Summary
A new high-resolution structure of a pain-sensing ion channel, TRPA1, provides a molecular scaffold to understand channel function. Unexpected structural features include a TRP-domain helix similar to TRPV1, a novel ligand-binding site, and an unusual C-terminal coiled coil stabilized by inositol hexakisphosphate (IP6). TRP-domain helices, which structurally act as a nexus for communication between the channel gates and its other domains, may thus be a feature conserved across the entire TRP family and, possibly, other allosterically-gated channels. Similarly, the TRPA1 antagonist-binding site could also represent a druggable location in other ion channels. Combined with known TRPA1 functional properties, the structural role for IP6 leads us to propose that polyphosphate unbinding could act as a molecular kill switch for TRPA1 inactivation. Finally, although packing of the TRPA1 membrane-proximal region hints at a mechanism for electrophile sensing, the details of how TRPA1 responds to noxious reactive electrophiles and temperature await future studies.
Keywords: electron cryomicroscopy, transient receptor potential ion channels, coiled coil, polyphosphates, ankyrin repeats, TRP domain, allosteric gating
Graphical abstract
A new high-resolution structure of a pain-sensing ion channel, TRPA1, reveals a novel small molecule binding site, unexpected intracellular architecture, and a structural role for phosphate-containing molecules. Armed with this structure, we infer more about channel workings and propose new hypotheses for the molecular bases behind features of TRPA1 function.
Introduction
In humans, many pungent and painful sensations are mediated by the transient receptor potential ankyrin 1 (TRPA1) ion channel expressed in peripheral nociceptor neurons [1–4]. TRPA1 is activated by many reactive irritants, including mustard oil and sulfur-containing compounds in onions and garlic. TRPA1’s irritant sensing ability is conserved from sea sponges to humans, suggesting an ancient origin [5]. TRPA1 activation in nociceptors leads to action potentials that signal pain and prompt aversive or protective responses [6]. Indeed, TRPA1 malfunction can lead to abnormal pain signaling. A human TRPA1 gain-of-function mutation is associated with familial episodic pain syndrome [7]. Another TRPA1 variant is linked to paradoxical heat sensation associated with neuropathic pain [8]. During tissue injury and inflammation, TRPA1 is activated by endogenous ligands, promoting pain and hypersensitivity [9–11]. Consequently, TRPA1 antagonists are pursued as drugs to combat pain, inflammation, itch, and even asthma [11].
Many TRPA1 homologs are also activated by specific hot or cold temperatures. Mammalian TRPA1s respond to temperatures below 17°C [1]. In some studies, TRPA1-deficient mice lack responses to burning cold on their extremities [12], although others report no cold-sensing defects [13]. This, along with other evidence [14], suggests that TRPA1 is not a primary cold sensor in mammals, a role partly filled by another cold-activated channel, TRP melastatin 8 (TRPM8) [15–17]. Still, TRPA1 is associated with injury-induced cold and mechanical hyperalgesia [18,19]. Conversely, insect and some snake TRPA1 homologs are activated by heat. In Drosophila, TRPA1 is necessary to set the preferred temperature range [20,21]. Pit snakes rely on TRPA1 expression in their pit organs for infrared-based prey detection [22].
Since its discovery in 2003 [1], numerous studies have probed the relationship between TRPA1 structure and function [4,23], identifying TRPA1 as a homotetrameric calcium-permeable cation channel that depends on calcium for both potentiation and inactivation [24]. Without high-resolution structures, the molecular mechanisms behind TRPA1 function and regulation remained a mystery. A new electron cryomicroscopy (cryoEM) structure reveals the first atomic-level view of TRPA1 [25]. This structure provides the context for many observations on TRPA1, allowing the information to be overlaid on an accurate spatial model (Fig. 1). Armed with this model, we can now infer more about the workings of the channel and propose new hypotheses for the molecular bases behind the prominent features of TRPA1 function. For example, the positioning of key cysteines near a potential gating handle, the TRP-domain helix, hints at a mechanism for coupling electrophile detection to channel gating. This review highlights several such insights provided by the TRPA1 structure, including a few surprises, and new questions that arise.
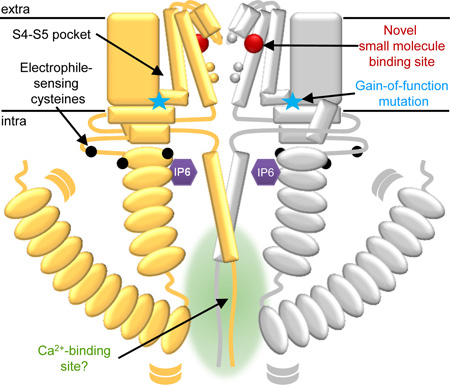
Figure 1.
A schematic of TRPA1 maps a number of demonstrated and proposed regulatory handles. The schematic follows the color scheme from N- to C-terminus: ankyrin repeats, turquoise; linker, yellow; S1–S4, lilac; S5–S6, blue; gate residues, orange; TRP-domain helix, red; and C-terminus, green. The regions absent in the structure are shown here in grey. The antagonist A967079 is represented as a green circle and the S4–S5 pocket is marked with a green asterisk. The positions of cysteines essential for response to reactive electrophiles are marked as black circles in the membrane-proximal N-terminal region that surrounds the TRP-domain helix (red). The position of the familial episodic pain variation N855S and the paradoxical heat syndrome variation E179K are marked with a yellow star and blue triangle, respectively. IP6 is represented as a purple hexagon between the membrane-proximal ankyrin repeats (turquoise) and coiled coil (green). The N-terminus and ankyrin repeats 1–11, which are missing from the TRPA1 structure, are in grey. Curved grey lines denote the suspected flexibility of the linkage between 1–11 and the remaining ankyrin repeats. Potential interactions between the N-terminus and the membrane or membrane-associated factors are indicated by a grey arrow. Intracellular mutations reported to disturb calcium-dependent potentiation or inactivation of TRPA1 are marked with red diamonds, which cluster at the intersection of the ARD and the C-terminus.
The general architecture of TRPA1 resembles that of other tetrameric ion channels
The human TRPA1 cryoEM structure shows four subunits assembling into a homotetrameric channel (Fig. 2). Each subunit includes six transmembrane segments (S1–S6) and large, intracellular N- and C-termini. Similar to distantly-related voltage-gated channels [26,27], TRPA1’s S1–S4 form bundles at the periphery of the channel, while the S5 and S6 segments surround the ion permeation pathway (Figs. 2A, 2B). Two helices between S5 and S6 form the extracellular-facing pore entrance and selectivity filter, which govern the channel’s cation-specific permeability. Select sidechains project into the pore, forming upper and lower gates that regulate channel opening: asparagine 915 from pore helix 1 is the upper gate, while hydrophobic S6 residues isoleucine 957 and valine 961 form the lower gate (Figs. 2B–D). TRPA1 agonists and antagonists allosterically regulate these gates to control channel opening and closing. Intracellularly, an unexpected “TRP-domain” α-helix radiates outward from the center of the channel, lying parallel to the membrane underneath the S1–S4 bundle within each respective subunit (Fig. 2B; also see Fig. 4A). As discussed below, this helix is structurally homologous to the TRP-domain helix first visualized in the homologous TRP vanilloid 1 (TRPV1) channel structure [28–30].
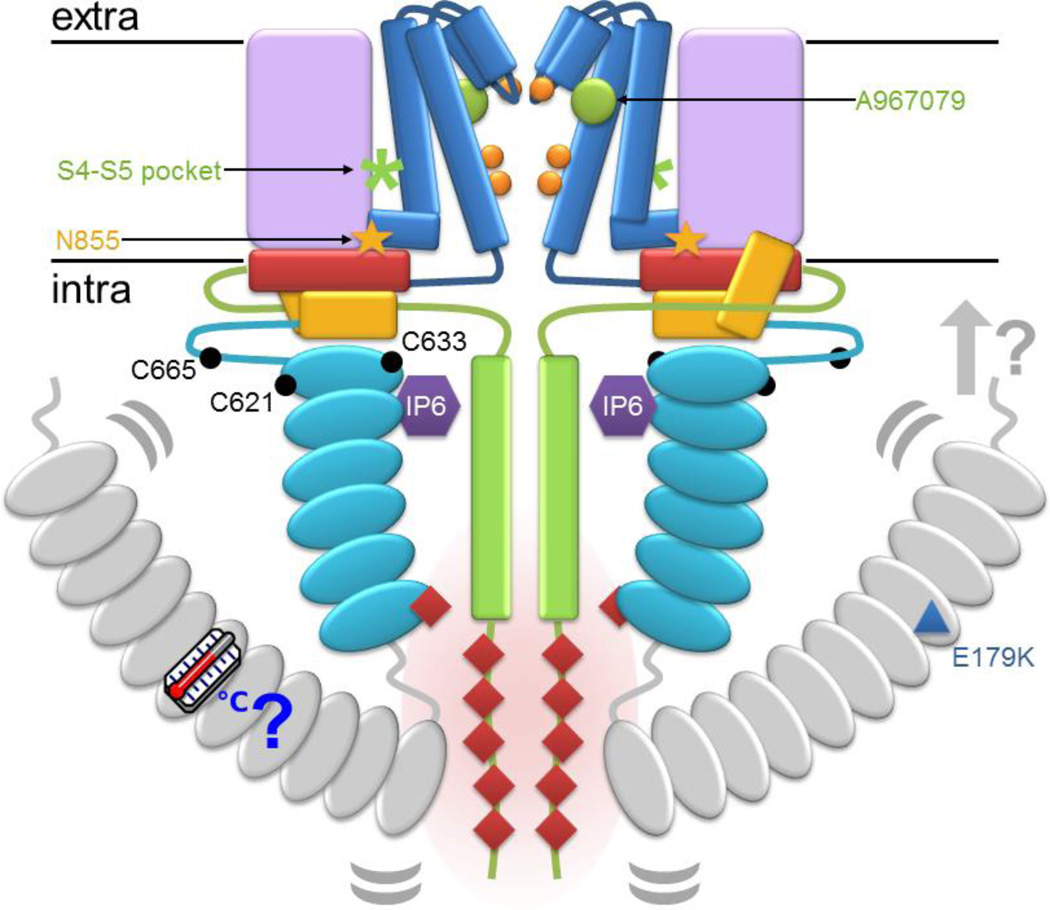
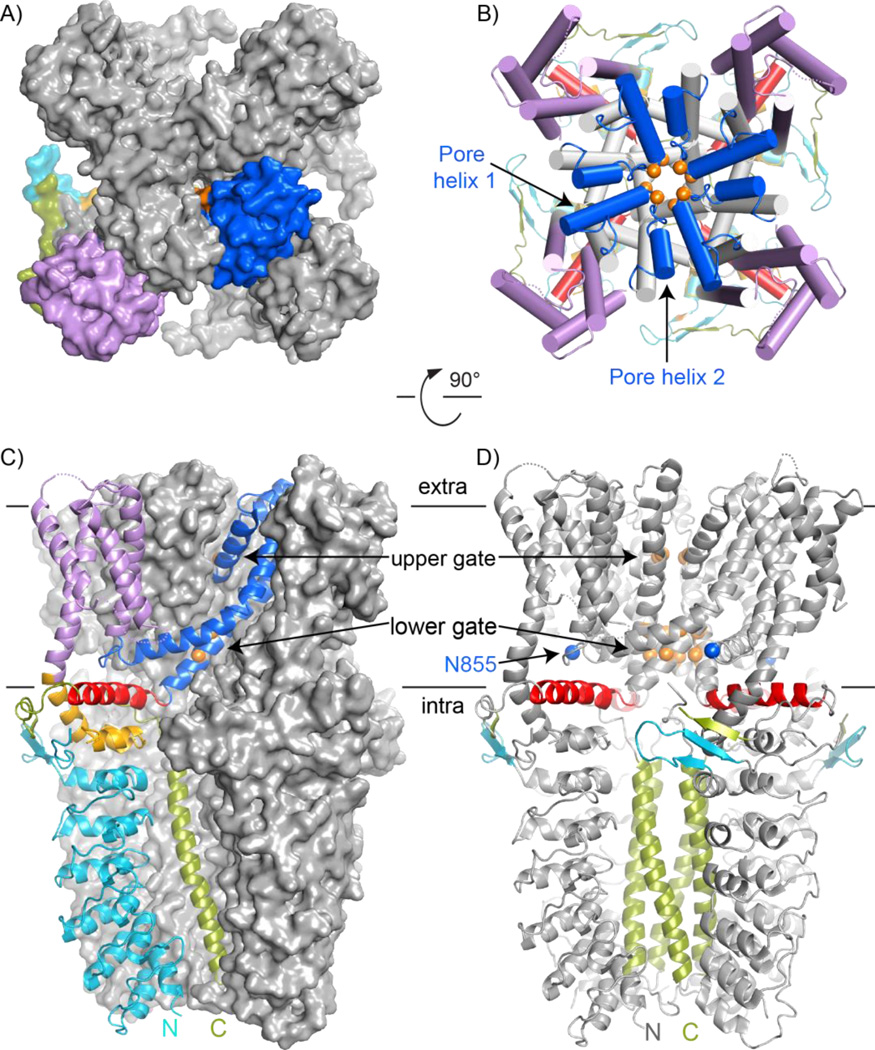
Figure 2.
CryoEM structure of TRPA1. All panels follow the color scheme outlined in Figure 1. A and B: Surface and cartoon views of the TRPA1 extracellular face, respectively. Only one subunit in (A) is colored, highlighting the domain swapping of the S1–S4 bundle (lilac) and pore-forming S5–S6 (blue). In (B) the S5 and S6 helices are grey to highlight the two pore helices, labeled in one subunit, at the pore entrance and the unexpected TRP-domain helices positioned like radiating spokes beneath the transmembrane domain. C: TRPA1 viewed from the side with one subunit as a colored cartoon to indicate the relative position of each element in the subunit. D: Key features of TRPA1 are colored on a cartoon side view, including the upper and lower gates (orange), asparagine 855 position involved in familial episodic pain syndrome (blue), tetrameric coiled coil (green), TRP-domain helix (red), and proposed β-sheet (turquoise and green).
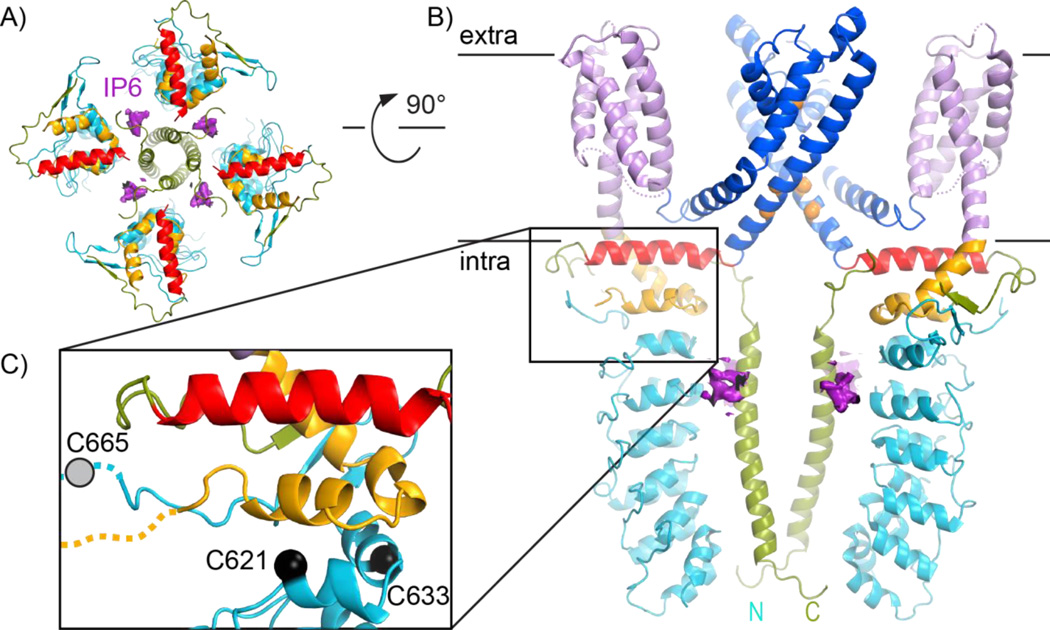
Figure 4.
Intracellular features of TRPA1. All panels follow the same color scheme as in Figure 1.A: The transmembrane domain is hidden from the view of the extracellular channel face, revealing the presence of four IP6 molecules (purple) at interfaces of the intracellular ankyrin repeats (turquoise) and coiled coil (green). B: A side view of TRPA1 with IP6 density has the front and back subunits removed for clarity. C: Zooming into the membrane-proximal region, the positions of cysteines important for TRPA1 activation by reactive electrophiles are marked with black spheres. The approximate position of cysteine 655, not modelled in the original structure, is marked with a grey circle.
The N- and C-terminal cytoplasmic domains comprise nearly three quarters of the TRPA1 protein. Although present in the cryoEM samples, only one third of the cytoplasmic mass was clearly resolved in the structure. Still, the available structure provides exciting clues about TRPA1 function. Interestingly, C-terminal α-helices from the four subunits form an unexpected coiled coil, extending below the pore within a cage formed by a portion of the N-termini. This N-terminal region, most proximal to the transmembrane domain, contains six of the seventeen predicted ankyrin repeats, structural motifs of two α-helices followed by a β-hairpin loop. A β-sheet formed by portions of the N- and C-termini may mediate interactions between adjacent subunits under some conditions; although, in this TRPA1 structure, no direct contacts are observed (Figs. 2C, 2D, 4A).
The TRPA1 structure reveals that the channel’s domains are remarkably interconnected. The intracellular domains are packed tightly against the transmembrane domain via the TRP-domain helix, which is directly attached to the gate-forming S6 helix (Figs. 2C, 2D). These connections suggest how intracellular structural changes may propagate from inside the cell to the pore and gates. Such interdomain interactions may be regulatory “handles” and part of the structural basis behind TRPA1’s remarkable polymodality, its ability to respond to multiple types of stimuli.
TRPA1 and TRPV1 have very similar transmembrane domains
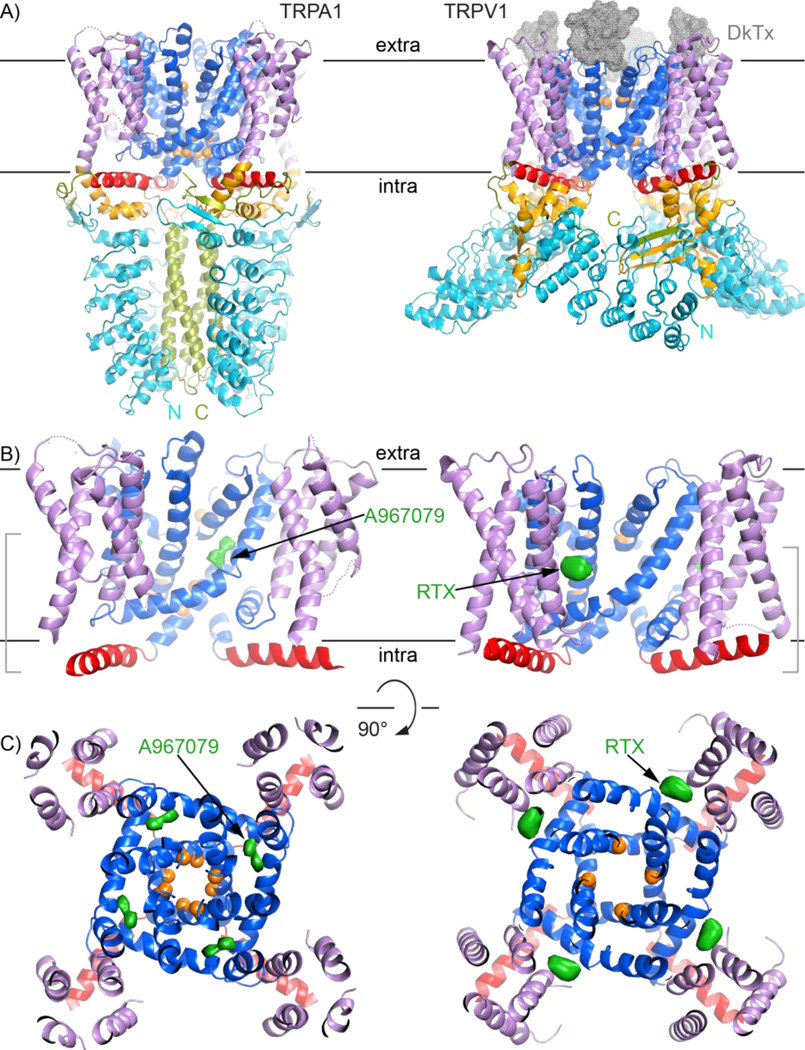
We can compare the TRPA1 structure bound to antagonists -- i.e. representing a closed state [25] -- to an open TRPV1 structure with two bound agonists: DkTx, a tarantula toxin bound to the extracellular pore region, and resiniferatoxin bound in the transmembrane region (Fig. 3)[29]. The TRPA1 transmembrane domain is generally organized as in TRPV1 (Fig. 3A), of which the high-resolution structure was also recently solved by cryoEM [28,29]. In both channels, stereotyped domain swapping entails one S1–S4 bundle contacting S5 and S6 of a neighboring subunit (Fig. 2A). This domain swapping is also observed in distant TRP channel cousins: voltage-gated channels [27,31–33].
Figure 3.
Comparisons of the TRPA1 and TRPV1 channels highlight different ligand-binding sites. All panels follow the same color scheme as Figure 1 to depict TRPA1 (PDB 3j9p) on the left and TRPV1 (3j5q) as cartoons. A: Side views of TRPA1 (left) and TRPV1 (right) show the high similarity between their transmembrane domains, despite drastically different intracellular regions. DkTx is depicted as a grey mesh. B: and C: Views of the TRPA1 and TRPV1 transmembrane domains from the side and extracellular face, respectively, include densities for TRPA1 antagonist A967079 and TRPV1 agonist resiniferatoxin as green surfaces. These molecules have different binding sites in the channels. For clarity, the views in (C) are clipped to include only the regions bracketed in (B).
As mentioned above, the TRPA1 TRP-domain helix was unexpected. The TRP domain is named after a characteristic tryptophan in the consensus TRP box sequence (EWKFAR) C-terminal to S6 in many TRP channels [34]. The presence of that motif predicted a TRP domain for TRPV, TRPC, and TRPM channels, but not TRPAs [35]. The TRP domain is important for channel gating [28,30,34]. The TRPV1 structure revealed that the TRP domain is an α-helix running from S6 beneath the S4–S5 linker and S1–S4 bundle [28–30]. In TRPA1, although the TRP box motif is absent [34], a structurally conserved α-helix is positioned just as in TRPV1 (Figs. 3B, 3C). This suggests TRP-domain helices may be present in more channels than originally suspected, perhaps spanning the entire TRP family and even beyond. These helices may enable layers of allosteric gating in these channels, providing the downstream link and a literal handle for distant sensors to affect the channel gates. In support of this idea, comparing antagonist-bound TRPA1 to agonist-bound TRPV1 highlights differences in tilt of the TRP-domain helix (Fig. 2A) and the rotation and constriction of the pore (Fig. 3C). These features are consistent with differences between ligand-free and agonist-bound TRPV1 [28–30,36], and may represent physiological conformational changes associated with channel gating.
Intriguingly, asparagine 855, mutated to serine in familial episodic pain syndrome [7], is in the S4–S5 linker, just above the TRP-domain helix. Asparagine 855 likely makes key contacts with the TRP domain or nearby residues. Similar interactions between the S4–S5 linker and TRP domain are predicted for other TRP channels [28,37]; disrupting the potential interaction results in channel hyperactivity in TRPV4 [37], and also impairs proper folding in TRPV channels [38].
There is one important difference in the transmembrane domain topology of TRPA1 and TRPV1: TRPA1 has an additional pore helix lining the extracellular face of the ion permeation pathway, and thus two pore helices per subunit (Fig. 2B). This pore architecture is more similar to that of bacterial sodium channels [31,32] than to bacterial and eukaryotic potassium channels [33,39]. The functional significance of this structural feature remains unclear, although it may be involved in attracting cations [32] or the observed pore dilation that can alter selectivity [40,41].
An antagonist highlights a novel ligand binding site in TRPA1: A new druggable site?
TRPA1’s involvement in pain and inflammation [42,43], partly via endogenous TRPA1 agonists generated during oxidative stress or tissue injury [9,10], makes it a prime target for pharmacological intervention [6,11]. Moreover, identified natural TRPA1 agonists and antagonists prove that TRPA1 is modulated by small molecules and, thus, druggable. Small-molecule ligands fall into two broad categories: electrophilic activators and nonelectophilic compounds. Allyl isothiocyanate (AITC) from wasabi, cinnamaldehyde, and allicin from garlic are among the natural electrophilic activators [3,23,44,45], discussed in more detail below. Nonelectrophilic ligands include several hydrophobic, cyclic molecules such as menthol and cannabinoids [3,46], which likely bind within the TRPA1 transmembrane domain. For example, S5 residues are important for responses to menthol [47] and several other nonelectrophilic ligands [48,49]. Known pharmacological TRPA1 agonists include common general anesthetics propofol, etomidate, and isoflurane [50]. Derivatization of small molecules and high-throughput screens have identified TRPA1 antagonists [19,51,52], most prominently A967079 [14] and HC-030031 [53]. Such antagonists have proven effective in mice, reducing pain and hypersensitivity associated with diabetic or chemotherapy-induced peripheral neuropathies [54,55]. A few TRPA1 antagonists are in clinical trials to treat neuropathic pain and asthma [11].
TRPA1 structures in complex with antagonists were also determined by cryoEM [29]. These structures, in combination with TRPV1 structures, identify potentially druggable sites. Due to similar topology, these insights may apply to the entire TRP channel family and, perhaps, more generally to other channels with the same transmembrane topology, e. g., voltage-gated calcium, potassium or sodium channels. In TRPV1 there is a pocket deep in the transmembrane domain, just above the S4–S5 linker helix and between the S1–S4 bundle and the pore; this pocket is the binding site for multiple ligands, including its potent activator resiniferatoxin (Figs. 3B, 3C) [29]. The analogous site in TRPA1 is thus a candidate ligand-binding site, particularly for nonelectrophilic compounds. The antagonist-bound TRPA1 structure revealed a second site near the pore [25]: A967079 binds between pore helix 1 and S5, a region distinct from the S4–S5 pocket (Figs. 3B, 3C). This binding site agrees with previous studies, which identified S5 and pore-helix residues affecting sensitivity to A967079 [56–58]. The binding site for the second antagonist, HC-030031, was not resolved in the structures [25].
As introduced above, several nonelectrophilic compounds modulate TRPA1. The observation that the resiniferatoxin-binding site in TRPV1 and A967079 site in TRPA1 are topologically different raises the question: do all noncovalent TRPA1 agonists and antagonists affect the channel through the same binding site, or do they bind at different locations? An answer will require additional structural studies, a combination of small molecule structure-function studies, and/or the use of crosslinkable analogs to directly identify binding-site residues. Understanding how known nonelectrophilic agonists and antagonists interact with TRPA1 will improve our understanding of this fascinating molecular machine, and assist in the rational design of new TRPA1-targeting analgesic and anti-inflammatory drugs.
The N-terminal ankyrin repeat domain contains a sharp and flexible kink
The TRPA1 N-terminus contains the large series of ankyrin repeats that inspired the channel’s name. An ankyrin repeat is a 33-residue structural motif consisting of two helices followed by a β-hairpin loop. In series, these repeats typically form a stack with an overall helical twist [35]. Other TRP channel subfamilies, namely TRPVs and TRPCs (canonical TRP channels), have ankyrin repeat domains (ARDs) that contain only 4–6 repeats. ARDs are typically protein- or ligand-interaction sites. The TRPV1 ARD binds ATP and the calcium-signaling protein calmodulin, known TRPV1 regulators [59,60]. No analogous endogenous ligands are identified for the TRPA1 ARD; therefore, the specific role of the extended ARD is unclear. It has been hypothesized based on molecular dynamics simulations and atomic force microscopy studies that long ankyrin repeat stacks are elastic and could play a role in mechanosensation [61–64]. But no such physiological role has been assigned to the TRPA1 ARD.
TRPA1 has 17 predicted ankyrin repeats; only repeats 12–17 were modelled in the structure. (Note that predictions vary between 16 [25,65,66] and 17 [35,64] because the most C-terminal repeat deviates from the consensus motif.) The repeat 10 sequence deviates from consensus [30], with two conserved prolines that would likely disrupt the second helix, suggesting a kink. Although rare, a kink has been observed in an ankyrin repeat-containing protein [67]. Interestingly, weak density for the missing eleven ankyrin repeats forms a bowl-like structure beneath rest of the channel (Fig. 1) [28]. This indicates that the TRPA1 ARD is not a complete, canonical stack [68,69], but instead contains the suspected kink before repeat 12. Furthermore, the weak density suggests this kink is flexible. A flexible kink means the ARD is less likely to act as a mechanical spring, and also poses a question as to how the TRPA1 N-terminal regions could communicate to the pore, a question we examine in a later section.
The TRPA1 N-terminus is important for temperature sensing and modulation
Multiple groups implicated the ARD and adjacent regions in TRPA1 temperature activation (Fig. 1). An E179K mutation in repeat 4 is associated with paradoxical heat syndrome in humans [8]. Studies of chimeras between heat-activated and non-heat-activated TRPA1 homologs highlighted two regions, repeats 3–8 and 10–15, as important modules for heat sensing in rattlesnake TRPA1 [65]. Also, point mutations in repeat 6 switch mouse TRPA1 from cold- to heat-activated [70]. For insect TRPA1, alternative splicing generates isoforms with different N-terminal sequences preceding the ARD; the N-termini dramatically alter temperature responses, effectively creating temperature-sensitive and -insensitive versions of TRPA1 [71,72]. Together these data identify the ARD and surrounding regions as important, highly-tunable players in TRPA1 temperature sensing. These regions were unresolved in the TRPA1 structure, suggesting they are only loosely associated with the rest of the channel, at least under the conditions in which the structure was determined. Therefore, additional experiments and high-resolution structures of the extreme TRPA1 N-terminus are needed to elucidate how those regions influence TRPA1 activity and temperature sensing.
The TRPA1 membrane-proximal region hints at mechanism for electrophile sensing
Various irritants act as TRPA1 agonists, and although their chemical structures differ widely, many are membrane-permeable reactive electrophiles [23]. These reactive electrophiles covalently modify intracellular cysteines in the TRPA1 N-terminus [66,73]. Three conserved cysteines in the ARD C-terminus and linker preceding the S1 segment are necessary for TRPA1 activation by electrophiles (Fig. 4C) [73,74]. The final, 17th, ankyrin repeat leads into an extended β-hairpin loop. This hairpin is followed by an ankyrin-repeat-like helix-turn-helix motif sandwiched between the 17th repeat and TRP-domain helix (Figs. 4B, 4C). Importantly, the cysteines essential for electrophile sensitivity are solvent-accessible (Fig. 4C). Notably, a previous low-resolution structure, particularly cysteine location and postulated intracellular disulfides [75,76] are inconsistent with the new, much higher resolution structure.
Although the TRPA1 samples used for structure determination were exposed to an excess of a reactive electrophile, AITC, no density corresponding to expected cysteine adduct(s) was observed [25]. Without further chemical analysis it is unclear whether the structure represents the AITC-bound or unmodified protein. Still, the proximity of the cysteines to the protein surface suggests they are indeed accessible to chemical modification. Finally, their location close to the TRP-domain helix provides a plausible link between cysteine modification status and channel gating (Figs. 4B, 4C). Additional structures clearly representing electrophile-free and electrophile-bound states will be required to understand the molecular mechanism of TRPA1 activation by electrophilic agonists.
Polyphosphates stabilize interactions between intracellular portions of TRPA1
TRPA1 currents rapidly run down in excised membrane patches, where the intracellular face of TRPA1 is exposed to the bath solution, unless this solution contains polyphosphates such as polyP3 or inositol hexakisphosphate (IP6) [24,77]. This suggests that soluble intracellular factors, perhaps polyphosphates, are required to maintain TRPA1 in an agonist-receptive state. Based on these studies, the cryoEM sample included IP6, which proved key to stabilize TRPA1 during purification [25].
The structure shows an IP6 molecule between the N-terminal ARD and C-terminal coiled coil, providing an explanation for the IP6 requirement. Unlike most coiled coils, which are stabilized by conserved hydrophobic residues at helix interfaces [78], the TRPA1 coiled coil has polar residues at several interface positions [25]. Additionally, the coiled coil outer surface has distinct patches of positively-charged residues near the transmembrane pore. These, together with ARD residues, bind one IP6 per subunit (Fig. 4A, 4B). IP6 is thus a bridging ligand between the intracellular N- and C-termini. Furthermore, without IP6, the high positive charge density could destabilize the coiled coil and weaken interactions with the ARD.
Are polyphosphates a kill switch for TRPA1 currents?
Since TRPA1 activation by reactive electrophiles occurs through covalent modification, removal, if at all possible, is very slow in comparison to non-covalent interactions. Unchecked, this would result in problematic sustained TRPA1 currents and pain signaling long after stimulus removal. Experimentally, however, TRPA1 displays inactivation dependent on an increase in intracellular calcium [24]. After inactivation, TRPA1 no longer responds to electrophilic or nonelectrophilic agonists [24,77]. The necessity of phosphate-containing molecules for both channel function and stability suggests they are important for physiological channel regulation. Furthermore, the structure shows IP6 bridging the coiled coil and ARD. Together these observations suggest that physiological ligands like IP6 may act as a molecular kill switch: displacement of phosphate-containing molecules from the TRPA1 intracellular regions by permeating cations, particularly divalents such as calcium, could be the molecular basis behind channel inactivation. Such an inactivation mechanism would provide a fast, simple way to shut off TRPA1 currents independent of the nature of the agonist. While the structure does not provide immediate answers, it does provide a framework to design mutations that further explore regulatory mechanisms that tune TRPA1 sensitivity, including the influence of polyphosphate compounds.
An intracellular nexus for Ca2+-dependent channel regulation?
Electrophysiological studies revealed that calcium promotes both TRPA1 potentiation and inactivation, and both processes rely on an increase in intracellular calcium passing through TRPA1 [24]. Potentiation and activation are disrupted when calcium permeability is reduced by mutating aspartate 918 in rat TRPA1 (aspartate 915 in human), the conserved upper gate residue [24]. The molecular details behind these processes remain unknown. Several mutations affect TRPA1 calcium-dependent regulation. In mouse TRPA1, mutating residues in the ankyrin repeat 12 loop abrogates potentiation or activation by calcium [79,80]. Chimeric studies implicated repeat 11 as important for calcium-dependent inactivation in human TRPA1 [65]. Also, mutating acidic residues just C-terminal of the coiled coil affects Ca2+-dependent potentiation kinetics [81]. The TRPA1 structure reveals that these regions should be proximal to each other (Fig. 1), although the C-terminal residues were not modeled. This intracellular nexus is important for regulation, perhaps through direct binding to calcium.
Conclusion
The high-resolution structure of TRPA1, a sensor of painful stimuli, presents new information about its workings, allowing us to generate new hypotheses about the molecular mechanisms of channel function (Fig. 1) and hence transmission of painful stimuli. From the similarities between the TRPA1 and TRPV1 transmembrane domains, we propose that the known principles and architecture of gating and allosteric activation may apply to a wider range of channels than expected. The TRPA1 transmembrane domain structure reveals a novel ligand-binding pocket in a TRP channel, which represents a druggable site. Intracellularly, the domain organization provides plausible explanations for how modification of the N-terminus by activating electrophiles leads to channel opening. Also, an essential structural role for polyphosphate-containing compounds was revealed as a bridge between intracellular domains. We suggest that following channel activation, removal of polyphosphate-containing compounds, promoted by entering calcium, could act a molecular kill switch to trigger inactivation. Additionally, the TRPA1 structure allows mapping of function-altering mutations onto a framework (Fig. 1). Consolidating those observations reveals the TRP-domain helix as a nexus for allosteric gating near the putative reactive-electrophile sensor and identifies a possible nexus for calcium-dependent channel regulation. Still, half of the TRPA1 protein is only a fuzzy vision, and many questions remain. In particular, little is known about the most mysterious aspect of TRPA1 and other TRP channels: how they sense temperature.
Acknowledgements
We thank Christina Zimanyi and other members of the Gaudet lab for discussions. This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5-F31-GM-109741 to M.S.J.B.
Abbreviations
- ARD
ankyrin repeat domain
- cryoEM
electron cryomicroscopy
- IP6
inositol hexakisphosphate
References
- 1.Story GM, Peier AM, Reeve AJ, Eid SR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 2.Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordt SE, Bautista DM, Chuang HH, McKemy DD, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 4.Zygmunt PM, Högestätt ED. Mammalian Transient Receptor Potential (TRP) Cation Channels. Springer; 2014. Trpa1; pp. 583–630. [Google Scholar]
- 5.Kang K, Pulver SR, Panzano VC, Chang EC, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koivisto A, Pertovaara A. Transient Receptor Potential Ankyrin 1 Channel Antagonists for Pain Relief. In: Szallasi A, editor. TRP channels as therapeutic targets: from science to clinical use. Academic Press; 2015. pp. 146–158. [Google Scholar]
- 7.Kremeyer B, Lopera F, Cox JJ, Momin A, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May D, Baastrup J, Nientit MR, Binder A, et al. Differential expression and functionality of TRPA1 protein genetic variants in conditions of thermal stimulation. J Biol Chem. 2012;287:27087–27094. doi: 10.1074/jbc.M112.341776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevisani M, Siemens J, Materazzi S, Bautista DM, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Hackos D. TRPA1 as a drug target—promise and challenges. Naunyn-Schmiedeberg's Arch Pharmacol. 2015;388:451–463. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Joshi SK, DiDomenico S, Perner RJ, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34C:14–19. doi: 10.1016/j.conb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhaka A, Murray AN, Mathur J, Earley TJ, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 18.del Camino D, Murphy S, Heiry M, Barrett LB, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrus M, Peier AM, Bandell M, Hwang SW, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:1–8. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & development. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada FN, Rosenzweig M, Kang K, Pulver SR, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laursen WJ, Bagriantsev SN, Gracheva EO. Chapter Four - TRPA1 Channels: Chemical and Temperature Sensitivity. In: León DI, Feng Q, editors. Current Topics in Membranes. Academic Press; 2014. pp. 89–112. [DOI] [PubMed] [Google Scholar]
- 24.Wang YY, Chang RB, Waters HN, McKemy DD, et al. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulsen CE, Armache JP, Gao Y, Cheng Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cellular and Molecular Life Sciences. 2015:1–17. doi: 10.1007/s00018-015-1948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catterall WA, Swanson TM. Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Molecular Pharmacology. 2015;88:141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellmich U, Gaudet R. High-Resolution Views of TRPV1 and Their Implications for the TRP Channel Superfamily. In: Nilius B, Flockerzi V, editors. Mammalian Transient Receptor Potential (TRP) Cation Channels. Springer International Publishing; 2014. pp. 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Ren W, DeCaen P, Yan C, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long SB, Campbell EB, MacKinnon R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 34.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson R. Structural biology: Ion channel seen by electron microscopy. Nature. 2013;504:93–94. doi: 10.1038/504093a. [DOI] [PubMed] [Google Scholar]
- 37.Teng J, Loukin SH, Anishkin A, Kung C. L596-W733 bond between the start of the S4–S5 linker and the TRP box stabilizes the closed state of TRPV4 channel. Proc Natl Acad Sci U S A. 2015;112:3386–3391. doi: 10.1073/pnas.1502366112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Elias A, Berna-Erro A, Rubio-Moscardo F, Pardo-Pastor C, et al. Interaction between the Linker, Pre-S1, and TRP Domains Determines Folding, Assembly, and Trafficking of TRPV Channels. Structure. 2015 doi: 10.1016/j.str.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Lee A, Chen J, Ruta V, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Kim D, Bianchi B, Cavanaugh E, et al. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Molecular Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. American Journal of Physiology - Cell Physiology. 2010;298:C1457–C1468. doi: 10.1152/ajpcell.00489.2009. [DOI] [PubMed] [Google Scholar]
- 42.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrison SR, Stucky CL. The dynamic TRPA1 channel: a suitable pharmacological pain target? Current pharmaceutical biotechnology. 2011;12:1689. doi: 10.2174/138920111798357302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandell M, Story GM, Hwang SW, Viswanath V, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 45.Bautista DM, Movahed P, Hinman A, Axelsson HE, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karashima Y, Damann N, Prenen J, Talavera K, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao B, Dubin AE, Bursulaya B, Viswanath V, et al. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohara K, Fukuda T, Okada H, Kitao S, et al. Identification of Significant Amino Acids in Multiple Transmembrane Domains of Human Transient Receptor Potential Ankyrin 1 (TRPA1) for Activation by Eudesmol, an Oxygenized Sesquiterpene in Hop Essential Oil. Journal of Biological Chemistry. 2015;290:3161–3171. doi: 10.1074/jbc.M114.600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takaishi M, Uchida K, Fujita F, Tominaga M. Inhibitory effects of monoterpenes on human TRPA1 and the structural basis of their activity. The Journal of Physiological Sciences. 2014;64:47–57. doi: 10.1007/s12576-013-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matta JA, Cornett PM, Miyares RL, Abe K, et al. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan J, Hunsberger G, Neipp C, McAlexander MA. TRPA1 antagonists as Potential Therapeutics for Respiratory Diseases. In: Szallasi A, editor. TRP channels as therapeutic targets: from basic science to clinical use. Academic Press; 2015. pp. 167–187. [Google Scholar]
- 52.Rooney L, Vidal A, D’Souza A-M, Devereux N, et al. Discovery, Optimization, and Biological Evaluation of 5-(2-(Trifluoromethyl)phenyl)indazoles as a Novel Class of Transient Receptor Potential A1 (TRPA1) Antagonists. Journal of Medicinal Chemistry. 2014;57:5129–5140. doi: 10.1021/jm401986p. [DOI] [PubMed] [Google Scholar]
- 53.Eid SR, Crown ED, Moore EL, Liang HA, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, et al. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- 55.Trevisan G, Materazzi S, Fusi C, Altomare A, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73:3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- 56.Banzawa N, Saito S, Imagawa T, Kashio M, et al. Molecular Basis Determining Inhibition/Activation of Nociceptive Receptor TRPA1 Protein A SINGLE AMINO ACID DICTATES SPECIES-SPECIFIC ACTIONS OF THE MOST POTENT MAMMALIAN TRPA1 ANTAGONIST. Journal of Biological Chemistry. 2014;289:31927–31939. doi: 10.1074/jbc.M114.586891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klement G, Eisele L, Malinowsky D, Nolting A, et al. Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore. Biophys J. 2013;104:798–806. doi: 10.1016/j.bpj.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakatsuka K, Gupta R, Saito S, Banzawa N, et al. Identification of molecular determinants for a potent mammalian TRPA1 antagonist by utilizing species differences. J Mol Neurosci. 2013;51:754–762. doi: 10.1007/s12031-013-0060-2. [DOI] [PubMed] [Google Scholar]
- 59.Lishko PV, Procko E, Jin X, Phelps CB, et al. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 60.Lau SY, Procko E, Gaudet R. Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J Gen Physiol. 2012;140:541–555. doi: 10.1085/jgp.201210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 62.Lee G, Abdi K, Jiang Y, Michaely P, et al. Nanospring behaviour of ankyrin repeats. Nature. 2006;440:246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 63.Liang X, Madrid J, Gartner R, Verbavatz JM, et al. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr Biol. 2013;23:755–763. doi: 10.1016/j.cub.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 64.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108:E1184–E1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macpherson LJ, Dubin AE, Evans MJ, Marr F, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 67.Terrak M, Kerff F, Langsetmo K, Tao T, et al. Structural basis of protein phosphatase 1 regulation. Nature. 2004;429:780–784. doi: 10.1038/nature02582. [DOI] [PubMed] [Google Scholar]
- 68.Michaely P, Tomchick DR, Machius M, Anderson RG. Crystal structure of a 12 ANK repeat stack from human ankyrinR. The EMBO journal. 2002;21:6387–6396. doi: 10.1093/emboj/cdf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Wei Z, Chen K, Ye F, et al. Structural basis of diverse membrane target recognitions by ankyrins. eLife. 2014;3:e04353. doi: 10.7554/eLife.04353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jabba S, Goyal R, Sosa-Pagan JO, Moldenhauer H, et al. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82:1017–1031. doi: 10.1016/j.neuron.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang K, Panzano VC, Chang EC, Ni L, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong L, Bellemer A, Yan H, Ken H, et al. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SH, Lee Y, Akitake B, Woodward OM, et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176. doi: 10.1074/jbc.M111.288993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY. Identification of in vivo disulfide conformation of TRPA1 ion channel. Journal of Biological Chemistry. 2012;287:6169–6176. doi: 10.1074/jbc.M111.329748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lupas AN, Gruber M. The structure of α-helical coiled coils. Advances in protein chemistry. 2005;70:37–38. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 79.Zurborg S, Yurgionas B, Jira JA, Caspani O, et al. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 80.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 81.Sura L, Zima V, Marsakova L, Hynkova A, et al. C-terminal acidic cluster is involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J Biol Chem. 2012;287:18067–18077. doi: 10.1074/jbc.M112.341859. [DOI] [PMC free article] [PubMed] [Google Scholar]