Abstract
While chemotherapy-induced nausea and vomiting is clinically controlled in the acute (<24h) phase following treatment, the anorexia, nausea, fatigue, and other illness-type behaviors during the delayed phase (>24h) of chemotherapy are largely uncontrolled. As the hindbrain glucagon-like peptide-1 (GLP-1) system contributes to energy balance and mediates aversive and stressful stimuli, here we examine the hypothesis that hindbrain GLP-1 signaling mediates aspects of chemotherapy-induced nausea and reductions in feeding behavior in rats. Specifically, hindbrain GLP-1 receptor (GLP-1R) blockade, via 4th intracerebroventricular (ICV) exendin-(9-39) injections, attenuates the anorexia, body weight reduction, and pica (nausea-induced ingestion of kaolin clay) elicited by cisplatin chemotherapy during the delayed phase (48hr) of chemotherapy-induced nausea. Additionally, the present data provide evidence that the central GLP-1-producing preproglucagon neurons in the nucleus tractus solitarius (NTS) of the caudal brainstem are activated by cisplatin during the delayed phase of chemotherapy-induced nausea, as cisplatin led to a significant increase in c-Fos immunoreactivity in NTS GLP-1-immunoreactive neurons. These data support a growing body of literature suggesting that the central GLP-1 system may be a potential pharmaceutical target for adjunct anti-emetics used to treat the delayed-phase of nausea and emesis, anorexia, and body weight loss that accompany chemotherapy treatments.
Introduction
Chemotherapy is accompanied by severe side effects such as nausea and vomiting [i.e. chemotherapy-induced nausea and vomiting (CINV)], anorexia, and weight loss which diminish quality of life and require effective management. Even with currently prescribed anti-emetic drugs [e.g., serotonin type-3 (5-HT3) antagonists, neurokinin-1 (NK-1) antagonists] that provide effective control of acute and delayed chemotherapy-induced vomiting [1, 2], a significant number of patients still exhibit treatment-induced anorexia, nausea, fatigue, and other illness-type behaviors, especially during the delayed phase (>24 h) following treatment [3–5]. Therefore, it is critical to investigate the neurobiological mechanisms mediating chemotherapy-induced nausea and disturbances in feeding behavior during the delayed phase to aid in the development of new anti-emetic targets to control malaise following chemotherapy.
The dorsal vagal complex (DVC), which is comprised of the nucleus tractus solitarius (NTS), the adjacent dorsal motor nucleus of the vagus, and area postrema, is often referred to as the chemoreceptor trigger zone in emesis literature [6]. DVC neural processing modulates descending vagal efferent communication to organs of the alimentary canal, thereby regulating gastric emptying and intestinal motility rates, as well as digestive enzymatic / hormonal secretions. Fluctuations in these physiological processes are thought to contribute greatly to emesis and the feeling of nausea and other illness behaviors [6, 7]. However, in addition to processing aversive, stressful, and emetic stimuli [8, 9], the DVC is also critically involved in the normal regulation of energy balance [10]. NTS neurons process within-meal gastrointestinal (GI)-derived vagally mediated satiation signals and integrate a multitude of circulating hormones and metabolites relevant to energy balance control. Axonal projections from NTS neurons then communicate monosynaptically and polysynaptically with various hindbrain, midbrain, and forebrain nuclei involved in energy balance regulation (see [10, 11] for review). Given that the DVC is a critical neural hub for both nausea and feeding behavior, it is logical that neuropeptide/neurotransmitter systems within the NTS may mediate a portion of the energy balance dysregulation and malaise side effects elicited by chemotherapy.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone synthesized and secreted principally from two locations: the L cells in the distal intestine and preproglucagon (PPG) neurons in the NTS [12], and plays an essential role in the regulation of glycemia and energy balance (see [13, 14] for review). Accordingly, multiple GLP-1 receptor (GLP-1R) agonists are now FDA-approved for the treatment of type II diabetes mellitus, and more recently for weight loss [15–17]. While these GLP-1R agonists are administered systemically, it is now well established that GLP-1R ligands penetrate the blood brain barrier and have direct action on GLP-1Rs that are expressed widely throughout the central nervous system (CNS) [18–20]. Of note, activation of a subset of these central GLP-1R-expressing nuclei, in particular those expressed within the NTS, suppress food intake in part by eliciting malaise [21]. To this end, GLP-1-expressing neurons in the caudal NTS stand out as an attractive candidate system that may mediate chemotherapy-induced nausea and energy balance-related effects.
While nausea and emesis have been known side effects of GLP-1R agonists for some time (see [14, 21] for review), research has only recently focused on whether blockade of GLP-1R can be used to alleviate illness behaviors elicited by aversive and nauseagenic agents [22, 23]. Of potential clinical relevance, recent work from Rudd and colleagues [22] has shown that forebrain intracerebroventricular (ICV) GLP-1R antagonist administration (thus providing widespread forebrain and hindbrain access) partially attenuates the acute emesis elicited by cisplatin therapy in the house musk shrew. However, it remains unclear whether central GLP-1Rs also mediate the anorexia and delayed emetic-like behaviors elicited by chemotherapy and whether the hindbrain GLP-1 system is involved in cisplatin-mediated illness behaviors. It is worth noting that while the acute (<24h)-phase of CINV (hallmarked by repetitive emetic events) is largely attenuated by 5-HT3R / NK1R antagonists, the delayed phase (>24hr) of CINV (hallmarked by nausea and sporadic emesis) is poorly studied and less controlled by 5-HT3R / NK1R-based drugs [24]. Therefore, using a combination of immunohistochemical and behavioral analyses, the present studies provide support for the hypothesis that endogenous GLP-1R signaling in the DVC is mediating, at least in part, the nausea and the delayed anorexia behaviors elicited by cisplatin chemotherapy.
Methods
Animals and Drugs
Adult male Sprague-Dawley rats (Charles River Laboratories; 250–265 at time of purchase), housed individually in hanging metal cages under a 12h light / 12h dark cycle (lights on 0900 h), had ad libitum access to rodent chow (Purina 5001; St. Louis, MO) and water except where noted. All procedures conformed to and receive approval from the institutional standards of The University of Pennsylvania Animal Care and Use Committee.
The GLP-1R antagonist, exendin-(9-39) (American Peptide Company) was dissolved in sterile artificial cerebrospinal fluid (aCSF) for ICV injections. Cisplatin (Sigma) was dissolved in sterile saline for IP injections at a volume of 3ml/kg body weight.
Intracerebroventricular Cannula Implantation Surgeries
Rats were anesthetized using a mixture of ketamine (90mg/kg), xylazine (2.7mg/kg), and acepromazine (0.64mg/kg) and were placed into a stereotaxic apparatus. Each rat was stereotaxically implanted with a guide cannula (26-gauge; Plastics One, Roanoke, VA) with its tip positioned 2.0 mm above the 4th ventricle (coordinates: on the midline, 2.5 mm anterior to the occipital suture and 5.2 mm ventral to the skull, with injector aimed 7.2 mm from skull) [21, 25–27]. Cannulae were attached to the skull with dental acrylic and jeweler’s screws. For all surgeries, analgesia was provided (meloxicam, 2mg/kg). At least five days after surgery, 4th icv injection placement was assessed by measurement of the sympathoadrenal-mediated hyperglycemic response to the cytoglucopenia induced by 5-thio-D-glucose (210 μg) dissolved in aCSF [25, 28]. Only data from rats showing at least a twofold increase in blood glucose level in response to this treatment were included in the analyses.
Immunohistochemical analyses of cisplatin-induced c-fos on NTS GLP-1-immunoreactive neurons
Following a week of daily IP injection habituations, ad libitum fed rats (n=6/drug treatment) received an IP injection of either saline vehicle (3ml/kg) or cisplatin (6mg/kg), two hours into the light cycle. 48h following IP injections, all rats were deeply anesthetized and transcardially perfused with 0.1 M PBS, pH 7.4, followed with 4% formalin in 0.1 M PBS. Brains were removed and post-fixed in 10% formalin overnight and then cryoprotected in 20% sucrose in 0.1 M PBS at 4°C for 3 days. Coronal sections (30μM) were cut from the hindbrain using a cryostat (Leica 3050S; Leica Corp., Deerfield, IL). Brain sections were stored in 0.1 M PBS at 4°C until processed. Immunofluorescence in the NTS was quantified in each brain (n=6/treatment) using 3 representative coronal sections from each animal taken at the level of the obex (-14.8mm from bregma according to the atlas of Paxinos and Watson [29]). Double immunostaining for GLP-1 and c-Fos was performed on free-floating coronal sections according to modified procedures from our previously published studies [30, 31]. Briefly, free-floating coronal sections were washed with 1% sodium borohydride followed by 0.1 M PBS. Sections were incubated on a shaker at room temperature for 1 h with a blocking solution consisting of 5% normal donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA) in 0.1 M PBS-Tx. Sections were subsequently incubated overnight at room temperature with the following primary antibodies: polyclonal goat anti-c-Fos (1:2,000, sc-52G, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-GLP-1(7-37) (1:2,000, T-4363, Bachem, Switzerland). Sections were washed with 0.1 M PBS-Tx and then incubated with respective secondary antibodies: donkey anti-goat Alexa Fluor 594 and donkey anti-rabbit Alexa Fluor 488 (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h. Brain sections were then washed and mounted onto glass slides and coverslipped using Fluorogel (Electron Microscopy Sciences; Hatfield, PA). Using fluorescent microscopy (Nikon 80i; NIS-Elements AR 3.0) at 20× magnification, neurons expressing GLP-1 and c-Fos immunoreactivity were quantified by a separate experimenter blinded to treatment conditions for all coronal sections of the caudal brain stem between −14.8 mm to −14.1 mm from bregma.
Hindbrain GLP-1R mediation of cisplatin-induced anorexia, pica and weight loss
Rats (n=24) implanted with 4th icv cannula had ad libitum access to chow and kaolin pellets (Research Diets, New Brunswick, NJ) for 1 week prior to testing and were habituated to handling and IP injections. Subsequently, rats’ daily food intake, body weight, and kaolin intake were recorded at 10:00h (1hr into the light cycle) for two days prior to receiving drug injections. Next, a between-subject design was used to evaluate the ability of hindbrain GLP-1R blockade to attenuate the acute (24h) and delayed (48h) anorexia and pica elicited by cisplatin treatment. Accordingly, on the injection day, four groups of rats (n=6) received a combination of 4th icv injections of either the GLP-1R antagonist exendin-(9-39) (20μg/1μl) or vehicle (1μl aCSF) followed by an IP injection of either cisplatin (6mg/kg) or 0.9% NaCl (vehicle: 3ml/kg). Food and kaolin intake, as well as spillage of both substances, were measured to the nearest 0.1g at 24h; body weight change over the 24h period was also recorded. At 24h, all rats received a second 4th icv injection of their assigned icv drug treatment; no IP injections were delivered at 24h. Food and kaolin intake (accounting for spillage) were then recorded, along with body weights, at the 48h time point.
Data analysis and statistics
All data are expressed as means ± SEM. For behavioral ICV and IP injection experiments, two-way ANOVAs were performed in a 2 × 2 between-subjects design to evaluate treatment group differences using IP drug treatment (cisplatin/vehicle) and 4th ICV drug treatment (Ex. 9/vehicle) as main effects for food intake, water intake, kaolin intake, and body weight change. For immunohistochemistry experiments one way ANOVAs were employed to compare unilateral counts between IP injection conditions (cisplatin vs. saline).When applicable, planned post-hoc comparisons were also made with least significant difference (LSD) tests. All statistical analyses of mean values (α=0.05) were made using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Cisplatin activates NTS GLP-1-immunoreactive neurons
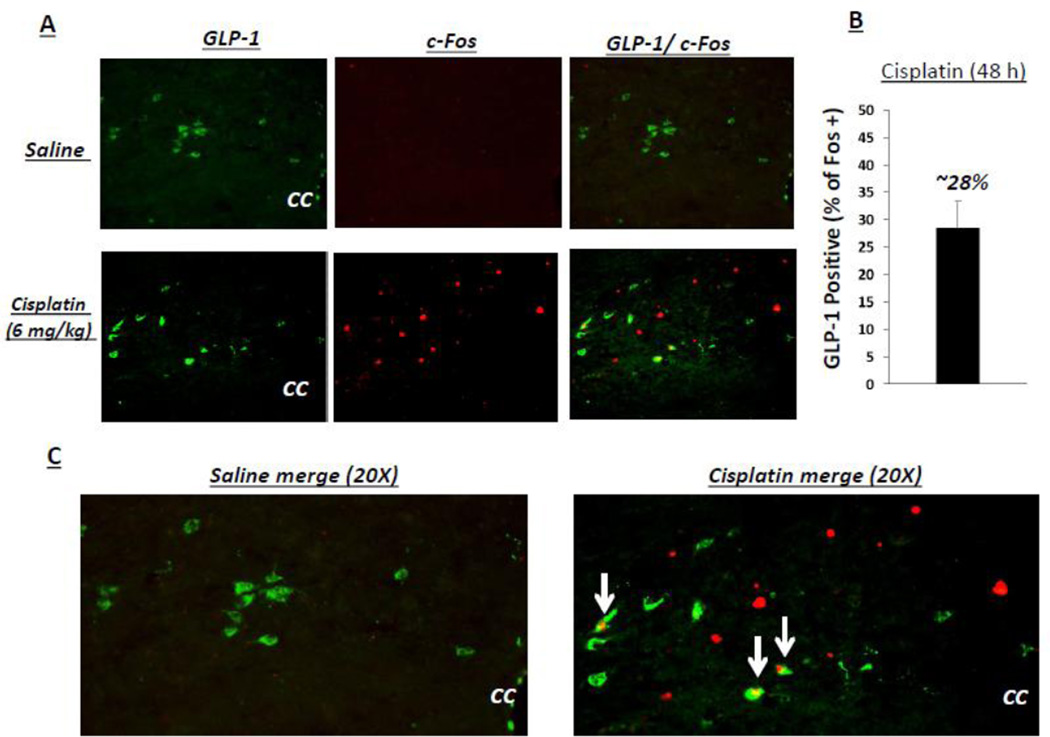
Representative images of neurons in the caudal NTS at the level of the obex double-labeled GLP-1 and c-Fos are shown in Figure 1. The total number of GLP-1 positive cells per brain section did not differ between treatments (cisplatin: 12.2 ±1.1; saline 11.8 ± 0.8: F(1,10)= 0.08; p= ns), however cisplatin treatment led to a significant increase in the number of c-fos-immunoreactive cells compared to nearly a complete absence of c-Fos in the saline vehicle condition (cisplatin: 10.8 ±0.9; saline 0.2 ± 0.04: F(1,10)= 47.2 p<0.0001) at 48h-post injection. Quantification of co-labeling in caudal NTS neurons revealed that 28 ± 3.1% of GLP-1-expressing neurons in the caudal NTS were c-Fos positive and 25% ± 2.3% of cisplatin-induced c-Fos-expressing cells in the NTS are GLP-1 positive at 48h-post treatment for cisplatin-treated rats, while no co-labeling was observed in saline treated animals.
Figure 1.
Cisplatin activates NTS GLP-1-immunoreactive neurons. A: Representative images of neurons in the caudal NTS at rostral-caudal level of obex for double-labeled GLP-1 and c-Fos are shown. B: Quantification of co-labeling in caudal NTS neurons revealed that 28 ± 3.1% of GLP-1-expressing neurons in the caudal NTS were c-Fos positive at 48h-post treatment for cisplatintreated rats. C: Zoomed image of representative merged results.
Blockade of hindbrain GLP-1R attenuates the anorexia, body weight suppression and pica produced by cisplatin treatment
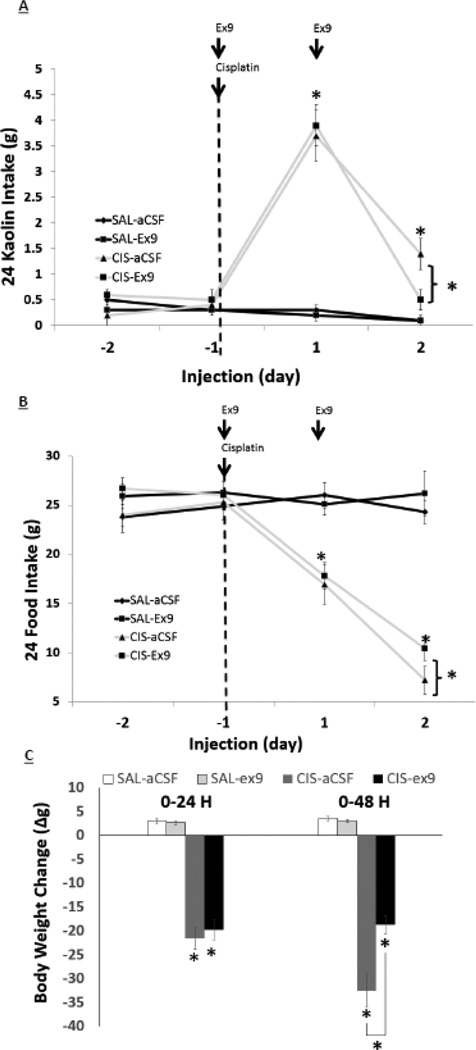
There was a significant interaction effect of IP drug X ICV drug at 48 hr on food intake [F(1,22 = 4.66; p<0.01], kaolin intake [F(1,22 = 3.75; p<0.03], and body weight [F(1,22 = 3.99; p<0.05], however, only a main effect of IP drug was noted at 24 hr for food intake [F(1,22) = 22.3; p<0.001], kaolin intake [F(1,22) = 14.1; p<0.001], and body weight change [F(1,22) = 11.05; p<0.01]. As shown in Figure 2, posthoc analyses reveal that 4th icv injection of the GLP-1R antagonist exendin-(9-39) significantly attenuated the food intake and body weight suppressive effects of cisplatin treatment at 48h, while blockade of hindbrain GLP-1R significantly attenuated cisplatin-induced increased kaolin intake at 48 h.
Figure 2.
Hindbrain administration of the GLP-1R antagonist exendin-(9-39) reduces anorexia, body weight loss, and pica following IP cisplatin treatment in rats. Exendin-(9-39) (20μg) when administered to the 4th ventricle reduces delayed pica (A), anorexia (B), and body weight loss (C) 48h following IP cisplatin (6mg/kg). Data expressed as mean ± SEM, n=6/treatment group. Arrows indicate injection day/agent. Asterisks denote significant differences between treatment groups in post-hoc comparisons within each time point (p<0.05).
Discussion
Despite tremendous strides in treating acute CINV over the past few decades, the control of delayed CINV remains poorly understood and less effectively treated [32]. Among the list of promising novel neurobiological targets involved in CINV is the central GLP-1 system, which has been intensely studied in the context of blood glucose, food intake and body weight regulation (see [12–14, 33, 34] for review), and also plays a role in modulating emesis and nausea [21–23, 35, 36]. It has been previously demonstrated that CNS blockade of GLP-1R partially attenuates the acute emesis elicited by cisplatin treatment in the house musk shrew [22]. Here, we extend these findings by identifying hindbrain GLP-1Rs as a critical locus for cisplatin-mediated clusters of illness-like feeding behaviors, as hindbrain GLP-1R blockade [via 4th ICV exendin-(9-39) injections] attenuates the anorexia, body weight suppression, and nausea responses elicited by cisplatin during the delayed phase of CINV. Additionally, current data provide evidence that the central GLP-1 system is activated by cisplatin during the delayed phase of CINV, as cisplatin caused a significant increase in c-Fos activation in a subset of NTS GLP-1-producing neurons.
Current data are consistent with previous reports [18, 22, 37] showing a contribution of the central GLP-1 system to not only food intake and body weight regulation, but also to illness behaviors. In fact, the most frequently reported side effects of GLP-1R agonists (e.g., exenatide and liraglutide) that are approved for the treatment of Type II Diabetes Mellitus and/or weight loss are emesis and nausea. Specifically, nausea has been reported in up to ~50% of subjects prescribed exenatide [16, 38–40] and up to ~30% of subjects prescribed liraglutide [16, 41] in clinical trials. These adverse events contribute to discontinuation of drug treatment in ~6–10% and reduced dose tolerance in another ~15% [38–40, 42, 43] of patients. We have previously shown that these GLP-1R agonist-induced illness behaviors are mediated by GLP-1Rs expressed within the CNS and are not mediated by GLP-1Rs expressed on vagal afferents when these compounds are delivered systemically [21]. Indeed, both liraglutide and exenatide are able to sufficiently penetrate into the CNS and activate central GLP-1R-expressing nuclei leading to hypophagia and weight loss [18, 44]. It is interesting to speculate as to whether current (e.g. exendin-(9-39) – which also crosses the blood-brain barrier [45]) or future generations of GLP-1R antagonists could be used as an adjunct anti-emetic to counteract the delayed phase CINV elicited by cisplatin.
While GLP-1Rs are expressed in various nuclei involved in the regulation of food intake and body weight regulation (e.g. NTS, VTA, NAc, PBN, CeA, PVH, ARH), only a subset of these GLP-1R-expressing nuclei has also been directly linked with mediating behaviors indicative of visceral malaise, emesis, nausea, and/or aversion (e.g. NTS, CeA) [14]. Here we examined the contribution of hindbrain GLP-1R-expressing nuclei (presumably those within the DVC) in mediating cisplatin-induced anorexia, body weight loss and pica behavior. The hindbrain/DVC focus in the present study was based on our previous work demonstrating that GLP-1R activation in NTS neurons produced a pica response in rats [21] as well as the clear role of this region in cisplatin-induced malaise [46]. However, it is likely that GLP-1R-expressing midbrain and forebrain nuclei may also mediate the adverse events of chemotherapy. Indeed, activation of GLP-1R expressed in the central nucleus of the amygdala (CeA) produces a conditioned flavor avoidance [21, 35] and cisplatin administration robustly increases c-Fos activation within the CeA at 48h [46]. We have also recently published that glutamatergic signaling within the CeA mediates cisplatin-induced malaise [47]. Future research is therefore warranted to investigate whether blockade of GLP-1R signaling in the CeA (or other CNS GLP-1R expressing nuclei) can attenuate the illness effects of chemotherapy. Such investigation is particularly important given that hindbrain GLP-1R blockade only partially attenuated the delayed phase illness-like behavioral responses following IP cisplatin injection, and therefore it is likely that any further GLP-1 mediation of CINV involves engagement of multiple nuclei throughout the neuraxis.
One limitation of the present study is that we only examined the anorexia, body weight loss, pica, and c-Fos activation for 48h following an acute cisplatin treatment. While our findings provide support for the hypothesis that the central GLP-1 system is mediating, in part, the delayed-phase of CINV elicited by cisplatin treatment, future studies examining longer time periods following a single injection of cisplatin are certainly warranted, as are studies in multiple species models to more confidently establish the translational potential of targeting the GLP-1 system for CINV. Another potential weakness of our c-Fos analyses is that animals were not food deprived for 48h. Instead, given the illness-like behaviors that occur by cisplatin alone, we chose not to further compromise the animals’ health with prolonged food deprivation, but rather chose to sacrifice the animals during their light cycle 48h following cisplatin to minimize the likelihood of meal ingestion-induced c-Fos in the NTS. However, our previous work has shown no DVC c-Fos induction in animals with 24-hr food restriction [48]. It will also be important to examine whether there is a potential interaction between hindbrain GLP-1R signaling and 5-HT3R-mediated vagal afferent signaling. Given that 5-HT3R are expressed on vagal afferent terminals innervating the stomach [49–51], that cisplatin produces pronounced gastric stasis and activates 5-HT3R (see [2] for review), and that hindbrain GLP-1R signaling is engaged by gastric distension [25], it is possible that there is an interaction between these two systems relevant to CINV responses. Indeed, the cisplatin-induced activation of GLP-1-producing neurons in the NTS may in fact be a 5-HT3R-vagally mediated excitation of PPG neurons. Equally possible is a direct cisplatin-induced activation of PPG neurons in the NTS. Thus future studies are certainly needed to examine the mechanism by which cisplatin activates GLP-1-producing neurons in the NTS and whether blockade of this activation can also attenuate CINV and delayed anorexia by cisplatin.
Collectively, the current set of data supports the hypothesis that the central GLP-1 system is activated by cisplatin chemotherapy and that hindbrain GLP-1Rs mediate, at least in part, the delayed phase anorexia and CINV behaviors of cisplatin treatment in rats. Together with previous reports highlighting a role for GLP-1R-mediation of cisplatin-induced emesis [22], current data support the need for further exploration of the GLP-1 system as a potential pharmaceutical target for adjunct anti-emetics used to treat the delayed-phase CINV, anorexia and body weight loss that accompany chemotherapy treatments.
Highlights.
Cisplatin chemotherapy activates central GLP-1-producing neurons in the NTS
Blockade of hindbrain GLP-1 receptors attenuates cisplatin induced delayed anorexia
Blockade of hindbrain GLP-1 receptors attenuates cisplatin induced delayed pica
Hindbrain GLP-1 receptor blockade attenuates cisplatin induced body weight loss
Acknowledgments
This work was support by K01-DK085435 (M.R.H.), R03-DK093874 (M.R.H.), R01- DK096139 (M.R.H.), and the American Cancer Society (B.C.D.). The authors would also like to thank James Valmer, Timothy Burch and Louis T. Dalton for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Percie du Sert N, Rudd JA, Apfel CC, Andrews PL. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer chemotherapy and pharmacology. 2011;67:667–686. doi: 10.1007/s00280-010-1339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–2268. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 4.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15:497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn CC, Meyers K, Lim A, Dye M, Pak D, Rinaman L, et al. Delineation of vagal emetic pathways: intragastric copper sulfate-induced emesis and viral tract tracing in musk shrews. Am J Physiol Regul Integr Comp Physiol. 2014;306:R341–R351. doi: 10.1152/ajpregu.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn CC. Measuring the nausea-to-emesis continuum in non-human animals: refocusing on gastrointestinal vagal signaling. Experimental brain research. 2014;232:2471–2481. doi: 10.1007/s00221-014-3985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress. 2015:1–19. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by "Silencing" Central Glucagon-Like Peptide 1 Signaling in Rats. J Neurosci. 2015;35:10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr. 2014;34:237–260. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 17.Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Therapeutic advances in endocrinology and metabolism. 2015;6:109–134. doi: 10.1177/2042018815580257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–1927. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SW, Lu Z, Lin G, Yew DT, Yeung CK, Rudd JA. The differential antiemetic properties of GLP-1 receptor antagonist, exendin (9-39) in Suncus murinus (house musk shrew) Neuropharmacology. 2014;83:71–78. doi: 10.1016/j.neuropharm.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Lachey JL, D'Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 24.Horn CC. The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting. Curr Pharm Des. 2014;20:2703–2712. doi: 10.2174/13816128113199990568. [DOI] [PubMed] [Google Scholar]
- 25.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain 5'-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology. 2009;150:2175–2182. doi: 10.1210/en.2008-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 30.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland RA, Leonard JJ, Kensey NA, Hannikainen PA, De Jonghe BC. Cisplatin induces neuronal activation and increases central AMPA and NMDA receptor subunit gene expression in mice. Physiology & behavior. 2014;136:79–85. doi: 10.1016/j.physbeh.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:1081–1090. doi: 10.1093/annonc/mdv138. [DOI] [PubMed] [Google Scholar]
- 33.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24:85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 37.Chan SW, Lin G, Yew DT, Yeung CK, Rudd JA. Separation of emetic and anorexic responses of exendin-4, a GLP-1 receptor agonist in Suncus murinus (house musk shrew) Neuropharmacology. 2013;70:141–147. doi: 10.1016/j.neuropharm.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 39.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 40.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 41.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 42.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 43.John LE, Kane MP, Busch RS, Hamilton RA. Expanded Use of Exenatide in the Management of Type 2 Diabetes. Diabetes Spectrum. 2007;20:59–63. [Google Scholar]
- 44.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J Pharmacol Exp Ther. 2004;309:469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 46.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus) Am J Physiol Regul Integr Comp Physiol. 2009;296:R902–R911. doi: 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate Receptors in the Central Nucleus of the Amygdala Mediate Cisplatin-Induced Malaise and Energy Balance Dysregulation through Direct Hindbrain Projections. J Neurosci. 2015;35:11094–11104. doi: 10.1523/JNEUROSCI.0440-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate Receptors in the Central Nucleus of the Amygdala Mediate Cisplatin-Induced Malaise and Energy Balance Dysregulation through Direct Hindbrain Projections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:11094–11104. doi: 10.1523/JNEUROSCI.0440-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 50.Hayes MR, Covasa M. Gastric distension enhances CCK-induced c-Fos expression in the dorsal hindbrain by activating 5-HT3 receptors (Abstract) Appetite. 2006 doi: 10.1016/j.brainres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distension-induced release of 5-HT stimulates c-fos expression in specific brain nuclei via 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2004;287:G228–G235. doi: 10.1152/ajpgi.00373.2003. [DOI] [PubMed] [Google Scholar]