretroviral therapy (ART)-naïve individuals (group 2), late HIV-infected ART-naïve individuals (group 3), and chronically HIV-infected individuals on ART with suppressed HIV RNA < 50 copies/ml (group 4). Represented are the cytokine/chemokine fold increase or decrease (ratio of medians) statistically significant between early HIV-1-infected (ART)-naïve individuals and HIV-1 uninfected controls (A), late HIV-infected ART-naïve individuals and HIV-1 uninfected controls (B), chronically HIV-infected individuals on ART and HIV-1 uninfected controls (C), and chronically HIV-infected individuals on ART or not (D). Comparisons between pairwise groups were done using the Mann Whitney U test. P values for pairwise comparisons were adjusted for multiple comparisons using the Benjamini-Hochberg method (resulting in a raw p<=0.0002 as a cutoff for statistical significance).
Similar to what we observed in blood plasma, ART did not result in normalization of the cytokine network in seminal plasma. In fact, 12 cytokines out of four different classes were different in seminal plasma of ART-suppressed HIV-1 infected individuals (Group 4) compared to uninfected controls (Group 1) (MIG, IP-10, RANTES, eotaxin, MIP-1α, MIP-1β, IL-22, GM-CSF, TGF-β, IL-2, IL-16, and IFN-γ) (Figure 2C; Supplemental Table 3, http://links.lww.com/QAD/A830). Among these 12 cytokines, three were downregulated. The extent of downregulation ranged from 1.1 to 4.5 times with MIG and TGF-β being the most downregulated (respectively 3, and 4.5 times). The upregulation of the remaining 9 cytokines ranged from 1 to 42.7 times with GM-CSF, IFN-γ, and IL-22 being the most upregulated (respectively 8.2, 29.1, and 42.7 times) (Figure 2C; Supplemental Table 3, http://links.lww.com/QAD/A830).
Comparing Group 3 and Group 4, we observed a significant impact of treatment on the cytokine network in seminal plasma as 15 cytokines (TNFα, MIG, IP-10, MIP-1α, eotaxin, IL-8, lactoferrin, MIP-1β, IL-22, GM-CSF, TGF-β, IL-12, IL-2, IL-15, and IL-16) were significantly modulated in seminal plasma. Specifically, six of these 15 cytokines were significantly downregulated (TNF-α, MIG, IP-10, TGF-β, and IL-12) (Figure 2D; Supplemental Table 3, http://links.lww.com/QAD/A830). The extent of the downregulations ranged between 2.2 and 9 times, with MIG, TNF-α, and IL-12 being the most downregulated cytokines (respectively 6.3, 8.5, and 9 times). On the other hand, the remaining 10 cytokines were upregulated in Group 4 compared to Group 3 (MIP-1α, eotaxin, IL-8, lactoferrin, MIP-1β, IL-22, GM-CSF, IL-2, IL-15, and IL-16). The extent of the upregulations ranged between 1.5, and 9.7 times, with IL-16, GM-CSF, and IL-22 being the most upregulated (respectively 8, 8.2, and 9.7 times).
Compartmentalization of the cytokines/chemokines network in blood and semen
Previous studies suggest that cytokine/chemokine profiles are different in blood and semen of healthy individuals and that these differences are greater in HIV-1 individuals[8, 9, 12]. Figure 3 displays the compartmentalization of each cytokine, by evaluating the ratio between levels of cytokines measured in seminal plasma versus blood plasma. Ratios for IL-17, IL-33, GM-CSF and IL-13 in which median levels remained under the limit of detection either in blood or seminal plasma were not reported.
Figure 3.
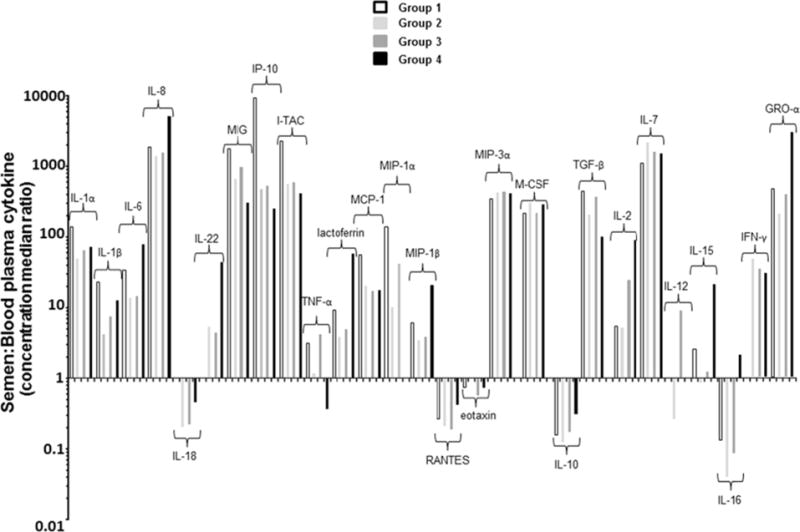
Cytokine spectra are different in blood and seminal plasma. Median of cytokine concentration ratios in seminal plasma and blood plasma (S:B) are shown for HIV-uninfected controls (group 1, white bars), HIV-infected individuals in early (group 2, light grey bars) or in a later stage (group 3, dark grey bars) of HIV infection and HIV-infected individuals under antiretroviral treatment (ART) (group 4, black bars). Ratios >1or <1 indicate enrichment of a cytokine in semen or blood plasma, respectively. Ratios for some of the cytokines in which concentration remained under the limit of detection either in blood or seminal plasma were not reported in Figure 11 (IL-17, IL-33, GM-CSF and IL-13).
We found that levels of all cytokines but five (i.e. IL-18, RANTES, eotaxin, IL-10, and IL-16) were significantly higher in semen than in matching blood samples in uninfected individuals. Cytokines or chemokines, such as IL-1α, IL-8, MIG, IP-10, I-TAC, MIP-3α, M-CSF, TGF-β, IL-7, and GRO-α were up to 100-fold more concentrated in semen than in blood. These cytokines are distributed across all four categories of cytokines, suggesting that higher concentration of cytokines in semen compared to blood is a general phenomenon and not linked to a specific subset of cytokines.
Generally, whether each given cytokine was more concentrated in blood or in semen, the occurrence of HIV-1 infection didn’t significantly affect its compartmentalization status between the blood and semen. However, for a subset of 4 cytokines (IL-18, IL-22, IL-12, and IFN-γ) that were equally present in blood and semen of HIV uninfected men (ratio=1), we observed a shift towards compartmentalization in case of untreated HIV-infection, with a relative increase of IL-18 in blood plasma (ratio=0.2) and a relative increase of IL-22 and IFN- γ in seminal levels (ratio>1). For IL-12, we observed enrichment in blood during the earliest phase of HIV infection (Group 2) but a relative increase in seminal plasma in subjects with late HIV-1 infection (Group 3).
With the exception of IL-12 and TNF-α, we didn’t observe any significant difference in the seminal to blood ratios between Group 2 and Group 3. On the other hand, the group of HIV-infected subjects on suppressive ART showed a relative increase in seminal levels for several cytokines compared to untreated HIV-infected and HIV-uninfected controls, including IL-6, IL-22, lactoferrin, MIP-1β, IL-2, IL-15 and GRO-α (Figure 3).
To evaluate the effect of bacterial STIs, we repeated this analysis after excluding five participants who tested positive for Chlamydia or Gonorrhea. There was no significant changes in the cytokine’s levels in blood and semen for the overall comparisons.
DISCUSSION
Cytokines and chemokines coordinate the host inflammatory response to infectious agents by modulating complex cell functions (i.e. cellular ‘activation’, migration, phagocytosis and antigen presentation). In this study, we evaluated the expression profile of selected mediators of immunity in blood and semen, providing new insights into how the cytokine/chemokine network is organized in HIV-uninfected individuals and how this network is altered during the course of HIV-1 infection and after ART initiation.
In agreement with previous smaller studies, we found that semen and blood are two distinct immunological compartments[8, 9, 12]. Indeed, in healthy control individuals, the cytokine profile was very different in seminal and blood plasma, with semen being enriched in several pro inflammatory cytokines and chemokines (mainly IL-1α, IL-8, MIG, IP-10, I-TAC, GRO-α), mediators of hematopoiesis such as M-CSF, anti–inflammatory cytokines such as TGF-β and mediators of activation, proliferation, and differentiation of adaptive lymphocytes such as IL-2 and IL-7. Semen-to-blood cytokine ratios observed in our study were generally comparable to those previously reported[8, 9, 12]. This enrichment of cytokines/chemokines in semen establishes a favorable immunologic milieu for fertilization in the female genital tract (i.e. TGF-β)[11, 22] and is likely involved in the maturation and proliferation of leukocytes and in their recruitment to the sites of inflammation[7, 9]. In particular, irrespective from HIV-1 infection, the levels of all three CXCR3 ligands (MIG, IP-10, and I-TAC) as well as the concentrations of IL-7 in semen are 1000 to 10,000 higher than the concentrations in blood plasma. Since IL-7 is a central regulator of naive and memory T cells homeostasis[23] and CXCR3 regulates chemotaxis and effector functions of Th1-type CD4+ T-cells and effector CD8+ T-cells[24, 25], our findings further indicate that the male genital tract is populated with highly specialized resident T-cells maintaining a constitutive state of immune activation in a mucosal compartment that is naturally exposed to sexually transmitted microbes, including HIV-1.
In HIV-1 infected individuals, the concentrations of most cytokines remained statistically different between semen and blood[8, 9]. However, we delineate for the first time specific patterns of modulation of cytokines/chemokines in blood and semen at the different stage of HIV-1 infection and upon initiation of ART. The concentration of 5 of the 31 measured cytokines was up-regulated in the blood of HIV-1 infected individuals in the earliest phase of HIV-infection (<90 days from infection) compared to healthy uninfected individuals. These cytokines were all mediators of innate immunity, inflammation or chemokines. Specifically, acute HIV-1 infection induced upregulation of all three CXCR3 ligands (MIG, IP-10 and ITAC), a CCR5 ligand (MIP-1α) and the CCR2 ligand (MCP-1). As mentioned earlier, CXCR3 is crucial for the trafficking of Th1 CD4 and effector CD8 T-cells to peripheral sites of inflammation and the development of Th1 amplification loop mediated by IFNγ and the IFNγ-inducible CXCR3 ligands (MIG, IP-10 and ITAC). Similarly, CCR5 and CCR2 are involved in chemotaxis of monocytes and T-cells. Therefore, although blood plasma levels may only partially reflect changes in peripheral sites, our data may reflect the intense mobilization of the innate and adaptive immune response in an attempt to control acute HIV-1 infection.
In HIV-1-infected individuals in a later stage of the disease, the same five cytokines and additionally IL-18, another potent pro-inflammatory cytokine involved in bridging innate with adaptive immunity[26], were up regulated in blood. Such observations suggest that the impact of HIV-1 infection on the immunological landscape (reflected by the cytokines in blood plasma) appears quantitatively and qualitative similar independently from the estimated time of infection during active viral replication.
In semen, HIV-1 infection was associated with lower levels of IL-1α, IL-1β, TGF-β and IL-15 in the early phase of infection. Such changes were modest and the most prominent were represented by downregulation of cytokines of the interleukin-1 family (IL-1α, IL-1β, downregulated 2–3 times). Therefore, acute HIV-1 infection does not appear to dramatically perturb the immunological homeostasis in the male genital tract. In the later phase of HIV-1 infection (> 90 days), higher levels of a few pro-inflammatory cytokines were observed in seminal plasma compared to uninfected controls. Some pro-inflammatory markers, such as IL-7, IL-22, MIP-1α, RANTES, eotaxin, MIP-3α, MIG, I-TAC and TNF-α were also up regulated in late phase of HIV-1 infection compared to controls, although they did not reach statistical differences. A significant change was related to the IL-12/IFN-γ axis with an upregulation of IFN-γ and IL-12 up to 30 and 9 fold, respectively. IL-12 produced by antigen presenting cells induces T-cell differentiation and proliferation and IFN-γ secretion from activated T-cells. Such changes may represent an attempt of the immune system to restore homeostasis in the genital compartment that could be greatly impacted by HIV-1 infection of immune cells of the male genital tract.
Our analysis indicates that an altered cytokine network is evident in blood and in semen from the earliest phase of HIV infection[27]. In blood, an intense pro-inflammatory reaction, reflecting the activation of both innate and adaptive immune systems was developed shortly after HIV-1 infection and further amplified thereafter. In semen, significant changes were apparent only at later time and were qualitatively very different from the blood plasma and initially limited only to a strong activation of the IL-12/IFN-γ axis.
Furthermore, in blood plasma, despite complete suppression of HIV-1 replication, we found that ART treatment did not normalize cytokines to levels comparable to that of uninfected controls. The reports on the effect of ART on blood plasma cytokine level in the literature are somehow conflicting: Haissman et al. [28] showed that 4 months after ART initiation, TNF-α, IL-1RA and IL-6 concentrations were significantly reduced and similar to those found in the uninfected group. However, other studies reported that chemokines and pro-inflammatory cytokines failed to return to the levels observed in uninfected individuals upon ART initiation[9, 29, 30]. In our study, we found that not only pro-inflammatory blood cytokines did not return to levels observed in uninfected controls, but rather were further modulated, suggesting further mobilization of the innate immune system (IL-8, eotaxin, MIP-3α and lactoferrin) or impairment of lymphocytes homeostasis (IL-15 and IL-16).
In semen, suppressive ART had even more profound effects: in contrast to the modest changes observed in levels of few cytokines in naïve early or late HIV-1 infected individuals compared to the uninfected group, upon suppression of viral replication in blood by ART, 12 cytokines were largely different compared to uninfected controls. These changes included mostly upregulation of cytokines of all functional groups including a striking upregulation of IL-22 (~ 40 fold increase). IL-22 is a cytokine produced by numerous lymphocytes, such as Th17, Th22, and mucosal associated invariant T-cells with regenerative properties for mucosal epithelium. Th17 and Th22 depletion in the gut mucosa is an important feature of HIV mucosal immunopathogenesis that is reversed after ART[31, 32]. We speculate here that such dramatic upregulation in the semen of ART treated individuals may reflect a repair of mucosal damage and repopulation of IL-22-producing cells in the male genital tract. Overall, despite complete suppression of plasma viremia with ART, we observed significant perturbations in the levels of cytokines associated with the function of the immune cells in the genital tract with possible consequence in the viral and transmission dynamics of HIV-1 as well as other local pathogens.
There are a number of limitations to the current study. Our analysis compared cross-sectional time points from different cohorts while longitudinal data collected from the same individuals during different stages of HIV infection would be preferred to study the complex association between clinical variables and biomarkers. Comparisons between group 2 (early) and group 3 (late) were limited by a relatively small difference in the EDI. Also, treated individuals started ART during the chronic phase of HIV-infection. Future studies should evaluate the impact of early ART initiation on the cytokine network. Lastly, the sample size limited power in discerning a significant effect in smaller groups of untreated and ART-naive acutely infected individuals. For example, five participants were positive for Chlamydia or Gonorrhea. This limited number of cases did not allow us to perform comparisons between participants with and without bacterial STI. However, there was no significant changes in the cytokine’s levels in blood and semen for the overall comparisons when eliminating these 5 participants.
In summary, our study highlights the role of HIV-1 infection and ART treatment in the modulation of cytokine/chemokine networks in blood and in semen. We found that profound changes in the cytokine profile and therefore in the coordination of complex immune functions are established very early upon HIV-1 infection. These changes are maintained throughout the chronic phase of the infection and are not reverted despite ART and full suppression of HIV-1 plasma replication. These changes are likely to be reflected in an altered ability of the innate and adaptive immune response of the male genital tract to maintain immunological homeostasis and prevent the acquisition and transmission of HIV-1 and other STIs. More research is needed to understand the interplays between pathogens and immune response, which could provide important information in the development of effective prevention strategies and complementary therapies to restore the failing immune systems during HIV infection.
Supplementary Material
Acknowledgments
We are grateful to all the study participants and all the nurses at all the enrollment sites. We are also thankful to Jean-Charles Grivel and Wendy Fitzgerald for their help in setting up the cytokine assay.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH; by the Department of Veterans Affairs; by the James Pendleton Charitable Trust; by amfAR grant 108537 with support from FAIR; by U.S.NIH awards P30-AI027763 (CNIHR), R24AI106039, AI69432, AI043638, MH62512, MH083552, MH101012, AI100665, AI077304, AI36214, AI047745, AI74621, GM093939, AI080353, AI306214 (CFAR), AI27670 (ACTU), AI43638, and 7-UM1 AI068636-07; by CTRI grant: UL1TR000100; by California HIV/AIDS Research Program RN07-SD-702, MC08-SD-700 and EI-11-SD-005; by the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124; and by National Institute of General Medical Sciences grant GM093939. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest statement:
CV, AI, SRM, LM, SJL, AL, LM, and SG do not have any commercial or other associations that might pose a conflict of interest. DMS has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe and Testing Talent Services. MPD has received grant support from Merck, Gilead, Serono, and ViiV and has served as a consultant to Serono. ESD has received grant support from Bristol Myers Squibb, Gilead, and ViiV, and has acted as a consultant for Abbvie, Bristol Myers Squibb, Gilead, Merck, Teva, and ViiV.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Ipp H, Zemlin A. The paradox of the immune response in HIV infection: when inflammation becomes harmful. Clin Chim Acta. 2013;416:96–99. doi: 10.1016/j.cca.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Alfano M, Crotti A, Vicenzi E, Poli G. New players in cytokine control of HIV infection. Curr HIV/AIDS Rep. 2008;5:27–32. doi: 10.1007/s11904-008-0005-5. [DOI] [PubMed] [Google Scholar]
- 5.Reuter MA, Pombo C, Betts MR. Cytokine production and dysregulation in HIV pathogenesis: lessons for development of therapeutics and vaccines. Cytokine Growth Factor Rev. 2012;23:181–191. doi: 10.1016/j.cytogfr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev. 2012;23:207–214. doi: 10.1016/j.cytogfr.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 8.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier AJ, Masson L, Ronacher K, Walzl G, Coetzee D, Lewis DA, et al. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis. 2014;209:1174–1184. doi: 10.1093/infdis/jit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman JC, Anton PA, Baldwin GC, Elliott J, Anisman-Posner D, Tanner K, et al. Seminal Plasma HIV-1 RNA Concentration Is Strongly Associated with Altered Levels of Seminal Plasma Interferon-gamma, Interleukin-17, and Interleukin-5. AIDS Res Hum Retroviruses. 2014;30:1082–1088. doi: 10.1089/aid.2013.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, et al. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS Pathog. 2010;6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth PM, Danesh A, Shahabi K, Rebbapragada A, Kovacs C, Dimayuga R, et al. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol. 2005;175:4789–4796. doi: 10.4049/jimmunol.175.7.4789. [DOI] [PubMed] [Google Scholar]
- 14.Berlier W, Bourlet T, Levy R, Lucht F, Pozzetto B, Delezay O. Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol. 2006;36:204–207. doi: 10.1016/j.jcv.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013;9:e1003148. doi: 10.1371/journal.ppat.1003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisco A, Introini A, Munawwar A, Vanpouille C, Grivel JC, Blank P, et al. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol. 2012;68:515–521. doi: 10.1111/aji.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianella S, Strain MC, Rought SE, Vargas MV, Little SJ, Richman DD, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol. 2012;86:1307–1315. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80:4741–4751. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianella S, Smith DM, Vargas MV, Little SJ, Richman DD, Daar ES, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis. 2013;57:441–447. doi: 10.1093/cid/cit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianella S, Morris SR, Anderson C, Spina CA, Vargas MV, Young JA, et al. Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS. 2013;27:39–47. doi: 10.1097/QAD.0b013e3283573305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, et al. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102:1959–1965. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- 25.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63. doi: 10.1016/s0952-7915(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 27.Keating SM, Jacobs ES, Norris PJ. Soluble mediators of inflammation in HIV and their implications for therapeutics and vaccine development. Cytokine Growth Factor Rev. 2012;23:193–206. doi: 10.1016/j.cytogfr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haissman JM, Vestergaard LS, Sembuche S, Erikstrup C, Mmbando B, Mtullu S, et al. Plasma cytokine levels in Tanzanian HIV-1-infected adults and the effect of antiretroviral treatment. J Acquir Immune Defic Syndr. 2009;52:493–497. doi: 10.1097/QAI.0b013e3181b627dc. [DOI] [PubMed] [Google Scholar]
- 29.Sachdeva RK, Wanchu A, Bagga R, Malla N, Sharma M. Effect of non-nucleoside reverse transcriptase inhibitors on cytokine, chemokine, and immunoglobulin profiles in serum and genital secretions of HIV-infected women. J Interferon Cytokine Res. 2010;30:299–310. doi: 10.1089/jir.2009.0056. [DOI] [PubMed] [Google Scholar]
- 30.Gay C, Dibben O, Anderson JA, Stacey A, Mayo AJ, Norris PJ, et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS One. 2011;6:e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 32.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


