Abstract
Malaria and schistosomiasis coinfections are common, and chronic schistosomiasis has been implicated in affecting the severity of acute malaria. However, whether it enhances or attenuates malaria has been controversial due the lack of appropriately controlled human studies and relevant animal models. To examine this interaction, we conducted a randomized controlled study using the baboon (Papio anubis) to analyze the effect of chronic schistosomiasis on severe malaria. Two groups of baboons (n = 8 each) and a schistosomiasis control group (n = 3) were infected with 500 Schistosoma mansoni cercariae. At 14 and 15 weeks postinfection, one group was given praziquantel to treat schistosomiasis infection. Four weeks later, the two groups plus a new malaria control group (n = 8) were intravenously inoculated with 105 Plasmodium knowlesi parasites and monitored daily for development of severe malaria. A total of 81% of baboons exposed to chronic S. mansoni infection with or without praziquantel treatment survived malaria, compared to only 25% of animals infected with P. knowlesi only (P = 0.01). Schistosome-infected animals also had significantly lower parasite burdens (P = 0.004) than the baboons in the P. knowlesi-only group and were protected from severe anemia. Coinfection was associated with increased spontaneous production of interleukin-6 (IL-6), suggesting an enhanced innate immune response, whereas animals infected with P. knowlesi alone failed to develop mitogen-driven tumor necrosis factor alpha and IL-10, indicating the inability to generate adequate protective and balancing immunoregulatory responses. These results indicate that chronic S. mansoni attenuates the severity of P. knowlesi coinfection in baboons by mechanisms that may enhance innate immunity to malaria.
INTRODUCTION
Polyparasitism is common in low-resource countries, especially in sub-Saharan Africa. Coinfections may alter disease outcomes and affect both drug and vaccine efficacies (1, 2). The majority of studies on coinfections have focused on interactions between malaria and chronic helminth infections. Interest in studying malaria and schistosomiasis, in particular, comes from the major overlap of the two diseases in areas where they are endemic and the fact that these infections account for a large proportion of global morbidity and mortality (3, 4). Because of the chronicity of schistosome infection, most studies have focused on its impact on susceptibility to clinical malaria.
Studies on malaria and schistosomiasis coinfections in human and animal models have generated contradicting results. Although some animal studies have shown that chronic schistosomiasis protects against severe malaria (5), others have reported that a concurrent schistosome infection enhances the severity of malaria (6–10). Many factors may account for these differences, including mouse strain, duration and intensity of the helminth infection, Plasmodium strain or species, inoculum size, or route of malaria infection (skin or blood stage) (11). An important limitation of murine studies is the difficulty of establishing chronic schistosome infections comparable to those observed in humans. Results in human studies have also been conflicting. Some studies have shown that helminth infections protect against malaria-associated morbidity (12–17), while others indicate that coinfections enhance clinical malaria (18–22) or have no influence on malaria outcome (23, 24). A number of factors can contribute to differing results, such as parasite exposure (large single or repeated smaller infections), age, the duration of prior infections, and the methods of data collection and analysis (25). In addition, due to ethical constraints of interventional trials in humans, most human studies are observational or associational, with many confounding variables. Therefore, determination of whether and how helminth infections affect the course of malaria infection requires randomized controlled studies performed using animal models that closely mimic human diseases.
Baboons (Papio anubis) are natural hosts of Schistosoma mansoni and can be experimentally infected with Plasmodium knowlesi; thus, baboons provide an excellent model for studying malaria and worm coinfections (26). The pathology and immune responses to chronic S. mansoni in baboons mimic those observed in humans (27, 28). The nonhuman primate malaria P. knowlesi readily infects baboons, producing symptoms of severe malaria similar to those seen in humans (29). Indeed, P. knowlesi has recently been identified as a major cause of human malaria in some areas of Southeast Asia (30, 31). This study was therefore designed to use nonhuman primates to determine the dynamic pathological and immunological interactions between a common chronic helminth infection (S. mansoni) and a malaria parasite (P. knowlesi).
MATERIALS AND METHODS
Parasites.
S. mansoni parasites used for experimental schistosomiasis infection were originally collected from school children in the Kibwezi district, Kenya, and maintained as long-term chronic infections in baboons at the Institute of Primate Research (IPR [www.primateresearch.org]). For malaria infection, cryopreserved P. knowlesi H strain parasites were retrieved from liquid nitrogen and cultured overnight to determine viability and inoculum size. The original parasite was clone Pk1(A+), which was initially passaged in rhesus monkeys (32).
Subjects.
Male and female subadult olive baboons (Papio anubis; ranging between 5.7 and 10.5 kg in body weight), originally from the Mount Kenya region of Kenya, were housed at the IPR according to institutional standards and guidelines for primate welfare and housing. These guidelines were developed based on the following references: the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences in 1985; appendix A of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Scientific Purposes (ETS 123, 2006); various international regulations and resources (e.g., the Convention for International Trade in Endangered Species, the U.S. Guide for the Care and Use of Laboratory Animals, and the European Primate Resources Network/Primate Vaccine Evaluation Network [PVEN]); the Association for Assessment and Accreditation of Laboratory Animal Centres; and the Statement of Compliance with Standards for Humane Care and Use of Laboratory Animals by Foreign Institutions (identification number A5796-01) issued by the National Institutes of Health (NIH) Office of Laboratory Animal Welfare, which covers all activities supported by the U.S. Public Health Service involving live vertebrate animals.
Animals were housed in outdoor group cages for the greater part of the experiment to allow for normal social behavior. Once a week they were caged individually to avoid contamination of stool samples collected for determination of schistosome ova burdens. Malaria infection was carried out in a biocontainment animal unit fitted with both group and single cages. Animals were moved to the unit 4 weeks before malaria infection (BMI) to allow animals to acclimatize to the new environment. All group animal houses and isolation units were designed to allow adequate natural light and dark cycles with ad libitum access to water. Animals were fed daily with monkey cubes (Unga Farm Care, Ltd., Nairobi, Kenya), supplemented with assorted vegetables and fruits.
At the beginning of the studies, baboons that were included in the experiments were subjected to general examination for ectoparasites and dermatophytosis, microscopic examination of stool and blood samples for helminth and protozoan infections, and tuberculosis (tuberculin) and simian immunodeficiency virus tests (by enzyme-linked immunosorbent assay [ELISA]). In addition, the absence of schistosome and P. knowlesi infection was determined by screening for antischistosome and anti-P. knowlesi antibodies using an ELISA technique.
Infection and treatment protocols.
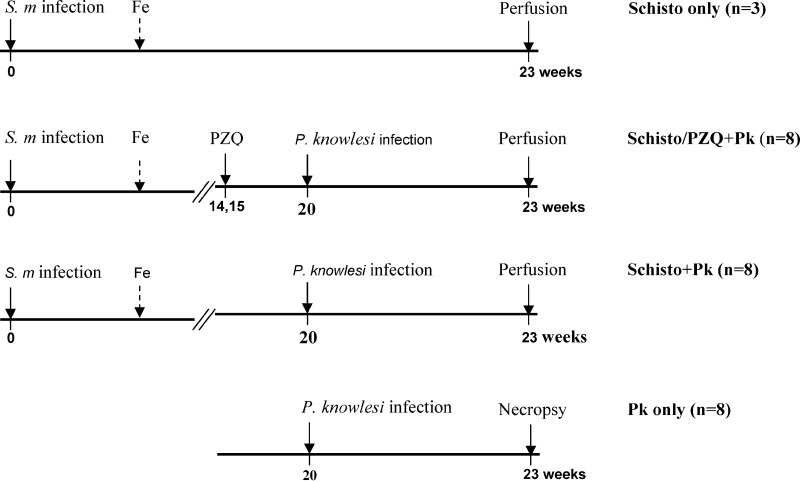
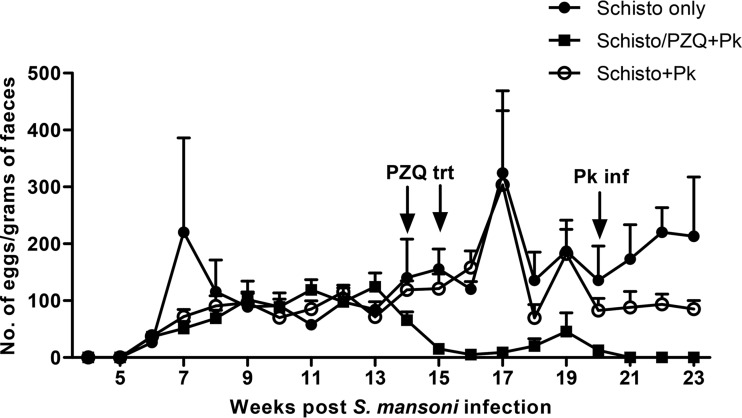
Twenty-seven baboons were randomly assigned into four experimental groups (Fig. 1). In three groups (S. mansoni only [Schisto only], n = 3; S. mansoni/praziquantel plus P. knowlesi [Schisto/PZQ+Pk], n = 8; Schisto+Pk, n = 8), baboons were initially infected percutaneously with 500 S. mansoni cercariae as previously described (33, 34). During the acute stage of the disease (8 to 10 weeks), animals were given iron supplements (1-ml iron dextran injection 10% [wt/vol]; Eagle Vet Tech. Co., Ltd., South Korea) for 7 days and 2 ml of multivitamins (Hebei Yuanzheng Pharmaceuticals Co., Ltd., Hebei Province, China) for 3 days to treat anemia and loss of appetite caused by schistosomiasis infection. At weeks 14 and 15 after infection, the Schisto/PZQ+PK group was treated twice with praziquantel (PZQ; Balcitricide, Bayer Schering Pharma) at a dose of 60 mg/kg (body weight) delivered by oral intubation. The presence and intensity of schistosome infection were monitored weekly (beginning at 4 weeks) by stool examination of ova by the Kato-Katz technique (35). Prior to P. knowlesi infection, seven of eight baboons became ova negative after PZQ treatment and one baboon had significant reduction in the ova recovered in the stools (peak of 124 eggs/g of feces to 53 eggs/g; Fig. 2).
FIG 1.
Experimental protocol. Four groups of experimental animals were included in the coinfection study: (i) animals infected with S. mansoni cercariae only (Schisto only); (ii) animals infected with schistosomiasis, followed by treatment with praziquantel (PZQ) and later infected with P. knowlesi (Schisto/PZQ+Pk); (iii) animals infected with S. mansoni (without PZQ treatment), followed by P. knowlesi infection (Schisto+Pk); and (iv) animals infected with P. knowlesi only (Pk only). Animals with a schistosome infection were treated with iron supplementation (Fe) between 8 and 10 weeks after schistosome infection.
FIG 2.
S. mansoni egg counts in baboons with schistosomiasis. Treatment with praziquantel (PZQ trt) was administered to the Schisto/PZQ+Pk group at weeks 14 and 15 postinfection. Infection with P. knowlesi (Pk inf) occurred at week 20 after schistosomiasis infection.
At week 20 after schistosome infection, the Schisto/PZQ+Pk group, the Schisto+Pk group, and an additional malaria control group (P. knowlesi only, n = 8) were inoculated intravenously (i.v.) with 105 P. knowlesi parasites as previously described (29) and monitored daily for clinical signs of severe malaria. During this period, parasitemia, loss of appetite, lethargy, pale mucous membranes, and the occurrence of hemoglobinuria, tachypnea, and fever were determined. As recommended by the attending veterinarian and international best practices for animal welfare, the animals were humanely euthanized once a parasitemia of >5% was recorded and the animals were lethargic and presented with one other clinical parameter indicative of severe malaria. Baboons that did not succumb to severe malaria were euthanized at the end of the experimental period (week 23 after schistosome infection). Euthanasia was induced by first sedating the animals with a mixture of ketamine hydrochloride and xylazine at a dose of 10 mg/kg ketamine-HCl and a dose of 0.5 mg/ml xylazine administered intramuscularly (adding 0.5 ml of 100 mg/ml xylazine to a 10-ml bottle of ketamine [100 mg/ml], dosed at 0.1 ml/kg). The animals were then injected with sodium pentobarbitone (Eutha-Naze; Bayer HealthCare) i.v. at 100 mg/kg. In order to recover adult schistosome worms, exsanguination by portal perfusion was performed on animals in the three groups infected with schistosomiasis. Necropsy was performed on baboons infected with P. knowlesi only. Blood for hematology, immunological, and growth inhibition assays was collected before schistosome and malaria infection, before and after treatment with PZQ, and at the time of euthanasia.
Assessment of severe malaria infection.
From day 3 after P. knowlesi infection, daily parasite counts were recorded by microscopic examination of Giemsa-stained thin blood smears collected by pricking the skin on the rear end of each animal. Parasitemia was expressed as number of parasites per 10,000 red blood cells (RBCs). Other gross observations included appetite, demeanor or prostration, the color of mucous membranes, the color and consistency of urine and stool, the depth and rate of breathing, vomiting, and the general body condition of the animal. Loss of appetite was measured qualitatively by observing the amount of food not eaten (>30%), and lethargy was (demeanor) determined based on whether animals were bright and active or dull and listless. The conjunctiva and oral mucosa were observed as pink and moist or pale. The presence of bloody urine indicated hematuria, raised fur suggested fever, and labored breathing indicated tachypnea. Defining clinical features of severe malaria for the baboons included parasite burdens of >500 parasite-infected erythrocytes per 10,000 RBCs (5%) and one or more of the listed features, including tachypnea with labored breathing, lethargy, loss of appetite, vomiting, and severe anemia hemoglobin (Hb; <5 g/dl), as previously observed by Ozwara et al. (29) and modified as published by the World Health Organization (36). After autopsy, evidence of cerebral malaria was determined by presence of sequestered red parasitized cells in the brain blood vessels as described by Ozwara et al. (29).
Hematology.
EDTA blood was collected at each sampling point, and Hb levels, as a measure of anemia, were recorded by use of a coulter counter (ACT5diffCP; Beckman Coulter, Inc., Villepinte, France).
Cell cultures.
Cell cultures for determination of T cell reactivity and cytokine production were set up as previously described (37). Briefly, peripheral blood mononuclear cells (PBMCs) were separated from whole blood mixed in 1:2 part Alsever solution (2.05% dextrose, 0.8% trisodium citrate, 0.055% citric acid, and 0.42% sodium chloride) by centrifugation at 2,000 rpm and 24°C for 20 min with Ficoll Paque (Pharmacia Ciotech, St. Albans, United Kingdom), washed twice in phosphate-buffered saline (PBS; 1,500 rpm and 4°C for 10 min), and resuspended in 10 ml of complete medium (RPMI 1640; Sigma-Aldrich, St. Louis, MO) enriched with 10% heat-inactivated fetal bovine serum (Gibco, Canada), 2 mM l-glutamine (Fisher Biotech, Wembley, West Australia, Australia), 25 mM HEPES (Sigma-Aldrich, Dorset, United Kingdom), 80 μg/ml gentamicin (Sigma-Aldrich, Dorset, United Kingdom), and 0.22% (vol/vol) sodium bicarbonate (Sigma-Aldrich, St. Louis, MO). For cytokine cell cultures, the cell concentration was adjusted to 2 × 106 cells/ml and aliquoted (1 ml/well) into flat-bottomed 24-well microtiter plates. Then, schistosome adult worm antigen (SWAP), schistosome egg antigen (SEA), soluble P. knowlesi lysate, and lipopolysaccharide (LPS; Sigma-Aldrich) at 20 μl/well (5 μg/ml) were added to the test wells. The same amount of concanavalin A (ConA; Sigma-Aldrich) at 5 μg/ml was added to the positive-control wells, whereas negative-control wells contained medium alone. Plates were incubated at 37°C in 5% CO2 for 72 h for the measurement of interferon gamma (IFN-γ), interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNF-α) and for 24 h for LPS stimulation. The supernatants were harvested and stored at −80°C for cytokine analysis.
Cytokine assays.
The protocols for measurement of cytokines in baboon cell cultures have previously been described (37). Briefly, cytokine cell culture supernatants were tested for the presence of IFN-γ, IL-6, IL-10, and TNF-α by sandwich enzyme-linked immunosorbent assay (ELISA). Flat-bottomed 96-well ELISA plates (Immunolon 4 HBX, USA) were coated with IFN-γ (2 μg/ml), IL-6 (2 μg/ml), IL-10 (1:1,000), and TNF-α (1:1,000) capture antibodies at 50 μl/well diluted in 1× PBS (0.8% NaCl, 0.02% KCl, 0.144% Na2HPO4, and 0.024% KH2PO4), followed by incubation overnight at 4°C. The plates were washed twice with PBS, blocked with 0.1% bovine serum albumin (Sigma-Aldrich) in PBS-Tween 20 (0.05% [PBS-T]) at 100 μl/well, and incubated for 1 h at 37°C. The plates were washed five times with PBS-T and 50 μl of standards/well, and then cytokine culture supernatants were added in duplicates, followed by incubation at 37°C for 2 h. The plates were washed five times, and then 50 μl of biotinylated capture antibody (IFN-γ, 1 μg/ml; IL-6, IL-10, and TNF-α, 1:1,000; diluted in blocking buffer) was added per well, followed by incubation for 1 h at 37°C. Next, 50 μl of streptavidin-labeled horseradish peroxidase (1:1,000 dilution) was added per well, followed by incubation for 1 h at 37°C. The plates were developed for 40 min at room temperature by adding the substrate TMB (3,3′,5,5′-tetramethylbenzidine; Kirkegaard and Perry Labs, Gaithersburg, MD) at 50 μl/well, and the absorbance was read at 630 nm. The cytokine concentration was calculated from standard curves using best-fit formulae. IFN-γ and IL-6 cytokine kits were human reagents from Mabtech AB (Nacka Strand, Sweden), and IL-10 and TNF-α cytokine kits were monkey reagents from U-Cy Tech Biosciences (Utrecht, Netherlands).
Growth inhibition assays.
Growth inhibition assays were performed to determine whether factors in serum of animals infected with schistosomiasis had the ability to prevent or significantly inhibit growth of P. knowlesi. Malaria cultures were set up at 100 μl of 0.7% parasitemia and 4% hematocrit and incubated for 48 h. Sera were heat inactivated at 56°C for 30 min and used at 5% test sera plus 5% human antibody (HuAb) sera. Both positive (growth inhibition, 100 ng/ml chloroquine [CQ]) and negative (growth promotion, 10% HuAb serum) controls were included in the assay. To record parasitemia, RBCs were stained using ethidium bromide as previously described (38). DiIC1-5 (1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide) was included in the staining protocol for the detection of live parasites (39). Parasitemia was read at 0 and 48 h on a Becton-Dickinson LSRII flow cytometer (Franklin Lakes, NJ). The gating strategy is shown in the supplemental material (see Fig. S1 in the supplemental material). The percent growth inhibition was calculated as follows: (%P of HuAb at 48 h − %P of HuAb at 0 h) − [(%P of test at 48 h − %P of test at 0 h)/(%P of HuAb at 48 h − %P of HuAb at 0 h) × 100], where %P represents the percent parasitemia.
Statistical analysis.
All statistical analysis was performed using Stata10 software (40). The survival outcome in the four groups of baboons was analyzed using the log-rank test. Differences in mean parasitemia and immunological values were analyzed using one-way analysis of variance with post hoc Bonferroni analysis. Values were termed significantly different at a P value of <0.05.
Ethical statement.
This study and all experimental protocols were approved by the Institutional Science and Ethics Committee (IRC-ACUC) of the IPR, Karen, Nairobi, Kenya (study IRC/12/2008), whose membership is constituted based on guidelines issued by the World Health Organization for committees that review biomedical research, by the NIH, by PVEN, and by the Helsinki Convention on the Humane Treatment of Animals for Scientific Purposes. The IRC-ACUC is nationally registered by the National Commission for Science, Technology, and Innovation, Kenya.
RESULTS
Coinfection with S. mansoni protects baboons from severe malaria.
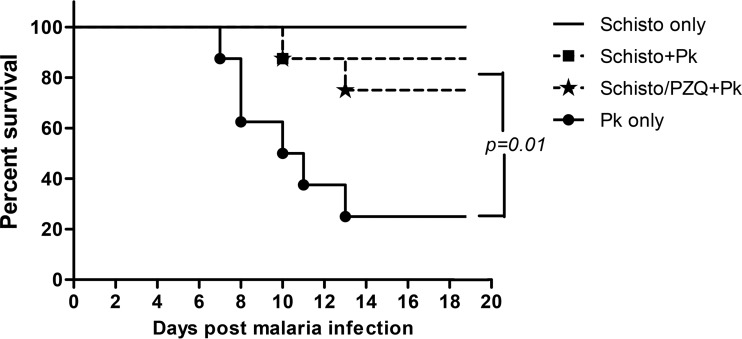
To investigate whether chronic schistosomiasis in malaria-naive animals can protect against severe P. knowlesi malaria, baboons with chronic schistosomiasis were challenged i.v. with 105 P. knowlesi blood-stage parasites. Six of eight animals (75%) infected with P. knowlesi alone (see Fig. 3) developed fever, loss of appetite, anemia, and organ failure associated with severe malaria between days 7 and 13 after infection. All six animals were euthanized when they attained parasitemia levels of >5% (this level corresponded to a degree of infection that would eventually lead to death, based on prior studies [29]). In contrast, only one of eight baboons (12.5%) chronically infected with schistosomiasis acquired severe malaria requiring euthanasia (Schisto+Pk, Fig. 3). A third group, schistosome-infected animals that were treated with praziquantel 5 weeks prior to P. knowlesi infection, produced results similar to those for the Schisto+Pk group, in that only two of eight animals (25%) developed severe malaria and were euthanized 10 and 13 days after infection. Overall, chronic schistosomiasis protected 13 of 16 animals (81.25%) from severe P. knowlesi, compared to two of eight animals (25%) without schistosomiasis that survived P. knowlesi infection (P = 0.01). Baboons infected only with schistosomiasis exhibited schistosomiasis-related morbidity, including a loss of appetite, lethargy, and bloody stool during the acute stage of the disease that resolved at the chronic phase of illness (i.e., ca. 10 weeks postinfection).
FIG 3.
Proportion of baboons surviving P. knowlesi malaria with or without prior S. mansoni infection. See the legend of Fig. 1 for a description of the various experimental groups.
Baboons chronically infected with schistosomiasis also demonstrated reduced parasitemia levels. Parasitemia reached levels that led to fatal malaria (>500 infected erythrocytes [iRBCs]/104 erythrocytes [RBCs], or >5%) in six of eight baboons infected with P. knowlesi only, whereas three of sixteen schistosome-coinfected baboons acquired fatal levels of malaria (Fig. 4). Based on these observations, baboons exposed to schistosomiasis exhibited significantly lower peak P. knowlesi parasitemia levels (Schisto+Pk, mean parasitemia = 80 iRBCs/104 RBC; Schisto/PZQ+Pk, mean parasitemia = 220 iRBCs/104 RBCs) than the P. knowlesi-only group (mean parasitemia = 1,410 iRBCs/104 RBCs) (P = 0.004). Thus, the presence of chronic schistosomiasis significantly protected against high parasitemia levels and death from an overwhelming P. knowlesi infection.
FIG 4.
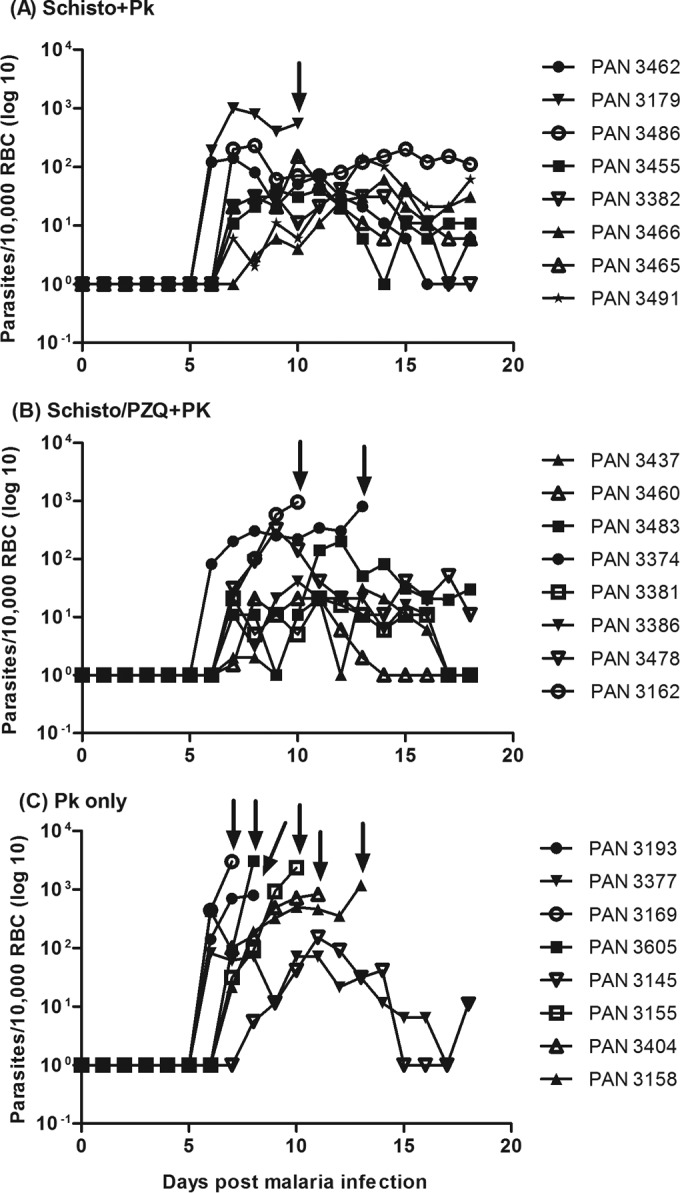
Daily P. knowlesi parasitemia after i.v. infection of 105 infected erythrocytes. Parasitemia was determined daily and expressed as number of parasites in 10,000 RBCs and graphed on a log scale. Truncated parasitemia levels (prior to day 18), indicated by arrows, show the results for a baboon that had to be euthanized (e.g., all but two animals in the P. knowlesi-only group). Animals that did not succumb to severe malaria were euthanized at day 18 after malaria infection.
Schistosomiasis protects animals from severe anemia.
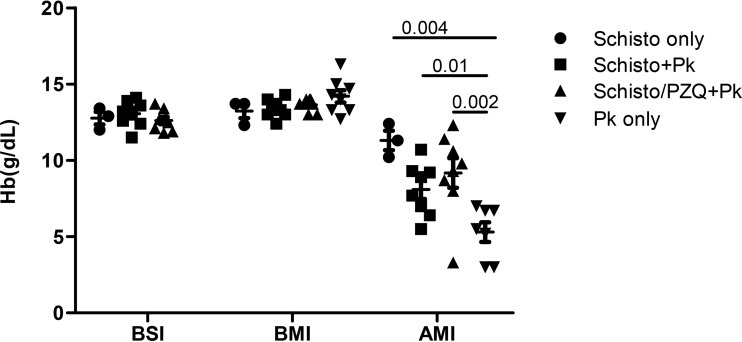
To assess the impact of schistosomiasis and subsequent P. knowlesi infection, hemoglobin (Hb) levels were measured before schistosomiasis infection, before malaria infection, and at the time of euthanasia, 7 to 23 days after P. knowlesi infection (Fig. 5). Animals infected with S. mansoni were given iron supplements to treat schistosomiasis-associated anemia. The mean Hb levels obtained before schistosome infection (BSI; week 0, Schisto only, 12.8 g/dl; Schisto+Pk, 13.1 g/dl; Schisto/PZQ+Pk, 12.6 g/dl) and before malaria infection (BMI; week 20, Schisto only, 13.2 g/dl; Schisto+Pk, 13.4 g/dl; Schisto/PZQ+Pk, 13.6 g/dl; P. knowlesi only, 14.2 g/dl) were similar among the four experimental groups as shown in Fig. 5. Schistosome and/or malaria infections reduced the Hb levels. This was most striking in baboons infected with P. knowlesi only, resulting in the development of severe anemia (average Hb level, 4.7 g/dl). Prior schistosome infection protected most animals from severe anemia. Compared to the P. knowlesi-only group, P. knowlesi-infected baboons with chronic schistosomiasis had almost twice the Hb levels, with averages of 8.7 and 8.6 g/dl (P = 0.01 and P = 0.002). The Hb levels negatively correlated with the P. knowlesi parasitemia levels at the time of euthanasia (r = −0.745, P = 0.007). Thus, schistosomiasis protected animals from developing severe anemia in response to P. knowlesi malaria.
FIG 5.
Impact of S. mansoni and P. knowlesi coinfection on Hb levels. Shown are the hemoglobin values in grams per deciliter for each animal, taken before S. mansoni infection (BSI), at week 20 after S. mansoni infection and before malaria infection (BMI) for the Schisto-only, Schisto+Pk, and Schisto/PZQ+Pk groups, at and time zero (naive) for the P. knowlesi-only group. The hemoglobin levels after P. knowlesi infection (AMI) were determined at endpoint (7 to 23 days after P. knowlesi infection) for the Schisto+Pk, Schisto/PZQ+Pk, and P. knowlesi-only (Pk only) groups. For the Schisto-only group, the final Hb levels were taken at week 23 after S. mansoni infection.
Chronic S. mansoni enhances innate immune and immunoregulatory responses.
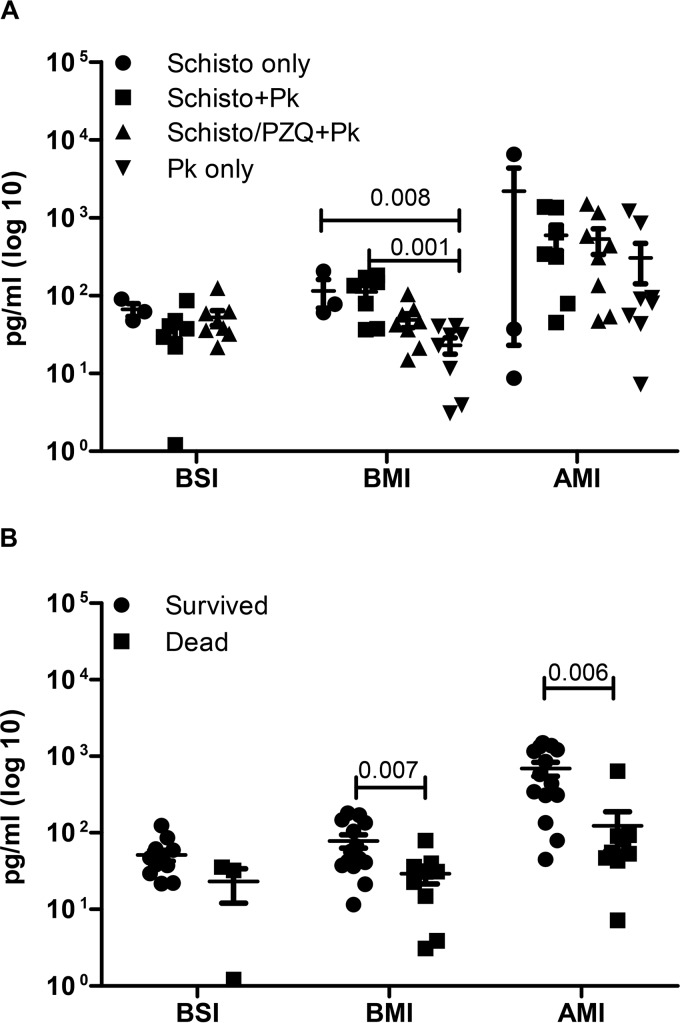
In order to determine the role of proinflammatory cytokines in protection or increased pathology to malaria infection, we measured the levels of IFN-γ, IL-6, IL-10, and TNF-α in PBMC supernatants after stimulation with schistosome and P. knowlesi antigens. The results showed very low net levels of cytokines produced in response to schistosome (SWAP and SEA) or crude P. knowlesi antigen preparations in vitro after these infections, with no significant differences at any of the time points (data not shown). However, animals with chronic schistosomiasis (i.e., the Schisto-only and Schisto+Pk groups) induced increased spontaneous levels of IL-6 from PBMC cultures (P = 0.009; Schisto only [P = 0.445] and Schisto+Pk [P = 0.014] compared to the period prior to schistosomiasis infection; Fig. 6A). Schistosome-infected animals treated with praziquantel failed to show increased spontaneously released IL-6 in culture supernatants (Schisto/PZQ+Pk, P = 0.831). Interestingly, animals that survived malaria had significantly higher IL-6 levels than animals that died (P = 0.006, Fig. 6B). This trend for higher IL-6 levels occurred in animals before they were challenged with malaria (BMI, P = 0.007). Thus, increased spontaneous IL-6 production in mixed-lymphocyte cultures with chronic schistosomiasis is associated with protection from death after P. knowlesi infection.
FIG 6.
Increased spontaneous IL-6 production is associated with active schistosomiasis infection and correlates with reduced risk of fatal malaria. IL-6 production in unstimulated PBMC cultures before S. mansoni infection (BSI), before malaria infection (BMI), and after P. knowlesi infection (AMI) was measured in culture supernatants from individual animals. (A) Cytokine production was compared between the different groups of animals. (B) Animals were grouped according to whether they succumbed to severe malaria (dead; AMI, 7 to 13 days after P. knowlesi infection) or recovered (survived; AMI, 23 days after P. knowlesi infection). The two groups were compared using a Student t test of log-transformed values.
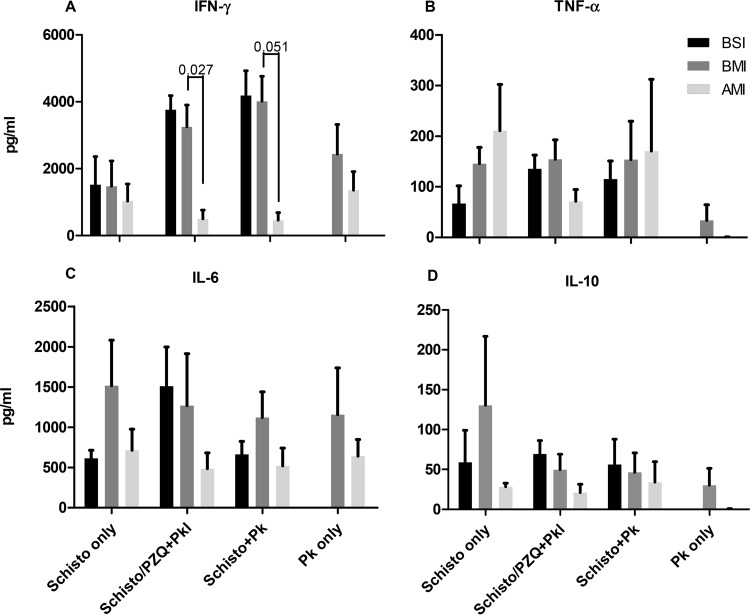
Since infections with multiple pathogens have the potential to produce generalized suppression of the host's immune system, we evaluated the effect of malaria and schistosomiasis coinfection on mitogen-induced cytokine production. Baboon PBMCs were stimulated with the T cell mitogen concanavalin A (ConA) and culture supernatants tested for IFN-γ, IL-6, IL-10, and TNF-α. There was no significance difference in cytokine levels in all groups before schistosome or malaria infection. However, the presence of chronic schistosomiasis was associated with significantly decreased IFN-γ levels after P. knowlesi infection compared to levels for schistosome-uninfected baboons (Fig. 7A, Schisto+Pk [P = 0.027] and Schisto/PZQ+Pk [P = 0.051]). Of note, P. knowlesi-infected animals without prior schistosome infection failed to produce detectable ConA-driven TNF-α and IL-10 responses compared to animals infected with schistosomiasis (Fig. 7B and D). This suggests a diminished expansion of immunoregulatory subset of T cells and failure to generate sufficient innate and early adaptive immune responses to protect animals from high P. knowlesi parasitemia.
FIG 7.
Schistosome-infected baboons demonstrate reduced ConA-induced IFN-γ production and sustained TNF-α and IL-10 release after P. knowlesi infection. Plotted are the average concentrations and standard errors of the mean for the IFN-γ (A), TNF-α (B), IL-6 (C), and IL-10 (D) levels for each group of animals, before schistosomiasis infection (BSI), before malaria infection (BMI), and after malaria infection (AMI). Cultures of PBMCs were stimulated with ConA for 3 days.
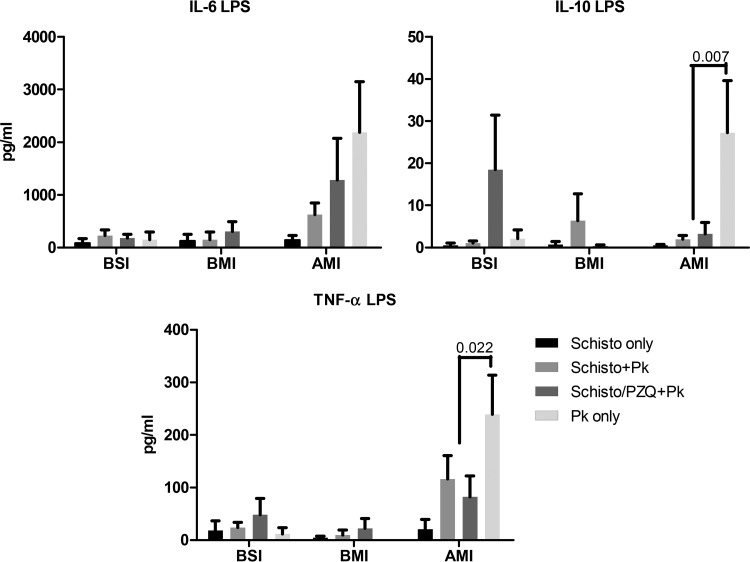
To determine the impact of chronic schistosomiasis on innate immune responses, PBMCs were collected both before schistosome infection and before and after malaria infection, and IL-6, IL-10, and TNF-α levels were measured 24 h after the addition of LPS. Chronic schistosomiasis infection failed to enhance LPS-induced cytokine response by PBMC prior to malaria infection (Fig. 8). LPS-stimulated PBMCs displayed increased IL-6 and TNF-α levels after malaria infection (AMI) compared to BMI in all three groups of baboons infected with malaria. On the other hand, the IL-10 levels were significantly increased AMI in the P. knowlesi-only group (P = 0.035). At this time point, the levels of LPS-driven IL-10 (P = 0.007; Schisto+Pk [P = 0.057] and Schisto/PZQ+Pk [P = 0.034]) and TNF-α (P = 0.022; Schisto+Pk [P = 0.1] and Schisto/PZQ+Pk [P = 0.037]) were significantly lower than in the P. knowlesi-only group. Thus, schistosomiasis infection may attenuate LPS induced immune responses after P. knowlesi infection.
FIG 8.
Prior schistosome infection attenuates LPS-induced TNF-α and IL-10 production after P. knowlesi infection. The average cytokine levels of IL-6, IL-10, and TNF-α were recorded before schistosome infection (BSI) and before (BMI) and after (AMI) malarial infection.
Serum from baboons with chronic schistosomiasis fails to block parasite invasion and growth in vitro.
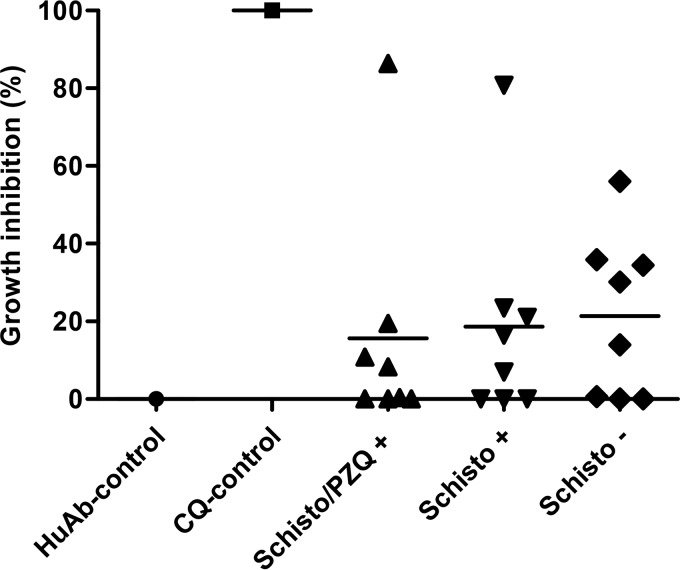
Since baboons with chronic schistosome infections were significantly protected from high-density parasitemia and anemia due to severe malaria, we examined whether chronic schistosome infections produced a serum factor associated with innate immune response that might inhibit P. knowlesi invasion and the growth of host erythrocytes in vitro. P. knowlesi was cultured with sera from baboons chronically infected with schistosomes, with or without PZQ treatment (i.e., the Schisto+Pk and Schisto/PZQ+Pk groups), or schistosomiasis-negative animals (i.e., the P. knowlesi-only group). Two animals exposed to chronic schistosomiasis with or without PZQ treatment exhibited high blocking activities (>80%), which correlated with a failure to develop severe malaria (Fig. 9). Conversely, all animals that succumbed to severe malaria from the Schisto+Pk and Schisto/PZQ+Pk groups demonstrated low or no inhibition capabilities (<21%). In the P. knowlesi-only group, the two animals without severe malaria and two that developed severe malaria moderately inhibited the growth of malaria parasites (35.8 to 56%). Although serum derived from animals before or after schistosome infections variably inhibited malaria parasite invasion or growth, this was not associated with chronic schistosomiasis.
FIG 9.
Growth inhibition of P. knowlesi parasites. Growth inhibition in the three groups of animals was determined at 20 weeks after infection with S. mansoni for the Schisto/PZQ+ and Schisto+ groups and before malaria infection in animals with malaria only (Schisto −). For the negative control (inducing growth), cultures were grown in commercially available HuAb serum. For the positive control (growth inhibition), chloroquine (CQ) was added to the culture medium.
DISCUSSION
This study has shown that chronic schistosomiasis protects against severe P. knowlesi malaria and death in a nonhuman primate model. Protection was directly related to a reduction in P. knowlesi parasitemia. Death was a consequence of the accumulation of large numbers of infected erythrocytes in critical organs, such as the brain and spleen, based on autopsies of euthanized animals that showed congestion, edema, and sequestration of parasites in the blood vessels, as previously described (29), and severe anemia. Animals with chronic schistosomiasis were protected from severe malaria by at least three possible inter-related mechanisms: (i) activation of innate immune responses, (ii) attenuation of potent proinflammatory responses, and (iii) protection from significant schistosomiasis-related anemia before malaria infection.
Activation of innate immune responses was evidenced by increased spontaneous production of IL-6 in schistosome-infected animals and was higher in animals that survived acute malaria infection. Notable was the failure of S. mansoni infection to enhance LPS-induced IL-6, TNF-α, and/or IL-10 release by PBMCs, indicating that enhanced IL-6 production resulting from schistosomiasis occurs by a Toll-like receptor 4-independent pathway. However, after malaria infection, the IL-6 levels were increased. In contrast, schistosome-infected baboons after malaria infection showed suppressed IL-10 and TNF-α production compared to animals not infected with schistosomes. This suggests that schistosome infection may attenuate the severe inflammatory response associated with acute malaria infection. In baboons infected with malaria only, high levels of LPS-stimulated IL-6, TNF-α, and the anti-inflammatory cytokine IL-10 were recorded. These animals developed severe malaria. A review by Pradhan and Ghosh (41) describes the role of the innate immune system in the production of proinflammatory cytokines that form an important basis for controlling parasite growth. The presence of IL-10, however, seems to have a negative effect in controlling malarial infection. Studies in mice have shown that the production of IL-10 and transforming growth factor β early in infection results in inhibition of the proinflammatory response, leading to high levels of parasitemia and severe anemia (42, 43). Hence, we speculate that the presence of low levels of IL-10 produced early during infection may have suppressed the production of sufficient amounts of proinflammatory cytokine necessary to facilitate parasite killing in animals with malaria only.
The spontaneous production of cytokines by unstimulated PBMCs has been described previously in healthy persons living in developing countries where there is high prevalence of human immunodeficiency virus, as well as mycobacterial, malarial, and other parasitic infections (44–47). Although IL-6 has been commonly associated with severe malaria (48–50), our study indicates the contrary, i.e., we found that significantly higher levels of this cytokine were present in animals that resisted severe malaria. Murine studies have yielded similar findings in which IL-6 mediated protection against the pre-erythrocytic stages of malaria by inducing the production of IL-1 and TNF-α, leading to increased levels of IgG antibodies which help in controlling parasitemia (51). Human studies have also reported a putatively protective role of IL-6 during malarial infection. For example, an early increase in IL-6 was recorded in volunteers vaccinated with irradiated sporozoites and later challenged with Plasmodium falciparum (52). These volunteers were fully protected from malarial infection. Other studies in Mali established an association between low circulating levels of IL-6 with hyperparasitemia and severe anemia (36), while another study in India found IL-6 levels to be inversely correlated with malaria severity (53). The elevated IL-6 levels associated with chronic schistosomiasis indicate enhanced activation of innate immune responses, and IL-6 may have a direct effect in protection against malaria infection. It cannot be excluded, however, that this relationship may only be an association and not causal.
Another aspect of the innate immune response is the activation of splenic macrophages critical for the clearance of malaria-infected erythrocytes (54). Splenic macrophages are one of the first lines of defense against blood-stage Plasmodium infection (54, 55). Chronic schistosomiasis is associated with elevation of alternatively activated macrophages (M2 macrophages) that are highly phagocytic (56). In addition, it has been shown that M2 macrophages exhibit immunoregulatory properties that can attenuate exaggerated inflammatory responses (57). In this study, we failed to demonstrate an increased phagocytosis of infected erythrocytes in the spleens of schistosome-coinfected animals at autopsy (data not shown), a finding probably due to the various times of autopsy (i.e., 23 days postinfection for protected animals with schistosomiasis and when parasitemia was beginning to resolve, whereas animals that succumbed from acute malaria were euthanized at 7 to 13 days after infection).
In the present study, protection against severe malaria was primarily related to a reduction in parasitemia. These results concur with human studies in Senegal, Mali, and Thailand, which showed protection against severe forms of malaria in individuals coinfected with schistosomes. Two studies in Senegal, looking at children aged 3 to 15 years with a light Schistosoma haematobium-Plasmodium falciparum coinfection showed decreased parasitemia compared to those with malaria only (15, 58). In Mali, low parasite density and delay in the first clinical symptoms were reported in the coinfected group (16), and in Thailand a similar group was protected from cerebral malaria (14). Protection against malaria parasitemia and disease requires both humoral and cellular immune responses, striking a balance between effector and immunoregulatory responses (59–61). There was no difference in cytokine production to crude S. mansoni or P. knowlesi antigens between the experimental groups, suggesting that adaptive immunity was unlikely to be involved. However, we noted that baboons infected with malaria only failed to develop ConA-driven TNF-α and IL-10, indicating impaired ability to stimulate a protective T-cell response. Interestingly, after malarial infection, ConA-induced IFN-γ was significantly reduced in the two groups of baboons with schistosomiasis compared to the malaria-only group, suggesting schistosome-induced immunoregulation. This immunoregulation does not appear to have impaired the ability of animals to clear parasites but may have attenuated some malaria-associated pathology, such as reduced immune-mediated RBC lysis of uninfected erythrocytes. Studies in Senegal, Ghana, and elsewhere have suggested a more anti-inflammatory environment in groups protected from severe malaria (24, 61–65). Thus, an early proinflammatory response may be important in controlling parasitemia. However, excessive and prolonged production of these cytokines may result in immunopathological reactions, as seen in animals with malaria only.
The acute stage of schistosomiasis is associated with bloody stools and chronic blood loss, resulting in mild anemia, as previously observed in baboons and humans (62). In the present study, iron supplementation in schistosome-infected animals served to partially maintain Hb levels. Iron supplementation itself may directly affect the course of malaria infection by increased risk (63), a protective role (64, 65), or no association (66, 67). These differences in outcomes may arise from the timing at which malaria supplementation is given (68). Iron given at the point of acute malaria may augment the severity of the disease by increasing the parasitemia levels, whereas iron given prior to infection may protect or reduce the risk of severe disease (e.g., yielding a higher RBC count) and/or enhanced immune effector function. It is possible that iron supplementation before malarial infection (>10 weeks) contributed to the protective effect of chronic schistosomiasis; however, the role of iron supplementation in otherwise healthy baboons is likely to be small.
A limitation of the present study is that the baboons were immunologically naive with respect to P. knowlesi infections and had insufficient time to acquire significant adaptive immune responses before they developed overwhelming parasitemias. Although in most cases of coinfections in humans, schistosome coinfections are likely to occur in individuals with various levels of naturally acquired immunity to malaria. How chronic schistosome infection affects the expression of existing immunity to malaria will be examined in upcoming experiments in baboons that have been previously exposed to P. knowlesi.
Although natural infection occurs via bites from infected mosquitoes, the malarial infection in the present study was achieved by i.v. inoculation of iRBCs. Our rationale for this approach derives from the fact that the primary targets of immunity are blood-stage infections responsible for malaria-associated morbidity, mortality, and disease transmission. Natural exposure to sporozoites induces a very weak immune response, and there is little evidence for naturally acquired immunity to sporozoites or early-stage infections (69, 70). Although there may be some innate immunity to sporozoites (71) that could be modified by chronic schistosome infection, even one successfully developing sporozoite can lead to a blood-stage infection, which was the focus of the present study.
In summary, our study shows that chronic schistosomiasis reduces P. knowlesi parasitemia and the risk of death associated with severe malaria in a nonhuman primate model of schistosomiasis and malaria. Such studies are important because they control for the first time important variables such the burden, duration, and stage of schistosome infections that confound human studies of schistosomiasis-malaria coinfections, leading to the variable results in the literature. How chronic schistosomiasis protects against severe malaria could not be clearly discerned from our studies. The observed increased levels of IL-6 in mixed-lymphocyte culture supernatants may be a biomarker of increased innate immune responses, leading to reduced parasitemia. There may also have been a role of chronic inflammation from persisting schistosome infection, leading to a reduced availability of free iron, and a component of immunoregulation, although most pathology was associated with overwhelming parasitemia. We also emphasize here the role of coinfections in understanding the spectrum of malarial disease in countries where malaria is endemic.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the contribution of South West National Primate Research Centre, Texas Biomedical Research Institute, San Antonio, TX, for providing the baboon blood used in the growth inhibition assays. We thank Paul Ogongo, Fred Nyundo, Simon Kiarie, James Ndung'u, Pricilla Kimiti, and the Animal Science Department at the IPR for their technical and animal welfare support.
Funding Statement
The project described was supported by award number 5R01AI075682 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00490-15.
REFERENCES
- 1.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. 2004. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun 72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 3.Mazigo HD, Nuwaha F, Kinung'hi SM, Morona D, Pinot de Moira A, Wilson S, Heukelbach J, Dunne DW. 2012. Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vectors 5:274. doi: 10.1186/1756-3305-5-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2013. World malaria report–2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Yan Y, Inuo G, Akao N, Tsukidate S, Fujita K. 1997. Downregulation of murine susceptibility to cerebral malaria by inoculation with third-stage larvae of the filarial nematode Brugia pahangi. Parasitology 114(Pt 4):333–338. doi: 10.1017/S0031182096008566. [DOI] [PubMed] [Google Scholar]
- 6.Graham AL, Lamb TJ, Read AF, Allen JE. 2005. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J Infect Dis 191:410–421. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- 7.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. 2005. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect Immun 73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legesse M, Erko B, Balcha F. 2004. Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghei-coinfected mice. Acta Trop 91:161–166. doi: 10.1016/j.actatropica.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida A, Maruyama H, Kumagai T, Amano T, Kobayashi F, Zhang M, Himeno K, Ohta N. 2000. Schistosoma mansoni infection cancels the susceptibility to Plasmodium chabaudi through induction of type 1 immune responses in A/J mice. Int Immunol 12:1117–1125. doi: 10.1093/intimm/12.8.1117. [DOI] [PubMed] [Google Scholar]
- 10.Lwin M, Last C, Targett GA, Doenhoff MJ. 1982. Infection of mice concurrently with Schistosoma mansoni and rodent malarias: contrasting effects of patent S. mansoni infections on Plasmodium chabaudi, P. yoelii, and P. berghei. Ann Trop Med Parasitol 76:265–273. [DOI] [PubMed] [Google Scholar]
- 11.Semenya AA, Sullivan JS, Barnwell JW, Secor WE. 2012. Schistosoma mansoni infection impairs antimalaria treatment and immune responses of rhesus macaques infected with mosquito-borne Plasmodium coatneyi. Infect Immun 80:3821–3827. doi: 10.1128/IAI.00590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. 2000. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol 22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 13.Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Vannaphan S, Traore B, Gay F, Looareesuwan S. 2001. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am J Trop Med Hyg 65:834–836. [DOI] [PubMed] [Google Scholar]
- 14.Nacher M, Singhasivanon P, Traore B, Vannaphan S, Gay F, Chindanond D, Franetich JF, Mazier D, Looareesuwan S. 2002. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am J Trop Med Hyg 66:304–309. [DOI] [PubMed] [Google Scholar]
- 15.Briand V, Watier L, Hesran J-Y, Garcia A, Cot M. 2005. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in Senegalese children? Am J Trop Med Hyg 72:702–707. [PubMed] [Google Scholar]
- 16.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, Guindo A, Traore K, Daou M, Diarra I, Sztein MB, Plowe CV, Doumbo OK. 2005. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg 73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 17.Murray J, Murray A, Murray M, Murray C. 1978. The biological suppression of malaria: an ecological and nutritional interrelationship of a host and two parasites. Am J Clin Nutr 31:1363–1366. [DOI] [PubMed] [Google Scholar]
- 18.Sokhna C, Le Hesran J-Y, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. 2004. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J 3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R, Looareesuwan S. 2002. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol 88:55–58. doi: 10.2307/3285390. [DOI] [PubMed] [Google Scholar]
- 20.Tshikuka JG, Scott ME, Gray-Donald K, Kalumba ON. 1996. Multiple infection with plasmodium and helminths in communities of low and relatively high socio-economic status. Ann Trop Med Parasitol 90:277–293. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. 2003. Increased frequency of malaria attacks in subjects coinfected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 97:198–199. doi: 10.1016/S0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- 22.Le Hesran J-Y, Akiana J, Ndiaye EHM, Dia M, Senghor P, Konate L. 2004. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans R Soc Trop Med Hyg 98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, Olsen A, Kabatereine NB, Ndyomugyenyi R, Brooker S. 2005. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg 99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier J-P, Pillet S, Garraud O, N′diaye AA, Capron A, Capron M, Riveau G. 2004. Schistosomiasis coinfection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol 26:365–369. doi: 10.1111/j.0141-9838.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson S, Dunne DW. 2012. Advances in our understanding of the epidemiology of Plasmodium and schistosome infection: informing coinfection studies. Curr Opin HIV AIDS 7:225–230. doi: 10.1097/COH.0b013e328351b9fb. [DOI] [PubMed] [Google Scholar]
- 26.Müller-Graf CD, Collins DA, Packer C, Woolhouse ME. 1997. Schistosoma mansoni infection in a natural population of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology 115(Pt 6):621–627. [DOI] [PubMed] [Google Scholar]
- 27.Kariuki TM, Farah IO, Yole DS, Mwenda JM, Van Dam GJ, Deelder AM, Wilson RA, Coulson PS. 2004. Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect Immun 72:5526–5529. doi: 10.1128/IAI.72.9.5526-5529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farah IO, Kariuki TM, King CL, Hau J. 2001. An overview of animal models in experimental schistosomiasis and refinements in the use of nonhuman primates. Lab Anim 35:205–212. doi: 10.1258/0023677011911570. [DOI] [PubMed] [Google Scholar]
- 29.Ozwara H, Langermans JAM, Maamun J, Farah IO, Yole DS, Mwenda JM, Weiler H, Thomas AW. 2003. Experimental infection of the olive baboon (Papio anubis) with Plasmodium knowlesi: severe disease accompanied by cerebral involvement. Am J Trop Med Hyg 69:188–194. [PubMed] [Google Scholar]
- 30.White NJ. 2008. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis 46:172–173. doi: 10.1086/524889. [DOI] [PubMed] [Google Scholar]
- 31.Sabbatani S, Fiorino S, Manfredi R. 2010. The emerging of the fifth malaria parasite (Plasmodium knowlesi): a public health concern? Braz J Infect Dis 14:299–309. doi: 10.1590/S1413-86702010000300019. [DOI] [PubMed] [Google Scholar]
- 32.Barnwell JW, Howard RJ, Coon HG, Miller LH. 1983. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun 40:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yole DS, Pemberton R, Reid GD, Wilson RA. 1996. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology 112(Pt 1):37–46. doi: 10.1017/S0031182000065057. [DOI] [PubMed] [Google Scholar]
- 34.Sturrock RF, Butterworth AE, Houba V. 1976. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology 73:239–252. doi: 10.1017/S003118200004693X. [DOI] [PubMed] [Google Scholar]
- 35.Katz N, Chaves A, Pellegrino J. 1972. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14:397–400. [PubMed] [Google Scholar]
- 36.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. 2004. Serum levels of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farah IO, Mola PW, Kariuki TM, Nyindo M, Blanton RE, King CL. 2000. Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-β and IL-4. J Immunol 164:5337–5343. doi: 10.4049/jimmunol.164.10.5337. [DOI] [PubMed] [Google Scholar]
- 38.Persson KEM, Lee CT, Marsh K, Beeson JG. 2006. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J Clin Microbiol 44:1665–1673. doi: 10.1128/JCM.44.5.1665-1673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimberg BT. 2011. Methodology and application of flow cytometry for investigation of human malaria parasites. J Immunol Methods 367:1–16. doi: 10.1016/j.jim.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.StataCorp. 2009. Stata statistical software, release 10. StataCorp LP, College Station, TX. [Google Scholar]
- 41.Pradhan V, Ghosh K. 2013. Immunological disturbances associated with malarial infection. J Parasit Dis 37:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi F, Ishida H, Matsui T, Tsuji M. 2000. Effects of in vivo administration of anti-IL-10 or anti-IFN-γ monoclonal antibody on the host defense mechanism against Plasmodium yoelii yoelii infection. J Vet Med Sci 62:583–587. doi: 10.1292/jvms.62.583. [DOI] [PubMed] [Google Scholar]
- 43.Omer FM, de Souza JB, Riley EM. 2003. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol 171:5430–5436. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- 44.Jason J, Archibald L, McDonald LC, Hart WM, Rheanppumikankit S, Tansuphwaswadikul S, Byrd MG, Larned J, Han A, Green TA, Jarvis WR. 1999. Immune determinants of organism and outcome in febrile hospitalized Thai patients with bloodstream infections. Clin Diagn Lab Immunol 6:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Buchanan I, Larned J, Kazembe PN, Dobbie H, Parekh B, Byrd MG, Eick A, Han A, Jarvis WR. 2001. Cytokines and malaria parasitemia. Clin Immunol 100:208–218. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- 46.Jason J, Buchanan I, Archibald LK, Nwanyanwu OC, Bell M, Green TA, Eick A, Han A, Razsi D, Kazembe PN, Dobbie H, Midathada M, Jarvis WR. 2000. Natural T, γδ, and NK cells in mycobacterial, Salmonella, and human immunodeficiency virus infections. J Infect Dis 182:474–481. doi: 10.1086/315740. [DOI] [PubMed] [Google Scholar]
- 47.Walker D, Jason J, Wallace K, Slaughter J, Whatley V, Han A, Nwanyanwu OC, Kazembe PN, Dobbie H, Archibald L, Jarvis WR. 2002. Spontaneous cytokine production and its effect on induced production. Clin Diagn Lab Immunol 9:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kremsner PG, Winkler S, Brandts C, Wildling E, Jenne L, Graninger W, Prada J, Bienzle U, Juillard P, Grau GE. 1995. Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am J Trop Med Hyg 53:532–538. [DOI] [PubMed] [Google Scholar]
- 49.Lyke KE, Dabo A, Arama C, Daou M, Diarra I, Wang A, Plowe CV, Doumbo OK, Sztein MB. 2012. Reduced T regulatory cell response during acute Plasmodium falciparum infection in Malian children coinfected with Schistosoma haematobium. PLoS One 7:e31647. doi: 10.1371/journal.pone.0031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. 2006. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis 194:837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 51.Pied S, Civas A, Berlot-Picard F, Renia L, Miltgen F, Gentilini M, Doly J, Mazier D. 1992. IL-6 induced by IL-1 inhibits malaria pre-erythrocytic stages but its secretion is downregulated by the parasite. J Immunol 148:197–201. [PubMed] [Google Scholar]
- 52.Harpaz R, Edelman R, Wasserman SS, Levine MM, Davis JR, Sztein MB. 1992. Serum cytokine profiles in experimental human malaria. Relationship to protection and disease course after challenge. J Clin Invest 90:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prakash D, Fesel C, Jain R, Cazenave P-A, Mishra GC, Pied S. 2006. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis 194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 54.Del Portillo HA, Ferrer M, Brugat T, Martin-Jaular L, Langhorne J, Lacerda MVG. 2012. The role of the spleen in malaria. Cell Microbiol 14:343–355. doi: 10.1111/j.1462-5822.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 55.Yadava A, Kumar S, Dvorak JA, Milon G, Miller LH. 1996. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc Natl Acad Sci U S A 93:4595–4599. doi: 10.1073/pnas.93.10.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Förster I, Brombacher F. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20:623–635. doi: 10.1016/S1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 57.Barron L, Wynn TA. 2011. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol 41:2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemaitre M, Watier L, Briand V, Garcia A, Le Hesran JY, Cot M. 2014. Coinfection with Plasmodium falciparum and Schistosoma haematobium: additional evidence of the protective effect of schistosomiasis on malaria in Senegalese children. Am J Trop Med Hyg 90:329–334. doi: 10.4269/ajtmh.12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crutcher JM, Stevenson MM, Sedegah M, Hoffman SL. 1995. Interleukin-12 and malaria. Res Immunol 146:552–559. doi: 10.1016/0923-2494(96)83031-8. [DOI] [PubMed] [Google Scholar]
- 60.Stevenson MM, Tam MF, Wolf SF, Sher A. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol 155:2545–2556. [PubMed] [Google Scholar]
- 61.Clark IA, Budd AC, Alleva LM, Cowden WB. 2006. Human malarial disease: a consequence of inflammatory cytokine release. Malar J 5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedman JF, Kanzaria HK, McGarvey ST. 2005. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol 21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. 2006. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 64.Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, Mahama E, Thorpe KE, Owusu-Agyei S. 2013. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 310:938–947. doi: 10.1001/jama.2013.277129. [DOI] [PubMed] [Google Scholar]
- 65.Okebe JU, Yahav D, Shbita R, Paul M. 2011. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev CD006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desai MR, Mei JV, Kariuki SK, Wannemuehler KA, Phillips-Howard PA, Nahlen BL, Kager PA, Vulule JM, ter Kuile FO. 2003. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine-pyrimethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis 187:658–666. doi: 10.1086/367986. [DOI] [PubMed] [Google Scholar]
- 67.Ouédraogo HZ, Dramaix-Wilmet M, Zeba AN, Hennart P, Donnen P. 2008. Effect of iron or multiple micronutrient supplements on the prevalence of anaemia among anaemic young children of a malaria-endemic area: a randomized double-blind trial. Trop Med Int Health 13:1257–1266. doi: 10.1111/j.1365-3156.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- 68.Spottiswoode N, Duffy PE, Drakesmith H. 2014. Iron, anemia and hepcidin in malaria. Front Pharmacol 5:125. doi: 10.3389/fphar.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riley EM, Stewart VA. 2013. Immune mechanisms in malaria: new insights in vaccine development. Nat Med 19:168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- 70.Khan ZM, Vanderberg JP. 1992. Specific inflammatory cell infiltration of hepatic schizonts in BALB/c mice immunized with attenuated Plasmodium yoelii sporozoites. Int Immunol 4:711–718. doi: 10.1093/intimm/4.7.711. [DOI] [PubMed] [Google Scholar]
- 71.Zheng H, Tan Z, Xu W. 2014. Immune evasion strategies of pre-erythrocytic malaria parasites. Mediators Inflamm 2014:362605. doi: 10.1155/2014/362605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.