Abstract
Apicomplexan parasites include those of the genera Plasmodium, Cryptosporidium, and Toxoplasma and those of the relatively understudied zoonotic genus Babesia. In humans, babesiosis, particularly transfusion-transmitted babesiosis, has been emerging as a major threat to public health. Like malaria, the disease pathology is a consequence of the parasitemia which develops through cyclical replication of Babesia parasites in host erythrocytes. However, there are no exoerythrocytic stages in Babesia, so targeting of the blood stage and associated proteins to directly prevent parasite invasion is the most desirable option for effective disease control. Especially promising among such molecules are the rhoptry neck proteins (RONs), whose homologs have been identified in many apicomplexan parasites. RONs are involved in the formation of the moving junction, along with AMA1, but no RON has been identified and characterized in any Babesia spp. Here we identify the RON2 proteins of Babesia divergens (BdRON2) and B. microti (BmRON2) and show that they are localized apically and that anti-BdRON2 antibodies are significant inhibitors of parasite invasion in vitro. Neither protein is immunodominant, as both proteins react only marginally with sera from infected animals. Further characterization of the direct role of both BdRON2 and BmRON2 in parasite invasion is required, but knowledge of the level of conformity of RON2 proteins within the apicomplexan phylum, particularly that of the AMA1-RON2 complex at the moving junction, along with the availability of an animal model for B. microti studies, provides a key to target this complex with a goal of preventing the erythrocytic invasion of these parasites and to further our understanding of the role of these conserved ligands in invasion.
INTRODUCTION
Human babesiosis is a zoonotic disease caused by protozoan parasites of the Babesia genus, primarily the bovine pathogen Babesia divergens in Europe (1, 2) and the rodent-borne parasite B. microti in the United States (3, 4). Parasites are transmitted by the bite of the ixodid tick when the insect takes a blood meal, and the current understanding of human babesiosis epidemiology is that B. divergens causes acute illness, usually in immunocompromised patients, whereas B. microti can also infect normosplenic immunocompetent individuals, resulting in infections that range from being asymptomatic to chronic. While many infections remain asymptomatic, especially in younger and immunocompetent individuals, the burden of severe pathology is in newborn infants and older or immunocompromised individuals, and fatality rates average 30% to 45% in these susceptible hosts (5). Transfusion-transmitted babesiosis is an emerging threat to public health, as asymptomatic carriers donate blood and there are no approved or regulated tests to screen blood products for this pathogen. As a consequence, since 2011, babesiosis has been a nationally notifiable disease in 18 states in the United States (6).
Additionally, reports of tick-borne cases within new geographical regions, such as in the Pacific Northwest of the United States (7–10), through Eastern Europe (11–13), and into China (14–17), are also on the rise. Further, new Babesia spp. have been identified to be agents of severe human babesiosis (18, 19), suggesting that the epidemiology of this disease is rapidly changing, and it is clear that human babesiosis is a serious public health concern that requires close monitoring and effective intervention measures.
The pathology of babesiosis, like that of malaria, is a consequence of the parasitemia which develops through the cyclical replication of Babesia parasites in a patient's erythrocytes (RBCs), though the symptoms are typically nonspecific (fever, headache, and myalgia) (20). It is the parasite's ability to first recognize and then invade host RBCs that is central to human babesiosis, and the parasites invade RBCs using multiple complex interactions between parasite proteins and the host cell surface, which are not fully elucidated yet (21–27). Thus, the Babesia-derived proteins involved in these recognition and invasion steps are of great interest for the development of an effective prophylaxis.
Although not as much is known about the invasion process for Babesia as is currently understood for Plasmodium, the basic steps of invasion are the same between the two parasites, in which invasion requires a series of steps at the molecular level, starting with initial contact and recognition between merozoites and erythrocytes, followed by reorientation so that the apical end of the parasite, where the micronemes, rhoptries, and dense granules are situated, is closest to the erythrocyte surface. Then, proteins are released from these organelles to bind to specific RBC surface receptors and directly contribute to the formation of a dynamic tight junction, which moves across the merozoite surface from fore to aft. Invasion finally concludes in the resealing of the erythrocyte membrane, encasing the parasite inside the host cell (28, 29). Crucially, then, the invasion of free merozoites into new RBCs is a critical pinch point in the life cycle. During this time, the parasites are exposed to the peripheral bloodstream, including immune cells and antibody, while they interact with and invade RBCs, although estimates have shown that Plasmodium merozoites can complete invasion within about a minute (29). Thus, although these parasites are technically exposed to host immune mechanisms, they have become extremely adept at protecting the key proteins that appear to be essential to their successful invasion.
Molecules secreted by rhoptries act at the host/parasite interface, and we need to identify them and determine their interactions and function, to define the steps in the invasion mechanism and identify new therapeutic targets. Especially promising among such molecules are the rhoptry neck proteins (RONs), which are invasion ligands with homologs in all apicomplexan parasites. In these parasites, part of the trigger that commits a parasite to invasion is the formation of the moving junction (MJ), key components of which are apical membrane antigen 1 (AMA1), which is initially stored in the micronemes, binding with rhoptry neck protein 2 (RON2) (30). The AMA1 proteins of both B. divergens (24) and B. microti (31) have been identified, and their general structures show high degrees of homology to those of the AMA1 proteins from other parasites. Here we report on the identification and characterization of the RON2 proteins from both major species involved in human babesiosis, B. divergens and B. microti, allowing further characterization of their role in invasion in comparison with the roles of the RON2 homologs of other apicomplexan parasites.
MATERIALS AND METHODS
Animal work and ethics statements.
Cattle and gerbil sera were produced in 1995 under license in the Republic of Ireland (license number B100/702, Department of Health, Cruelty to Animal Act, 1876 [European Directive 86/609/EC]) as fully described elsewhere (27). Rabbit sera were produced by Genmed Synthesis, Inc., San Antonio, TX (OLAW assurance number A3669-01) and by Strategic Diagnostics, Inc., a subsidiary of SDIX, Newark, DE (OLAW assurance number A3975-01). The New York Blood Center Institutional Animal Care and Use Committee reviewed and approved the protocols of the studies with mice (protocol number 337.02) to ensure that they were in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council (32) and were in accordance with U.S. Public Health Service policy at the New York Blood Center. Isoflurane was used to sedate the mice for intraperitoneal infection of the B. microti Peabody strain, which was initially obtained from ATCC (catalog number PRA-99), and for the collection of serum. Isoflurane and carbon dioxide were used to sedate the animals before they were euthanized, and all efforts were made to minimize suffering at all times. The use of blood samples from anonymous human blood donors for in vitro culture and screening for B. microti was approved by the New York Blood Center Institutional Review Board (number 618-10).
Parasite propagation.
Asexual erythrocytic cultures of B. divergens (strain BdRouen1987, isolated from a French patient) (33) were maintained in vitro in human type A-positive blood using RPMI 1640 medium (Life Technologies Corporation, Carlsbad, CA) supplemented with 10% human serum and 0.25% (wt/vol) sodium bicarbonate solution (Life Technologies Corporation, Carlsbad, CA). Cells were cultured at 37°C in 90% CO2, 5% nitrogen, and 5% oxygen. B. microti Peabody strain parasites (catalog number PRA-99; ATCC) were maintained in vivo in 8- to 12-week-old male DBA/2 mice.
Identification and confirmation of the full-length BdRON2 sequence.
The partial B. divergens RON2 (BdRON2) sequence was obtained by initially screening a B. divergens cDNA library (34) by PCR with the universal primers T3 and T7 in various combinations with degenerate primers whose sequences are based upon the published sequences of the RON2 genes of closely related species. Amplified PCR products representing partial BdRON2 sequences were separated on 1% agarose gels, cleaned using a QIAquick gel extraction kit (Qiagen), cloned into the TOPO TA vector (Life Technologies Corporation, Carlsbad, CA), and transformed into Escherichia coli strain TOP10 cells (Life Technologies Corporation, Carlsbad, CA) prior to sequencing. The partial BdRON2 sequences obtained were aligned using the ClustalW program (http://www.ebi.ac.uk/Tools/msa/clustalw2/) to design a BdRON2-specific degenerate primer, BdRON2-T7 (5′-GCSGTSTGGTTTGGTGT-3′), which was used with the universal T7 primer to obtain a more specific BdRON2 sequence, which was used to design the following BdRON2-specific primers: BdRON2R1 (5′-AATTCCTGGTTTGATATAACAGCC-3′), BdRON2R2 (5′-GACGATTCTATGATGTTGTATA-3′), BdRON2R3 (5′-TGCACCAATGCTGTGTTCATA-3′), and BdRON4 (5′-TGAGGTTGATTACATTGCGG-3′). Using various combinations of the T3 universal primer and the BdRON2-specific primers, the full-length sequence of the cDNA of the BdRON2 gene was obtained using the same amplification and cloning strategy described above. The PCR product was separated on a 1% agarose gel and cleaned using the QIAquick gel extraction kit (Qiagen, Hilden, Germany), cloned into the TOPO TA vector (Life Technologies Corporation, Carlsbad, CA), and transformed into E. coli strain TOP10 cells (Life Technologies Corporation, Carlsbad, CA) prior to sequencing.
Phylogenetic comparison of BdRON2 sequences with RON2 sequences of closely related species.
The B. divergens RON2 protein sequence obtained here (GenBank accession number ADM34975.2) was used in a protein BLAST search to identify the full-length RON2 protein sequences of closely related organisms with high degrees of identity, namely, those of Babesia bovis (GenBank accession number XP_001608815), Toxoplasma gondii (GenBank accession number AAZ38163), Theileria parva (GenBank accession number XP_765541), Neospora caninum (GenBank accession number XP_003886062), Plasmodium vivax (GenBank accession number XP_001615869), Plasmodium falciparum (GenBank accession number XP_001348669), Plasmodium reichenowi (GenBank accession number XP_012765563), Eimeria tenella (GenBank accession number XP_013231132), and Babesia microti (GenBank accession number XP_012649548). A phylogenetic tree showing the relatedness of each RON2 protein was constructed using the free phylogeny software at http://www.phylogeny.fr/index.cgi, which simultaneously utilizes various statistical methods, such as maximum likelihood, Bayesian phylogeny, and bootstrapping, to construct phylogenetic trees (35, 36).
Anti-BdRON2 antibody production.
An anti-B. bovis RON2 (BbRON2) serum was generated in two rabbits by Genemed Synthesis by use of its proprietary immunization regimen and a synthetically generated 21-amino-acid (aa) peptide (CQAPYFGNMIVRWDREREKSR; referred to here as BbRON2peptide), also manufactured by Genemed Synthesis, encompassing amino acids 1159 to 1179 of the B. bovis RON2 sequence (GenBank accession number XP_001608815, PiroplasmaDB database accession number BBOV_I001630) as the immunogen. For the solubility of the peptide, it was necessary to replace the first residue at the amino terminus of the peptide, isoleucine, with a cysteine residue.
Anti-B. divergens RON2 antiserum was generated in two rabbits by use of the polyclonal genomic antibody technology (GAT) from Strategic Diagnostics Inc., using its proprietary immunization regimen, where specific DNA is used as the immunogen and the antigen against which antibodies are targeted is produced in vivo. These antibodies are active against the region encoding amino acids 503 to 602 of B. divergens RON2, which make up the target antigen, which is referred to here as BdRON2503–602.
Immunoblotting.
Saponin-lysed pellets from B. divergens in vitro cultures with high levels of parasitemia or from B. microti in vivo infections with high levels of parasitemia were separated under reducing conditions on 6% SDS-polyacrylamide gels and blotted onto nitrocellulose, and the blots were probed with anti-BdRON2503–602 or anti-BbRON2peptide serum, along with rabbit preimmune serum and appropriate secondary antibodies, to detect specific immunoreactivity.
Immunoprecipitation (IP) with 35S-labeled parasites.
B. divergens cells in cultures with ∼60% parasitemia were washed and resuspended in methionine-free RPMI 1640 medium (MP Biomedicals Inc., Aurora, OH). 35S-labeled methionine-cysteine (200 mCi/ml; PerkinElmer, Boston, MA) was added, and the parasites were incubated at 37°C overnight. The parasites were lysed in NETT buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, and 0.1% Triton X-1000) using a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and centrifuged to collect the supernatant. Lysates were precleared with protein G-Sepharose beads (GE Healthcare, Waukesha, WI) before antibody addition. Protein G-Sepharose beads were added and washed extensively with NETTS buffer (10 mM Tris, pH 7.5, 500 mM NaCl, 5 mM EDTA, 0.1% Triton X-100) and NETT buffer. Protein was eluted from the beads using elution buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA), boiled, and run on an SDS-polyacrylamide gel. The gels were fixed with fixing solution (25% isopropanol, 10% acetic acid) for 30 min, enhanced with Amplify fluorographic solution (GE Healthcare, Waukesha, WI) for 45 min, dried under vacuum, and exposed to X-ray film for autoradiography.
IFA.
Cultured B. divergens parasites at a high level of parasitemia (∼70%) were smeared on glass slides and fixed in cold 90% acetone–10% methanol. B. microti-infected mouse RBCs (∼50% parasitemia) were harvested from mice, pelleted, and washed before they were smeared to create immunofluorescence assay (IFA) slides. The slides were incubated for 30 min with purified antibodies diluted 1:200 in 1× phosphate-buffered saline (PBS)–1% bovine serum albumin (BSA). The slides were washed three times in 1× PBS and incubated for 30 min with a polyclonal anti-rabbit IgG (Dako, Denmark) diluted 1:200 in 1× PBS–1% BSA. The slides were washed again three times in 1× PBS, rinsed once in distilled water, and mounted using Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole) solution for microscopy (Vector Laboratories Inc., Burlingame, CA).
ELISAs.
For all enzyme-linked immunosorbent assays (ELISAs), microtiter ELISA plates (Costar; Corning Life Sciences, Corning, NY) were coated with 1 μg/ml of recombinant BbRON2peptide diluted in 0.05 M carbonate buffer, pH 9.6. After incubation overnight at 4°C, the plates were washed 5 times with 1× PBS with 0.05% Tween 20 (1× PBS-T) and blocked with blocking buffer (3% BSA in 1× PBS-T) for 1 h at 37°C. Serum samples, diluted in blocking buffer, were reacted with the bound antigens by incubation for 1 h at 37°C in triplicate wells. Tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) was used as the substrate for 30 min for all ELISAs. Sulfuric acid (2 M) was used to stop the substrate reaction, and the optical density (OD) at 450 nm was immediately read on a SpectraMax 190 ELISA reader (Molecular Devices, Sunnyvale, CA). Serum from the cows was diluted from 1:100 to 1:1,600, and serum from the gerbils was diluted 1:200 to 1:1,600. Bound anti-B. divergens antibodies in cow serum were detected after incubation for 1 h at 37°C with goat anti-cow immunoglobulin conjugated to horseradish peroxidase (HRP; Abcam, Cambridge, MA) diluted 1:1,000 in blocking buffer; anti-B. divergens antibodies in gerbil serum were detected after incubation for 1 h at 37°C with goat anti-rat immunoglobulin conjugated to HRP (Pierce Antibody Products, Thermo Fisher Scientific Inc., Rockford, IL) diluted 1:10,000 in blocking buffer. Human serum was diluted from 1:50 to 1:800 in blocking buffer, and goat anti-human IgG conjugated to HRP (KPL, Gaithersburg, MD) diluted 1:1,000 was used as the secondary antibody. Mouse serum was diluted from 1:50 to 1:1,600, and anti-goat mouse IgG (GE Healthcare, Bucks, United Kingdom) diluted 1:5,000 was used as the secondary antibody.
In vitro growth inhibition assays.
IgG from the sera from animals that had been immunized with the BdRON2503–602 antigen was purified using protein G-Sepharose (GE Healthcare, Waukesha, WI) with IgG binding and elution buffers (Thermo Fisher Scientific Inc., Rockford, IL) according to the manufacturer's recommendations and dialyzed with 1× Dulbecco PBS overnight. Rabbit IgG from preimmune sera was used as the negative control, and controls with no serum were also established. IgG from both mice and rabbits was added to warmed medium at 5% hematocrit. Free merozoites were isolated from unsynchronized cultures with a high level of parasitemia on the basis of previously described protocols (37–39) with the following modifications: the hematocrit of the cultures was reduced to ∼30% by aspiration, the culture was filtered once with a 5-μm-pore-size syringe filter and twice with a 2-μm-pore-size syringe filter (Versapor membranes), and the supernatant (the suspension of free merozoites) was added to fresh uninfected RBCs in a 9:1 supernatant/cell ratio. Free merozoites were allowed to invade for 5 min before the RBCs were washed twice in a 10× cell volume with RPMI 1640 medium to remove any uninvaded merozoites. Cultures for growth inhibition assays were immediately established with these freshly synchronized cells and antibodies at a final concentration of 1 mg/ml in a 500-μl final volume. Each antibody was tested in triplicate. The culture medium, containing purified IgG to a final concentration of 1 mg/ml, was replenished at 12 h and 24 h. Samples were incubated at 37°C with 90% CO2, 5% nitrogen, and 5% oxygen. Smears were made at 12 h, 24 h, and 36 h. The slides were fixed in 100% methanol and stained with Giemsa (Sigma-Aldrich, St. Louis, MO). The level of parasitemia was determined after the total number of intracellular parasites present in 1 × 104 RBCs was counted using a Nikon Eclipse E 600 microscope at a ×100 magnification, and the level of inhibition of invasion with respect to the level of inhibition for the controls was determined for each antibody tested. The parasitemia of the no-serum control was considered 100% invasion/growth, and the level of inhibition of invasion/growth obtained by the use of either the purified preimmune serum or anti-BdRON2503–602 IgG was determined by comparing their observed levels of parasitemia to the level of parasitemia for the no-serum control.
Nucleotide sequence accession numbers.
The contiguous full-length sequence of the gene for BdRON2 and the translated protein have been submitted to GenBank under accession numbers GU198499.2 and ADM34975.2, respectively.
RESULTS
Cloning and sequencing of the gene encoding BdRON2.
To clone the gene encoding BdRON2, a B. divergens cDNA library, created from material obtained from a strain isolated from a human infected with B. divergens and maintained in culture (33, 34), was screened by PCR with the universal primers T3 and T7 in conjunction with degenerate primers based upon homology with the published sequences of RON2 genes of related species. The resulting band of ∼1 kb was obtained and sequenced and was used to design BdRON2-specific primers, which were used with the universal primers to continue screening the cDNA library. Amplified fragments were cloned into the TOPO TA vector and sequenced multiple times with a sequence coverage of at least 3-fold. Nucleotide sequences were aligned using the ClustalW2 program (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and assembled into a contiguous full-length sequence of 4,053 bp. Alignment of the BbRON2 and BdRON2 sequences located the 21-amino-acid BbRON2 peptide to residues 1144 to 1164 in our BdRON2 sequence, and 17/21 amino acids were found to match between BbRON2 and BdRON2 (81% identity).
BdRON2 is an ∼170-kDa antigen.
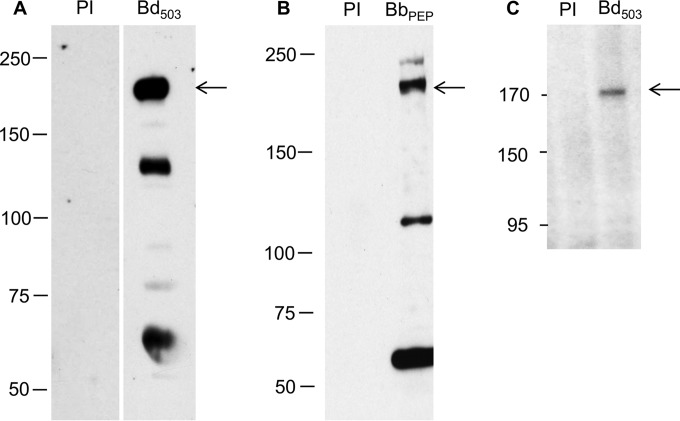
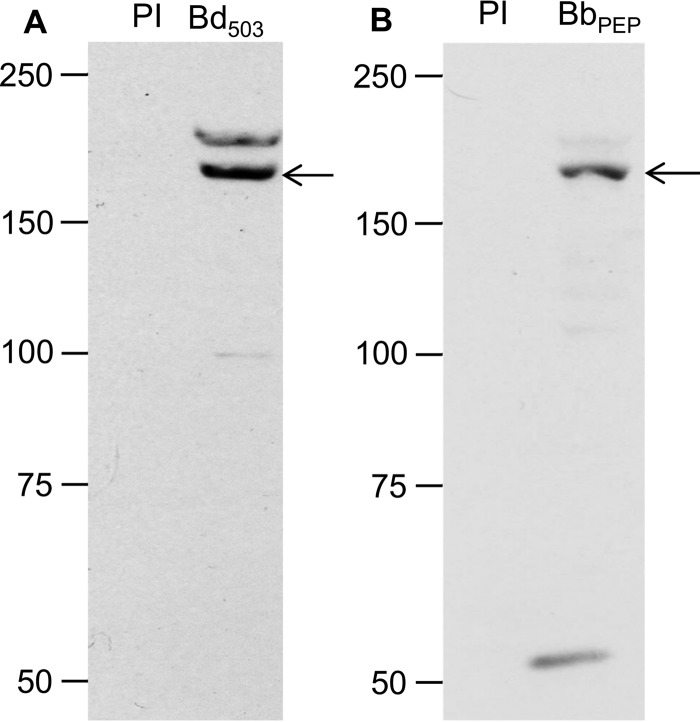
Serum from rabbits immunized with the BdRON2503–602 antigen was used to identify the BdRON2 protein from in vitro culture supernatants by immunoblotting. A distinct band of ∼170 kDa (Fig. 1A) was observed in the parasite lysate. Secondary bands of ∼130 kDa and ∼60 kDa each were also observed. Immunoblotting with anti-BdRON2peptide serum also detected a distinct band at ∼170 kDa (Fig. 1B), and additional lower bands were present. These lower-molecular-mass bands are believed to be processed BdRON2 products, although the possibility of nonspecific reactivity cannot be ruled out. Immunoprecipitation (IP) with the 35S-radiolabeled parasite lysate from infected cells detected the same single band of ∼170 kDa, confirming the size of the native BdRON2 protein (Fig. 1C). Immunoblotting and IP with preimmune sera did not detect any products. Synchronization of B. divergens in vitro is extremely difficult, and although the overall level of parasitemia may be the same between the lysates used, the presence and intensity of the other bands obtained from immunoblotting vary to some degree, depending on the relative proportion of the different parasite forms present in culture (39).
FIG 1.
Anti-BdRON2 antibodies identify a specific ∼170-kDa parasite antigen. (A) Immunoblotting of proteins from in vitro-cultured B. divergens cells show that anti-BdRON2503–602 antibodies (lane Bd503) are able to detect native BdRON2, as shown by the dominant band at ∼170 kDa (arrow) and secondary products of ∼130 kDa and ∼60 kDa each. (B) Anti-BbRON2peptide antibodies (lane BbPEP) show a profile similar to that shown in panel A with the predicted ∼170-kDa product (arrow). (C) Immunoprecipitation with anti-BdRON2503–602 serum and lysate from B. divergens cultures labeled with 35S-labeled methionine-cysteine (lane Bb503) confirms that native BdRON2 is present in the culture pellet, as observed by the presence of a single band at ∼170 kDa (arrow). Preimmune rabbit serum (lanes PI) did not react with any native antigens. Numbers to the left of the gels are molecular masses (in kilodaltons).
BdRON2 localizes to the apical end of the parasite.
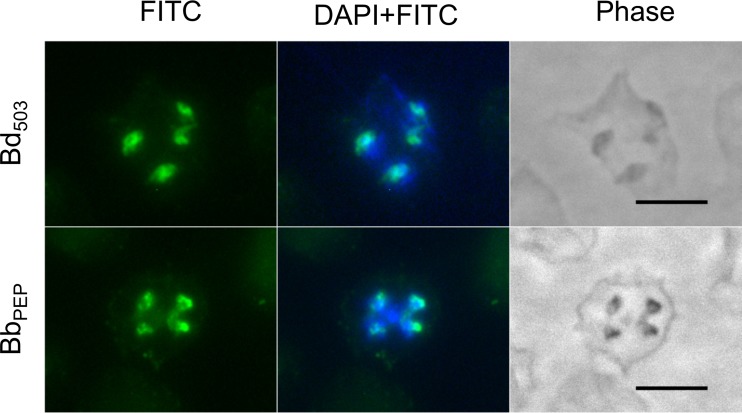
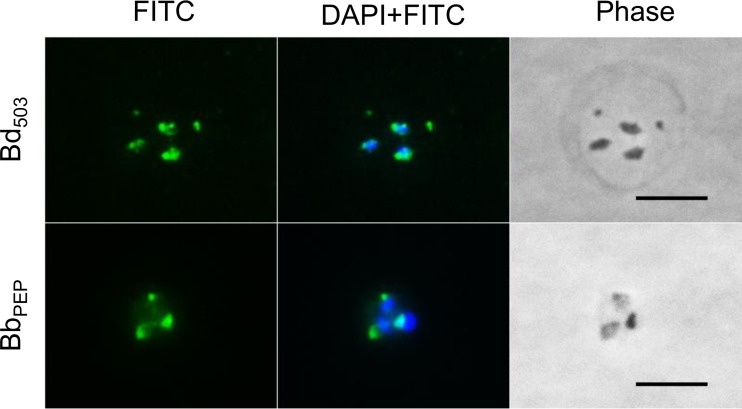
All RONs identified to date are housed in the rhoptries at the apical end of the parasites. The subcellular localization of BdRON2 was determined by an immunofluorescence assay (IFA) using anti-BdRON2503–602 and anti-BbRON2peptide antibodies (Fig. 2). IFA analysis shows that BdRON2 is localized to the apical end of merozoites, as predicted for an antigen involved in parasite invasion. Phase-contrast microscopy confirmed the number and location of the parasites within the RBC.
FIG 2.
Native BdRON2 localizes to the apical complex. Immunofluorescence staining of anti-BdRON2503–602 antibodies (Bd503) and anti-BbRON2peptide antibodies (BbPEP) with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin secondary antibodies on fixed red blood cells infected with B. divergens parasites shows that native BdRON2 is localized mainly at the apical ends of the intracellular parasites. Staining with DAPI in mounting medium identifies the nucleus of the parasite. Phase-contrast microscopy images confirm the location and number of individual intracellular parasites. Bars, 5 μm.
BdRON2 does not appear to be an immunodominant antigen recognized by infected sera.
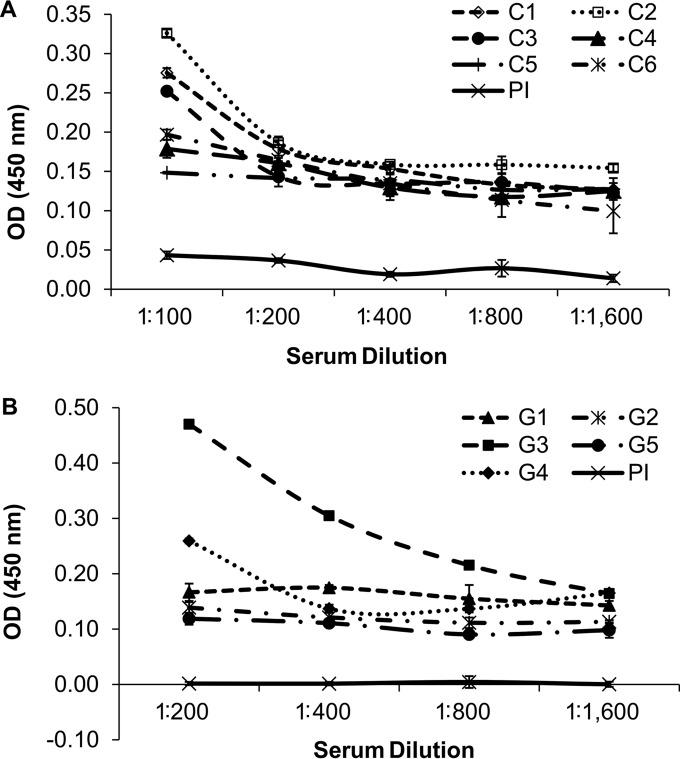
One of the major thrusts in Babesia studies is the identification of immunodominant antigens that can be used in serological diagnostics. To assess if BdRON2 is recognized by sera from infected animals, ELISAs were carried out using sera from B. divergens-infected cows and gerbils (27). Sera from six cows and five gerbils infected with B. divergens and preimmune sera pooled from each group of animals were assayed for reactivity against BbRON2peptide using an enzyme-linked immunosorbent assay (ELISA). The level of reactivity against BbRON2peptide, as determined from the OD reading at 450 nm, was low in all serum samples tested compared to the levels of reactivity of the same serum samples against another B. divergens antigen (27). Of the six cow serum samples tested, the OD at the lowest dilution of 1:100 ranged from 0.33 for cow serum sample C2, which was ∼9-fold higher than that for the preimmune serum sample, to 0.15 for cow serum sample C5, which was ∼4-fold higher than that for the preimmune serum sample (Fig. 3A). However, by dilution 1:200, the observed reactivity of cow serum sample C2 was not greater than that of the other serum samples, and at the highest dilution assayed of 1:1,600, the observed ODs of all cow serum samples had plateaued within the range of 0.10 to 0.15. All these ODs were about ∼3-fold higher than those of the preimmune serum samples at this dilution. The OD readings of the cow preimmune serum samples fell from 0.04 to 0.00 at dilutions of 1:100 to 1:1,600, respectively. Of the five gerbil serum samples, the OD at the lowest dilution of 1:200 ranged from 0.47 for gerbil serum sample G3, which was ∼47-fold higher than that for the preimmune serum sample, to 0.12 for gerbil serum sample G5, which was ∼12-fold higher than that for the preimmune serum sample (Fig. 3B). Gerbil serum sample G3 continued to provide an OD reading higher than the OD readings of the other gerbil serum samples at 1:400 and 1:800 dilutions. The OD readings of the serum from the next-highest responder, gerbil G4, however, were no different from those of the sera from the other gerbils by the 1:400 dilution, and at the highest dilution assayed of 1:1,600, the observed ODs of all the gerbil serum samples had plateaued at OD values within the range of 0.10 to 0.17. These ODs are 10- to 17-fold higher than those of the preimmune serum samples at this dilution. The OD readings of the gerbil preimmune sera indicated no reactivity at all at any concentration, with the OD readings constantly being 0.00. This analysis showed that BdRON2 may not be an effective immunodiagnostic tool, although other regions of the antigen, including the full-length protein, should be tested as the substrates before its use is ruled out. Testing of the reactivity of the same set of serum samples against B. divergens RAP1 (BdRAP1) (27) indicated the relatively high immunodominance of BdRAP1, suggesting that in comparison to BdRAP1, BdRON2 may not be exposed to the host immune system for a period of time long enough to generate substantial antibody.
FIG 3.
Antigenicity of BdRON2 against sera from B. divergens-infected cows and gerbils. The reactivity of BdRON2 against recombinant BbRON2peptide was determined by ELISA with preimmune sera (PI) and sera from cows (A) or gerbils (B) experimentally infected with B. divergens. Three bovine serum samples showed higher OD values than the others at a 1:100 dilution, but then the reactivity dropped to the basal level, with all serum samples showing the same reactivity to BbRON2peptide at the remaining dilutions. One of the five serum samples from gerbils experimentally infected with B. divergens showed much higher OD values than noninfected serum samples in a dilution-dependent manner at dilutions of 1:200 to 1:6,400 (serum sample G3), and another gerbil serum sample (serum sample G4) showed higher reactivity than the others at the 1:200 dilution. However, no reactivity of the three bovine or three gerbil serum samples against BbRON2peptide was observed at any dilution, suggesting that the B. bovis RON2 peptide used here either is not specific enough for native BdRON2 or is within a region of the native antigen that is not exposed to the host immune mechanisms to generate naturally acquired antibodies against this peptide. Error bars show the standard error of the mean ODs.
Anti-BdRON2 antibodies significantly abolish invasion in vitro.
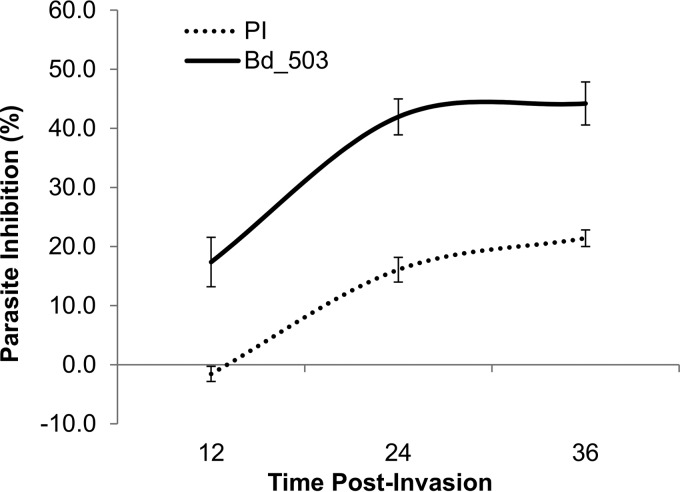
The other major goal of research on parasites is the identification of immunoprotective antigens that could be exploited as vaccine candidates to halt infections. The effectiveness of anti-BdRON2503–602 antibodies for the direct prevention of parasite invasion of RBCs was determined by the invasion/growth inhibition assay. Due to limited availability, anti-BbRON2peptide antibodies were not used for this assay. We have shown that the life cycle of B. divergens is highly variable, with egress expected no sooner than 5 h after initial invasion (39). B. divergens cannot be synchronized using the same methods applied to other parasites, such as P. falciparum, but allowing the merozoites to invade initially provides a tightly synchronized culture that can be used to directly assess the bioactivity of antibodies in the invasion process. To synchronize the parasite culture, freshly purified B. divergens merozoites were allowed to invade uninfected RBCs for 5 min before being constantly cultured for 36 h in the presence of purified anti-BdRON2503–602 IgG at a final concentration of 1 mg/ml. Preimmune rabbit IgG at the same concentration was used as a control, antibodies were added immediately after merozoites were allowed to invade (0 h), and at 12 h and 24 h the culture medium was refreshed and antibody was replenished. Cultures with no serum at all were also established in parallel as controls, and the inhibition of invasion by both IgGs was determined in these cultures. Smears were made at 12 h, 24 h, and 36 h. The rabbit preimmune serum control showed no inhibition compared to the level of inhibition achieved with the no-IgG control after 12 h (−1%) and compared to the relatively high level of inhibition achieved with the anti-BdRON2503–602 antibodies (17%). After 36 h, the preimmune serum inhibited invasion and/or growth by only ∼28% (Fig. 4); however, the invasion and/or growth inhibition due to anti-BdRON2503–602 purified IgG was 44% (Fig. 4), which is a level of inhibition significantly greater than that by the preimmune serum at this time point (t test, P = 0.03). After the first cycle of parasite entry into the RBC, it is difficult to separate the effects of the antibody on invasion and/or growth, as both processes could be affected. The clear inhibition that was obtained after the first 12 h of culture points to a role for BdRON2 in invasion, and this reflects the effect seen in other apicomplexan parasites, confirming the homology of both the structure and function of RON2 among parasites (40).
FIG 4.
Anti-RON2 antibodies inhibit growth in vivo. Highly synchronized B. divergens parasites were cultured at low levels of parasitemia in the continued presence of purified IgG from anti-BdRON2503–602 antibodies (Bd_503) or IgG from preimmune sera (PI) at 1 mg/ml for 36 h. After 12 h, anti-BdRON2503–602 antibodies had inhibited parasite invasion by 18%, but after 36 h, a maximum parasite inhibition of 45%, which is significantly higher than the level of inhibition achieved with the preimmune serum (t test, P = 0.03), was observed. After 36 h, the maximum inhibition from the preimmune IgG was 18%. Error bars show the standard error of the mean levels of parasitemia.
Molecular characterization of BmRON2.
Studies of the other human babesial parasite, B. microti, have been neglected owing to the lack of an in vitro culture system for the parasite. However, as cases of B. microti-caused babesiosis are on the rise (6, 41–43), efforts have to be made to identify immunodominant and immunoprotective antigens in this parasite. The recent publication of the B. microti genome (44) has aided the efforts of researchers to identify and target orthologous proteins that appear to maintain essential functions within the life cycle of apicomplexans. To confirm whether a RON2 protein was present in the B. microti genome, we used the full-length BdRON2 protein sequence to perform a protein-protein search of the sequences within the recently available B. microti sequence project by use of the BLAST program and identified a B. microti antigen (GenBank accession number XP_012649548) of 1,480 aa with a predicted molecular mass of 165.3 kDa and 38% identity to the sequence of BdRON2, confirming that the protein product identified is likely to be the B. microti rhoptry neck protein 2 (BmRON2). The identity match is also good across the homologous regions of BdRON2 and BmRON2 corresponding to the locus used to generate the BdRON2503–602 antibody, suggesting that this antibody that was already generated would be a suitable resource for identification and characterization of the native BmRON2 antigen. Using the anti-BdRON2503–602 antibody, we were indeed able to identify the native BmRON2 from the supernatant of B. microti-infected mouse RBCs kept in culture overnight, as shown by the presence of a band of ∼170 kDa (Fig. 5A). Immunoblotting with anti-BbRON2peptide antibodies also identified the same ∼170-kDa product. Another band of ∼55 kDa could also be detected with this serum sample, suggesting the proteolysis of BmRON2. Preimmune rabbit serum did not react with any native antigens. This anti-BdRON2503–602 antibody was also used to show that native BmRON2 localizes to the apical organelles of the parasite, as shown by IFA staining of mature intracellular B. microti parasites in fixed infected mouse RBCs (Fig. 6). The same immunoblotting and IFA profiles were observed when the anti-BbRON2peptide antibodies were used (Fig. 6).
FIG 5.
B. microti RON2 is an ∼170-kDa antigen. (A) Immunoblotting with B. microti-infected lysates from mouse RBCs and anti-BdRON2503–602 antibodies (lane Bd503) detected a doublet, with the predominant band being of the predicted size of ∼170 kDa (arrow). (B) Blotting with anti-BbRON2peptide antibodies (lane BbPEP) also detected the predominant band of ∼170 kDa (arrow) and a product of ∼55 kDa. Preimmune rabbit serum (lanes PI) did not react with any native antigens. Numbers to the left of the gels are molecular masses (in kilodaltons).
FIG 6.
B. microti RON2 localizes to the apical end of merozoites. Immunofluorescence staining with anti-BdRON2503–602 antibody (Bd503) and anti-BbRON2peptide antibody (BbPEP) as the primary antibody with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG as the secondary antibody on fixed mouse RBCs infected with B. microti parasites from in vivo infections clearly shows that BmRON2 is also localized to the apical ends of the intracellular parasite. Staining with DAPI in the mounting medium identifies the nucleus of the parasite. The phase-contrast microscopy images confirm the location and number of individual intracellular parasites. Bars, 5 μm.
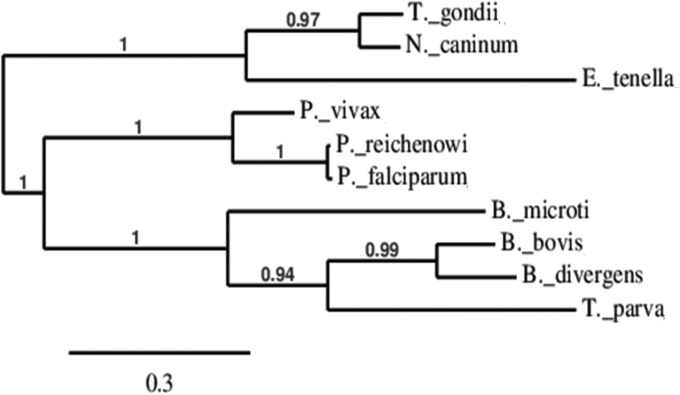
Phylogenetic analysis of the RON2 protein sequence from our B. divergens isolate presented here, the B. microti RON2 protein sequence identified in the search with the BLAST program, and the RON2 protein sequences from other closely related species, namely, B. bovis, T. gondii, T. parva, N. caninum, P. vivax, P. falciparum, P. reichenowi, and E. tenella, was performed to identify how closely related these RON2 proteins are to each other. The resulting tree shows three broad clades, but each branch was grouped together significantly (P > 0.94) according to the maximum likelihood or bootstrap values observed (Fig. 7). The first clade shows that the RON2 protein sequences of T. gondii and N. caninum are very similar to each other, with the RON2 protein sequences of E. tenella also being grouped with those of T. gondii and N. caninum, although E. tenella RON2 has a longer branch length, indicating that it has evolved some differences from the RON2 proteins of T. gondii and N. caninum. The other two clades are one subset containing all the RON2 sequences of the three Plasmodium spp. used in the analysis and another subset that groups together the Babesia proteins with the T. parva protein. Interestingly, the maximum likelihood and bootstrap analyses predict that the RON2 protein of B. divergens is more closely related to the RON2 proteins of B. bovis and T. parva than the RON2 protein of B. microti.
FIG 7.
Relationship of BdRON2 and BmRON2 proteins with other RON2 proteins from closely related species. A phylogenetic tree constructed using RON2 protein sequences shows three broad relationships between the sequences used. The sequences of the RON2 proteins of T. gondii, N. caninum, and E. tenella are the most distinct from those of the proteins from the other parasites, forming a subgroup of their own. The RON2 proteins of the Plasmodium spp. used, especially the P. falciparum and P. reichenowi proteins, are most like each other. Although the sequences of the B. divergens and B. microti proteins group together, the RON2 protein sequence of B. divergens is most like that of B. bovis and T. parva, and the RON2 protein sequence of B. microti is distinct. The scale bar shows the length of the branch that relates to each 0.3 nucleotide difference per 100 nucleotides. The maximum likelihood values of each branch are indicated.
Analysis of immunogenicity of the RON2 peptide using sera from B. microti-infected mice and human sera.
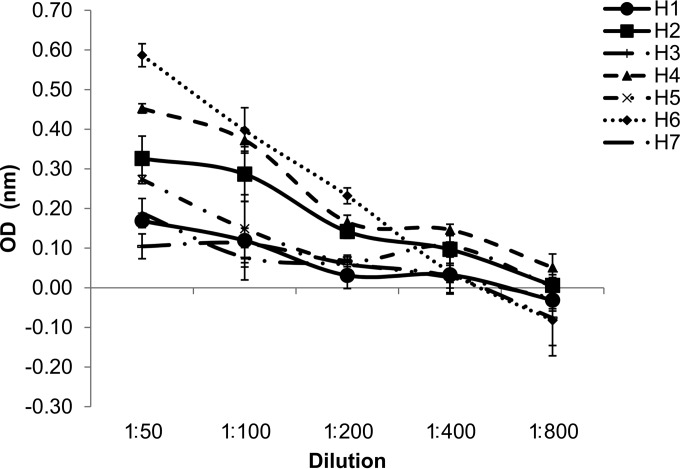
As both our anti-RON2 antibodies successfully identified the BmRON2 antigen because of sufficient homology with native BmRON2, we used BbRON2peptide in an ELISA to assess whether there was sufficient homology to determine cross-reactivity with sera from humans known to be infected with B. microti parasites. Human blood was collected as part of ongoing screening programs and volunteer donation programs at the New York Blood Center, and sera were collected from anonymized blood samples from these programs. All human samples were screened for the presence of parasites by microscopy and B. microti-specific PCR, and two samples (samples H1 and H2) had previously been identified to be B. microti positive by examination of Giemsa-stained smears (Fig. 8). The OD readings of samples H1 and H2 at the highest concentration tested in the ELISA were 0.17 and 0.33, respectively. Although parasites were not observed in the remaining five human serum samples, the samples tested positive by PCR for the B. microti 18S rRNA gene. Both sample H6 and sample H4 presented a greater reactivity at the 1:50 serum dilution than at the other dilutions, as evidenced by their OD readings of 0.59 and 0.45, respectively (Fig. 8), showing that although no parasites were visible in the peripheral blood of these patients, the patients had been infected with Babesia parasites to generate circulating anti-BmRON2 antibodies. However, when the mean of the observed OD values of sera from individuals who were parasite positive at the time of serum collection (samples H1 and H2; mean OD = 0.25, standard error [SE] = 0.08 [n = 2]) was compared with the observed mean OD of sera from those who were parasite negative (samples H3 to H7; mean OD = 0.31, SE = 0.08 [n = 5]) at the highest concentration of 1:50, we found there was no real difference between these two groups (t test, P = 0.708).
FIG 8.
Reactivity of sera from B. microti-infected individuals against the recombinant BbRON2peptide. Human blood samples were screened for the presence of parasites by microscopy and PCR, and then the reactivity of the sera against the recombinant BbRON2peptide was determined for all available samples. Only samples H1 and H2 were identified to be positive for B. microti by Giemsa staining; however, the OD readings of serum samples H1 and H2 at the highest concentration tested in the ELISA were only 0.17 and 0.33, respectively. Parasites were never observed in the remaining five human samples, but the samples were PCR positive. Both serum sample H6 and serum sample H4 showed a greater reactivity at the 1:50 dilution, with ODs of 0.59 and 0.45, respectively, suggesting that the patients from whom these samples were obtained had recently been infected with Babesia parasites or had been harboring subpatent parasite loads for a long period of time, allowing anti-RON2 antibodies to accrue to levels higher than those in parasite-positive individuals. Error bars show the standard error of the mean ODs.
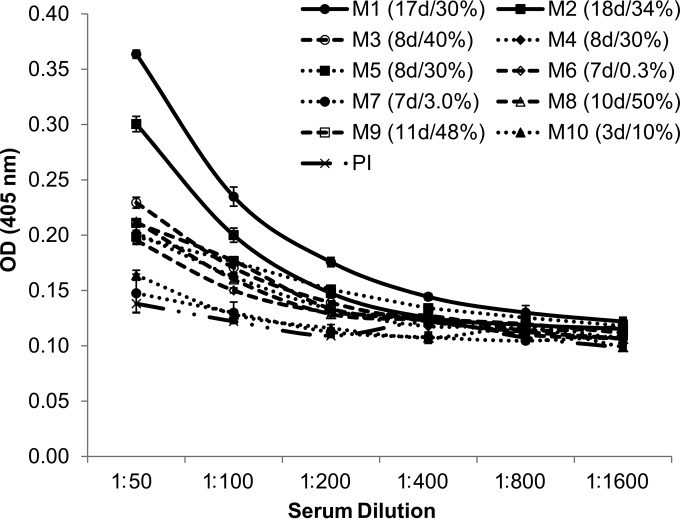
Preimmune serum samples were collected from multiple mice before infection and pooled. Serum samples were collected from individual B. microti-infected DBA/2 mice with levels of parasitemia ranging from 0.3% to 50%. The average level of parasitemia in mice from which these sera were obtained was 28%. To determine if the parasite burden was a contributor to anti-RON2 production, as classified by the observed OD values, the sera were defined to originate from infections with high levels of parasitemia (>15%, n = 7) or low levels of parasitemia (<15%, n = 3). On the basis of this stratification, no difference in the mean OD values between the infections with high levels of parasitemia (mean OD = 0.25, SE = 0.02) and low levels of parasitemia (mean OD = 0.17, SE = 0.01) (t test, P = 0.082) was observed at a 1:50 dilution. The length of infection from the time of initial inoculum to the time of killing and collection of blood samples depended on whether the inoculum was from frozen parasites, which averaged 17 days (animals M1 and M2), or was from fresh infected blood, which averaged 8 days (animals M3 to M10) (Fig. 9). The B. microti-infected sera from all mice showed a weak cross-reactivity against BbRON2peptide, though the sera appeared to cluster into three general groups at the highest concentration tested of 1:50. Here these mice have been nominated high (n = 2), medium (n = 6), and low (n = 2) responders in relation to each other. We observed that the sera from animals in the high responder group (animals M1 and M2) were those with the longest period of infection since the time of inoculation (17 days and 18 days, respectively), and these sera gave the greatest reactivity, especially at the highest serum concentrations of 1:50 to 1:200. It was observed that animals forming the medium responder group (animals M3 to M6 and M8 to M9) had been infected for times ranging from 7 to 11 days, with the average being 8.6 days. The average length of infection of animals forming the low-responder group (animals M7 and M10, which were infected for 7 days and 3 days, respectively) was 5 days. The mean OD values obtained for the 1:50 dilution of sera from each of the three groups were independently compared with those of sera from the other groups. It was shown that sera from animals with the longest infections, i.e., high responders (mean OD = 0.33, SE = 0.03), were significantly more reactive to BbRON2peptide than sera from animals that were medium responders (mean OD = 0.21, SE = 0.01; t test, P = 0.000) and the sera from medium responders were significantly more reactive to BbRON2peptide than sera from the low responders (mean OD = 0.16, SE = 0.1; t test, P = 0.002). These data combined suggest that RON2 is not a highly immunodominant antigen and that effective anti-RON2 antibody production is more dependent on exposure to parasites over time than on the parasite burden per se, with a critical minimum exposure time being necessary before anti-RON2 antibodies are detectable. Careful experimentation dissecting the length of infection using various inocula of parasites and various anti-RON titers should shed light on this relationship.
FIG 9.
Weak reactivity of sera from mice infected with B. microti with BbRON2peptide. Sera were collected from 10 individual DBA/2 mice infected with B. microti with levels of parasitemia ranging from 0.3% to 50%. The length of infection from the time of inoculation to the time of serum collection also varied from 3 to 10 days. Although the sera from B. microti-infected mice showed weak cross-reactivity against BbRON2peptide, any reactivity that was observed was not associated with the level of parasitemia but was associated with the length of infection, as the sera from animals that had been infected for the longest period of time since the time of inoculation, serum samples M1 and M2 (17 days and 18 days, respectively), gave the greatest reactivity, especially at the highest serum concentrations, suggesting that effective anti-RON2 antibody production is more sensitive to exposure over time than to parasite burden. Preimmune serum (PI) was collected from multiple animals before inoculation and pooled. For each animal, the length of infection (in days [d]) and the level of parasitemia (in percent) at the time of serum collection are shown in parentheses. Error bars show the standard errors of the mean ODs.
DISCUSSION
Parasites of the phylum Apicomplexa are responsible for a huge burden of disease in humans and animals and also a loss of economic productivity. They include Plasmodium spp., which cause malaria; Cryptosporidium spp., which cause diarrheal disease; Toxoplasma gondii, which causes toxoplasmosis; and the relatively understudied Babesia spp., which cause veterinary and human babesiosis. The rhoptry is one of the three important secretory organelles in the Apicomplexa. Rhoptry contents separate into two intraorganellar compartments of the bulb and the neck. While the bulb proteins are less conserved and function to suit a specific parasite life cycle across the phylum, the rhoptry neck proteins (RONs) are relatively conserved between species (45) and are involved in various functions, such as host cell invasion.
This is the first study to identify and characterize the ∼170-kDa RON2 proteins of B. divergens and B. microti, the main causal agents of human babesiosis. Both antigens were localized to the apical end of the parasite, as expected for proteins involved in parasite invasion. We have shown that purified IgG from anti-BdRON2 antibodies is able to inhibit B. divergens invasion by up to 44% over 36 h. The RON2 peptide does not appear to be an immunodominant target and, hence, may not be suitable as a diagnostic. Our results also show that a longer length of parasite infection may be of greater importance than the actual level of parasitemia in the production of circulating anti-RON2 antibody.
After contact and reorientation of the invading parasite at the host cell surface, various adhesins are released from the apical organelles in a sequential manner and are used to attach the parasite to the host cell surface. The micronemes, including AMA1, release their contents first, followed by the rhoptry neck, including the RONs, and then the rhoptry bulb. Although all the parasite ligands and host cell receptors to which they bind have yet to be identified, these adhesins establish the first specific link between the parasite and the host cell. Irreversible attachment of the parasite to the host cell occurs when the moving junction (MJ) is formed (30) and the parasite begins to dynamically enter the host cell and simultaneously form the parasitophorous vacuole (PV) (46–48).
The MJ is characterized by the formation of a complex between AMA1 and RON2. AMA1 remains associated with the parasite surface and RON2 inserts itself into the host cell membrane. The tight connection between the parasite and the host cell needed for the MJ occurs when RON2 physically binds to the hydrophobic groove of AMA1. In Plasmodium, RON4 and RON5 also constitute part of the AMA-RON complex of the MJ (49–53), with RON8 being added in Toxoplasma (40, 54–58).
There are no exoerythrocytic stages of Babesia like there are in P. falciparum infections, and clinical disease is a direct result of blood-stage parasites invading and destroying RBCs, so targeting of this stage and the direct prevention of parasite invasion are the most desirable for effective disease control, yet targeting of the blood-stage antigens is not an easy process, primarily due to the antigenic polymorphism of known antigens or due to the lack of available data for these less studied parasites. In P. falciparum, AMA1 was considered a potential blood-stage vaccine candidate, as it was able to induce invasion-blocking antibodies in vitro (59) and in immunized nonhuman primates in vivo (60, 61), and we have shown that B. divergens invasion can be inhibited by ∼50% in vitro by anti-B. divergens AMA1 antibodies (24) and ∼80% in vitro inhibition of B. microti has been achieved with anti-B. microti AMA1 antibodies (31), confirming that AMA1 is certainly a key player in Babesia invasion. However, in a controlled human malaria challenge trial, the P. falciparum AMA1 (PfAMA1) vaccine conferred little protection even against the homologous parasite (62–64), showing that AMA1 alone may not be suitable as a candidate on its own.
Fortunately, a study has shown, using an animal model of Plasmodium yoelii infection, that vaccination of mice with the AMA1-RON2 peptide complex but not with AMA1 alone provides complete protection against a lethal P. yoelii challenge (65). Although all the minute details of the invasion processes and the molecules involved at each step are not fully elucidated for each apicomplexan parasite, the conservation of these proteins among apicomplexan parasites strongly suggests that the protein functions and interactions are similar and limited to host cell invasion throughout the phylum. Further characterization of the direct role of both BdRON2 and BmRON2 in parasite invasion certainly remains to be accomplished, but knowledge of this conformity within the AMA1-RON2 complex and the availability of an animal model for B. microti studies provide keys for continuing to target this protein complex and thus potentially prevent erythrocytic invasion by these parasites.
Funding Statement
This work was funded by the George Link Jr. Foundation and the HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID) (HL105694 and HL129215), awarded to Cheryl A. Lobo.
REFERENCES
- 1.Garnham PC. 1980. Human babesiosis: European aspects. Trans R Soc Trop Med Hyg 74:153–155. doi: 10.1016/0035-9203(80)90232-1. [DOI] [PubMed] [Google Scholar]
- 2.Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. 2003. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev 16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dammin GJ, Spielman A, Benach JL, Piesman J. 1981. The rising incidence of clinical Babesia microti infection. Hum Pathol 12:398–400. doi: 10.1016/S0046-8177(81)80020-2. [DOI] [PubMed] [Google Scholar]
- 4.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. 2015. Babesiosis. Infect Dis Clin North Am 29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ru YX, Mao BY, Zhang FK, Pang TX, Zhao SX, Liu JH, Wickramasinghe SN. 2009. Invasion of erythroblasts by Plasmodium vivax: a new mechanism contributing to malarial anemia. Ultrastruct Pathol 33:236–242. doi: 10.3109/01913120903251643. [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt BL, Montgomery S, Woodhall D, Bosserman E. 2012. 2011. Babesiosis surveillance—18 states. MMWR Morb Mortal Wkly Rep 61:505–509. [PubMed] [Google Scholar]
- 7.Persing DH, Herwaldt BL, Glaser C, Lane RS, Thomford JW, Mathiesen D, Krause PJ, Phillip DF, Conrad PA. 1995. Infection with a babesia-like organism in northern California. N Engl J Med 332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 8.Kjemtrup AM, Conrad PA. 2000. Human babesiosis: an emerging tick-borne disease. Int J Parasitol 30:1323–1337. doi: 10.1016/S0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 9.Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR III, Herwaldt BL. 2006. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 36:779–789. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, Reed W, Hunter R, Ryals R, Hagar W, Xayavong MV, Slemenda SB, Pieniazek NJ, Wilkins PP, Kjemtrup AM. 2012. The third described case of transfusion-transmitted Babesia duncani. Transfusion 52:1517–1522. doi: 10.1111/j.1537-2995.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, Kiehntopf M, Fricke HJ, Straube E. 2007. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis 26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 12.Morch K, Holmaas G, Frolander PS, Kristoffersen EK. 2015. Severe human Babesia divergens infection in Norway. Int J Infect Dis 33:37–38. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Welc-Faleciak R, Pawelczyk A, Radkowski M, Pancewicz SA, Zajkowska J, Sinski E. 2015. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med 22:51–54. doi: 10.5604/12321966.1141394. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, Ge HX, Chen JH, Hu W. 2013. Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty 2:24. doi: 10.1186/2049-9957-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN. 2014. Human babesiosis, an emerging tick-borne disease in the People's Republic of China. Parasit Vectors 7:509. doi: 10.1186/s13071-014-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, Sun Y, Jia N, Wang YW, Ma L, Liu HB, Chu YL, Ni XB, Liu K, Song YD, Yao NN, Wang H, Sun T, Cao WC. 2015. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis 15:196–203. doi: 10.1016/S1473-3099(14)71046-1. [DOI] [PubMed] [Google Scholar]
- 17.Vannier E, Krause PJ. 2015. Babesiosis in China, an emerging threat. Lancet Infect Dis 15:137–139. doi: 10.1016/S1473-3099(14)71062-X. [DOI] [PubMed] [Google Scholar]
- 18.Vannier E, Krause PJ. 2012. Human babesiosis. N Engl J Med 366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 19.Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, Fritsche TR, Persing DH, Limaye AP. 2004. Babesia divergens-like infection, Washington State. Emerg Infect Dis 10:622–629. doi: 10.3201/eid1004.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruebush TK II, Juranek DD, Chisholm ES, Snow PC, Healy GR, Sulzer AJ. 1977. Human babesiosis on Nantucket Island. Evidence for self-limited and subclinical infections. N Engl J Med 297:825–827. [DOI] [PubMed] [Google Scholar]
- 21.Lobo CA. 2005. Babesia divergens and Plasmodium falciparum use common receptors, glycophorins A and B, to invade the human red blood cell. Infect Immun 73:649–651. doi: 10.1128/IAI.73.1.649-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero E, Rafiq S, Heck S, Lobo CA. 2007. Inhibition of human erythrocyte invasion by Babesia divergens using serine protease inhibitors. Mol Biochem Parasitol 153:80–84. doi: 10.1016/j.molbiopara.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Montero E, Rodriguez M, Gonzalez LM, Lobo CA. 2008. Babesia divergens: identification and characterization of BdHSP-20, a small heat shock protein. Exp Parasitol 119:238–245. doi: 10.1016/j.exppara.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Montero E, Rodriguez M, Oksov Y, Lobo CA. 2009. Babesia divergens apical membrane antigen 1 and its interaction with the human red blood cell. Infect Immun 77:4783–4793. doi: 10.1128/IAI.00969-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobo CA, Rodriguez M, Cursino-Santos JR. 2012. Babesia and red cell invasion. Curr Opin Hematol 19:170–175. doi: 10.1097/MOH.0b013e328352245a. [DOI] [PubMed] [Google Scholar]
- 26.Cursino-Santos JR, Halverson G, Rodriguez M, Narla M, Lobo CA. 2014. Identification of binding domains on red blood cell glycophorins for Babesia divergens. Transfusion 54:982–989. doi: 10.1111/trf.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez M, Alhassan A, Ord RL, Cursino-Santos JR, Singh M, Gray J, Lobo CA. 2014. Identification and characterization of the RouenBd1987 Babesia divergens rhopty-associated protein 1. PLoS One 9:e107727. doi: 10.1371/journal.pone.0107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. 1975. Invasion of erythrocytes by malaria merozoites. Science 187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 29.Aikawa M, Miller LH, Johnson J, Rabbege J. 1978. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 77:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, Moch JK, Tyler JS, Narum DL, Pierce SK, Boothroyd JC, Haynes JD, Miller LH. 2011. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A 108:13275–13280. doi: 10.1073/pnas.1110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moitra P, Zheng H, Anantharaman V, Banerjee R, Takeda K, Kozakai Y, Lepore T, Krause PJ, Aravind L, Kumar S. 2015. Expression, purification, and biological characterization of Babesia microti apical membrane antigen 1. Infect Immun 83:3890–3901. doi: 10.1128/IAI.00168-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 33.Gorenflot A, Brasseur P, Precigout E, L'Hostis M, Marchand A, Schrevel J. 1991. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res 77:3–12. doi: 10.1007/BF00934377. [DOI] [PubMed] [Google Scholar]
- 34.Florin-Christensen M, Suarez CE, Hines SA, Palmer GH, Brown WC, McElwain TF. 2002. The Babesia bovis merozoite surface antigen 2 locus contains four tandemly arranged and expressed genes encoding immunologically distinct proteins. Infect Immun 70:3566–3575. doi: 10.1128/IAI.70.7.3566-3575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dereeper A, Audic S, Claverie JM, Blanc G. 2010. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol 10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(Web Server issue):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Precigout E, Valentin A, Carcy B, Gorenflot A, Nakamura K, Aikawa M, Schrevel J. 1993. Babesia divergens: characterization of a 17-kDa merozoite membrane protein. Exp Parasitol 77:425–434. doi: 10.1006/expr.1993.1102. [DOI] [PubMed] [Google Scholar]
- 38.Montero E, Gonzalez LM, Rodriguez M, Oksov Y, Blackman MJ, Lobo CA. 2006. A conserved subtilisin protease identified in Babesia divergens merozoites. J Biol Chem 281:35717–35726. doi: 10.1074/jbc.M604344200. [DOI] [PubMed] [Google Scholar]
- 39.Cursino-Santos JR, Singh M, Pham P, Rodriguez M, Lobo CA. 10 December 2015. Babesia divergens builds a complex population structure composed of specific ratios of infected cells to ensure a prompt response to changing environmental conditions. Cell Microbiol. doi: 10.1111/cmi.12555. [DOI] [PubMed] [Google Scholar]
- 40.Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, Dubremetz JF, Fauquenoy S, Tomavo S, Faber BW, Kocken CH, Thomas AW, Boulanger MJ, Bentley GA, Lebrun M. 2011. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog 7:e1001276. doi: 10.1371/journal.ppat.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apostolou A, Sorhage F, Tan C. 2014. Babesiosis surveillance, New Jersey, USA, 2006-2011. Emerg Infect Dis 20:1407–1409. doi: 10.3201/eid2008.131591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bullard JM, Ahsanuddin AN, Perry AM, Lindsay LR, Iranpour M, Dibernardo A, Van Caeseele PG. 2014. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol 25:e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta ME, Ender PT, Smith EM, Jahre JA. 2013. Babesia microti infection, eastern Pennsylvania, USA. Emerg Infect Dis 19:1105–1107. doi: 10.3201/eid1907.121593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, Augagneur Y, Bres V, Duclos A, Randazzo S, Carcy B, Debierre-Grockiego F, Delbecq S, Moubri-Menage K, Shams-Eldin H, Usmani-Brown S, Bringaud F, Wincker P, Vivares CP, Schwarz RT, Schetters TP, Krause PJ, Gorenflot A, Berry V, Barbe V, Ben Mamoun C. 2012. Sequencing of the smallest apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res 40:9102–9114. doi: 10.1093/nar/gks700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, Boothroyd JC. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem 280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, Leroy C, Blazquez S, Menard R, Tardieux I. 2009. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe 5:259–272. doi: 10.1016/j.chom.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Mordue DG, Desai N, Dustin M, Sibley LD. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinai AP. 2008. Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane. Subcell Biochem 47:155–164. doi: 10.1007/978-0-387-78267-6_12. [DOI] [PubMed] [Google Scholar]
- 49.Morahan BJ, Sallmann GB, Huestis R, Dubljevic V, Waller KL. 2009. Plasmodium falciparum: genetic and immunogenic characterisation of the rhoptry neck protein PfRON4. Exp Parasitol 122:280–288. doi: 10.1016/j.exppara.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Narum DL, Nguyen V, Zhang Y, Glen J, Shimp RL, Lambert L, Ling IT, Reiter K, Ogun SA, Long C, Holder AA, Herrera R. 2008. Identification and characterization of the Plasmodium yoelii PyP140/RON4 protein, an orthologue of Toxoplasma gondii RON4, whose cysteine-rich domain does not protect against lethal parasite challenge infection. Infect Immun 76:4876–4882. doi: 10.1128/IAI.01717-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutungi JK, Yahata K, Sakaguchi M, Kaneko O. 2014. Expression and localization of rhoptry neck protein 5 in merozoites and sporozoites of Plasmodium yoelii. Parasitol Int 63:794–801. doi: 10.1016/j.parint.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. 2011. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Cao J, Kaneko O, Thongkukiatkul A, Tachibana M, Otsuki H, Gao Q, Tsuboi T, Torii M. 2009. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol Int 58:29–35. doi: 10.1016/j.parint.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog 1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M. 2009. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5:e1000309. doi: 10.1371/journal.ppat.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Straub KW, Peng ED, Hajagos BE, Tyler JS, Bradley PJ. 2011. The moving junction protein RON8 facilitates firm attachment and host cell invasion in Toxoplasma gondii. PLoS Pathog 7:e1002007. doi: 10.1371/journal.ppat.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial H, Dubremetz JF. 2005. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol 7:1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 58.Tyler JS, Boothroyd JC. 2011. The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog 7:e1001282. doi: 10.1371/journal.ppat.1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deans JA, Alderson T, Thomas AW, Mitchell GH, Lennox ES, Cohen S. 1982. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol 49:297–309. [PMC free article] [PubMed] [Google Scholar]
- 60.Stowers AW, Miller LH. 2001. Are trials in New World monkeys on the critical path for blood-stage malaria vaccine development? Trends Parasitol 17:415–419. doi: 10.1016/S1471-4922(01)02011-6. [DOI] [PubMed] [Google Scholar]
- 61.Dutta S, Sullivan JS, Grady KK, Haynes JD, Komisar J, Batchelor AH, Soisson L, Diggs CL, Heppner DG, Lanar DE, Collins WE, Barnwell JW. 2009. High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model. PLoS One 4:e8138. doi: 10.1371/journal.pone.0008138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, Bergmann-Leitner E, Stewart VA, Bittner S, Juompan L, Kortepeter MG, Nielsen R, Krzych U, Tierney E, Ware LA, Dowler M, Hermsen CC, Sauerwein RW, de Vlas SJ, Ofori-Anyinam O, Lanar DE, Williams JL, Kester KE, Tucker K, Shi M, Malkin E, Long C, Diggs CL, Soisson L, Dubois MC, Ballou WR, Cohen J, Heppner DG Jr. 2009. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One 4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG Jr, Plowe CV. 2011. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouattara A, Mu J, Takala-Harrison S, Saye R, Sagara I, Dicko A, Niangaly A, Duan J, Ellis RD, Miller LH, Su XZ, Plowe CV, Doumbo OK. 2010. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malar J 9:175. doi: 10.1186/1475-2875-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srinivasan P, Ekanem E, Diouf A, Tonkin ML, Miura K, Boulanger MJ, Long CA, Narum DL, Miller LH. 2014. Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Natl Acad Sci U S A 111:10311–10316. doi: 10.1073/pnas.1409928111. [DOI] [PMC free article] [PubMed] [Google Scholar]