Abstract
Dendritic cells (DCs) are sentinels of the immune system that uniquely prime naive cells and initiate adaptive immune responses. CD1c (BDCA-1) myeloid DCs (CD1c+ mDCs) highly express HLA-DR, have a broad Toll-like receptor (TLR) repertoire, and secrete immune modulatory cytokines. To better understand immune responses to malaria, CD1c+ mDC maturation and cytokine production were examined in healthy volunteers before and after experimental intravenous Plasmodium falciparum infection with 150- or 1,800-parasite-infected red blood cells (pRBCs). After either dose, CD1c+ mDCs significantly reduced HLA-DR expression in prepatent infections. Circulating CD1c+ mDCs did not upregulate HLA-DR after pRBC or TLR ligand stimulation and exhibited reduced CD86 expression. At peak parasitemia, CD1c+ mDCs produced significantly more tumor necrosis factor (TNF), whereas interleukin-12 (IL-12) production was unchanged. Interestingly, only the 1,800-pRBC dose caused a reduction in the circulating CD1c+ mDC count with evidence of apoptosis. The 1,800-pRBC dose produced no change in T cell IFN-γ or IL-2 production at peak parasitemia or at 3 weeks posttreatment. Overall, CD1c+ mDCs are compromised by P. falciparum exposure, with impaired HLA-DR and CD86 expression, and have an increased capacity for TNF but not IL-12 production. A first prepatent P. falciparum infection is sufficient to modulate CD1c+ mDC responsiveness, likely contributing to hampered effector T cell cytokine responses and assisting parasite immune evasion.
INTRODUCTION
Malaria caused by Plasmodium spp. remains a major global health problem, with 584,000 deaths in 2013 (1). Repeat Plasmodium infections are common. Among the reasons cited for lack of sterile protective immunity is the ability of parasites to subvert host immune responses. Early effects include the impaired function of dendritic cells (DCs) (2), the only cells capable of priming naive T cells. DCs are a heterogeneous population composed of several subsets distinguished by phenotype, location, and functional properties (3). Circulating CD1c+ myeloid DCs (mDCs) represent ∼20% of total blood DCs (4), express Toll-like receptors (TLRs) 1 to 7 (5), and produce immunoregulatory cytokines (interleukin-12 [IL-12] and IL-10) (6–8) and the proinflammatory cytokine tumor necrosis factor (TNF) (9). CD1c+ mDCs express high levels of HLA-DR compared to other circulating DC subsets (8, 10), suggesting a specialized ability to initiate adaptive immune responses.
We previously reported the loss of total mDCs and reduced phagocytosis by total blood DCs during prepatent experimental human blood-stage Plasmodium falciparum infection (11), but CD1c+ mDCs were not individually examined. In acute P. falciparum malaria, CD1c+ mDCs decline (12) and have reduced major histocompatibility complex (MHC) class II (HLA-DR) expression in both uncomplicated (13) and severe malaria (14). However, it remains to be determined whether this impairment is evident in prepatent blood-stage P. falciparum infection, the effect of different pRBC inoculating doses, and whether CD1c+ mDC cytokine production is impacted by Plasmodium, informing whether CD1c+ mDCs can contribute to protective host adaptive immune responses.
CD1c+ mDC cytokine production and TLR response in prepatent Plasmodium infections have not been previously evaluated. Key immunomodulatory cytokines produced by CD1c+ mDCs include IL-12, TNF, and IL-10. These cytokines facilitate immune priming and can influence whether the immune response promotes the onset of immunity or assists immune escape. DC-generated IL-12 can drive T cell IFN-γ secretion and promote cytotoxic capacity (15), as well as facilitate the development of clinical immunity to malaria (16–19). TNF can promote the maturation and survival of DCs in vitro (20, 21), but in circulating blood TNF is not sufficient for maturation of CD1c+ mDCs (9). The function and influence of TNF production by CD1c+ mDCs in the immune response to malaria is unclear. IL-10 is a regulatory cytokine that plays a key role in host survival, pathogen control, and the prevention of hyperinflammatory responses (22). In acute malarial infection, IL-10 has been implicated in mediating DC apoptosis (12). We sought here to understand whether CD1c+ mDCs produce these cytokines and whether prepatent P. falciparum infection altered their production.
Experimental human P. falciparum infection of malaria-naive healthy volunteers is a valuable model to evaluate immune cell maturation and function. First, this approach allows the assessment of responses before exposure and at subsequent time points after inoculation and, second, it allows comparison of the responses after infection with different doses of parasite-infected red blood cells (pRBCs) (150 pRBCs versus 1,800 pRBCs) (23). Because of limited current understanding of Plasmodium antigens processed by DCs and presented in the context of HLA-DR to CD4+ T cells, we measured cytokine production ex vivo and after stimulation with TLR ligands or pRBCs. TLRs are key pathogen recognition receptors involved in the initiation of the innate immune response (24). Differential expression of TLRs on DCs confers functional specialization of DC subsets. CD1c+ mDCs express a broad TLR repertoire, including TLR2 and TLR4 (5). P. falciparum glycosylphosphatidylinositol (GPI) is known to mediate inflammatory responses via TLR2 and TLR4 (25). Furthermore, changes in TLR expression and responses to the disease manifestation of malaria emphasize a role for TLRs in malaria pathogenesis (26–28). To better understand the response of CD1c+ mDCs in prepatent P. falciparum infection, we assessed CD1c+ mDCs directly ex vivo and after stimulation of three TLRs (TLR1/2, TLR4, and TLR7) with appropriate agonists or pRBCs.
Our data show CD1c+ mDCs are compromised during prepatent blood-stage Plasmodium infection, with reduced HLA-DR expression, at both infecting pRBC doses. CD1c+ mDCs exhibited reduced CD86 expression and increased production of TNF, with no change in IL-12 and no detectable IL-10 production. Furthermore, CD4+ and CD8+ T cell IL-2 and IFN-γ cytokine responses at peak parasitemia and 3 weeks after curative treatment remained stable from baseline. Taken together, results demonstrate the modulation of CD1c+ mDCs by Plasmodium associated with static effector T cell responses, which likely assists immune evasion and parasite expansion.
MATERIALS AND METHODS
Infection cohorts.
A total of 62 volunteers aged 19 to 41 years (median age, 25 years; interquartile range [IQR], 22 to 28 years; 34% female, 66% male) consented to participate in a phase Ib clinical trial to test the efficacy of antimalarial drugs. Volunteers enrolled in separate cohorts and received two different-sized parasite inocula as determined by quantitative PCR (qPCR), an objective of exploratory drug studies. The first cohorts (150 pRBCs, n = 12, 20%), received an intravenous inoculation of red cells containing ∼150 ring-stage parasites, and subsequent cohorts received ∼1,800 pRBCs (1,800 pRBCs, n = 50, 80%). Antimalarial drugs were administered when a threshold of ≥1,000 parasites/ml was confirmed by qPCR (29, 30) on day 10 (n = 1, 8%) or day 11 (n = 11, 91%) after the inoculation of 150 pRBCs and on day 7 (n = 19, 38%), day 8 (n = 30, 60%), or day 9 (n = 1, 2%) after the inoculation of 1,800 pRBCs (Fig. 1A). The protocol for the controlled human malaria infection studies has previously been reported (30); the details of the clinical trials and therapeutic response to the antimalarials in the present study will be reported elsewhere. Blood anticoagulated with lithium heparin was collected before inoculation and at the same time on days 7, 9, 10, and 11 (150 pRBCs) and on days 6, 7, and 8 (1,800 pRBCs) (Fig. 1A). Functional and flow cytometric assays used fresh whole blood processed <2 h after collection. Full blood counts were determined by an automated cell counter using EDTA blood (Beckman Coulter, USA).
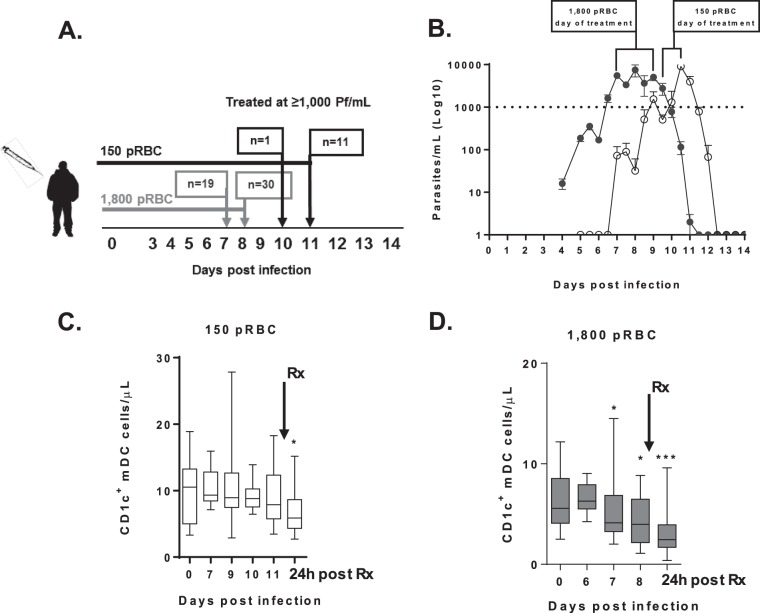
FIG 1.
Parasitemia and CD1c+ mDC absolute counts in participants infected with 150 pRBCs (white) or 1,800 pRBCs (gray). (A) Schematic of clinical trial cohorts, 150 pRBCs (black) and 1,800 pRBCs (gray). On the days specified, PCR, full blood counts, and immunological assays were performed. Arrows indicate the day of antimalarial treatment, and “n” represents the number of volunteers treated. (B) Parasitemia was determined by qPCR in participants infected with 150 pRBCs (white circles) or 1,800 pRBCs (gray circles). The dotted line indicates the predetermined parasite treatment threshold of 1,000 parasites/ml. Brackets represent the day of antimalarial treatment after infection with 1,800 pRBCs on day 7 (n = 19), day 8 (n = 30), or day 9 (n = 1) or with 150 pRBCs on day 10 (n = 1) or day 11 (n = 11). The mean parasitemia ± the standard error is presented. (C) Absolute number of circulating CD1c+ mDCs after 150-pRBC infection in 12 participants (24 h after drug treatment, P = 0.04; the exception being six individuals on days 7 and 10). (D) Absolute number of circulating CD1c+ mDCs after 1,800-pRBC infection in 21 participants (day 7, P = 0.05; day 8, P = 0.04; 24 h after drug treatment, P = 0.0002; the exception being 7 individuals on day 6 and 14 individuals on day 8 and after antimalarial drug treatment [Rx]). The box plot shows the minimum, maximum, median, and interquartile range for the data from all of the subjects.
Ethics.
Studies were approved by the Human Research Ethics Committees of QIMR Berghofer Medical Research Institute (P1479) and the Human Research Ethics Committee of Menzies School of Health Research, Australia (HREC 10/1431). Written and informed consent was obtained from all participants in the clinical trials. The clinical trial registration numbers for this study are ACTRN12611001203943, ACTRN12612000323820, ACTRN12612000814875, ACTRN12613000565741, ACTRN12613001040752, and NCT02281344.
CD1c+ mDC enumeration.
Two hundred microliters of blood was stained with surface antibodies (lineage markers CD3 [HIT3a], CD14 [HCD14], CD19 [HIB19], CD56 [HCD56], HLA-DR [L243], CD11c [B-Ly6], CD123 [6H6], CD1c [LI6I], CD16 [3G8], and CD141 [M80]), RBCs lysed with fluorescence-activated cell sorting (FACS) lysing solution (BD Biosciences), and cells fixed with 1% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS). Absolute numbers of CD1c+ mDCs were determined by adding automated lymphocyte and monocyte counts (109 cells/liter), dividing the sum by 100, multiplying the percentage of CD1c+ mDCs, and multiplying the product by 1,000 to give the cell count per microliter. For the 150-pRBC cohort CD1c+ mDCs were characterized as lineage negative (Lin−), HLA-DR+, CD11c+, CD123−, CD16−, and CD141− mononuclear cells (see Fig. S1A in the supplemental material) and for the 1,800-pRBC cohorts CD1c+ mDCs were characterized as Lin−, HLA-DR+, CD11c+, CD123−, and CD1c+ (BDCA-1; see Fig. S1B in the supplemental material). Lymphocyte subsets were assessed in whole blood. T cells, B cells, and NK cells were gated by forward- and side-scatter properties and the lack of CD14 expression and differentiated by HLA-DR expression (see Fig. S1C in the supplemental material).
Apoptosis.
Intracellular active caspase-3 staining was assessed as previously described (31). Briefly, 1,000 μl of blood was stained with surface antibodies, RBCs were lysed with FACS lysing solution (BD), and the cells were permeabilized using 1× BD Perm/Wash (BD) and stained with active caspase-3 antibody (C92-605; BD).
Antigen uptake.
CD1c+ mDC phagocytosis was assessed by the uptake of 1 mg/ml fluorescein isothiocyanate (FITC)-dextran (Sigma, USA) after 60 min at 37°C or on ice as a control and expressed as the change in the median fluorescence intensity (ΔMFI; i.e., ΔMFI = the MFI of treated cells at 37°C − the MFI of control cells on ice).
T cell activation.
T cell proliferation was assessed by the expression of Ki-67 (B56). In brief, 1 million peripheral blood mononuclear cells (PBMCs) were stained with surface antibodies (CD4 [SK4], CD3 [UCHT1], and CD8 [RPA-T8]), washed with 2% fetal calf serum (FCS)/PBS, permeabilized with 1× BD Perm/Wash, and stained with intracellular Ki-67.
Intracellular cytokine staining.
Cytokine production was assessed in 300 μl (T cells) or 1,000 μl (CD1c+ mDC) of blood stimulated with TLR agonists (TLR1, Pam3CSK4, 100 ng/ml; TLR2, HKLM, 108 cells/ml; TLR4, Escherichia coli K-12 lipopolysaccharide, 200 ng/ml, or TLR7, imiquimod, 2.5 μg/ml [Sigma-Aldrich]), pRBCs, or unparasitized RBCs (uRBCs) at a final concentration of 5 million (CD1c+ mDCs) or 1 million (T cells) pRBCs or uRBCs/ml in the presence of anti-CD28 and anti-CD49d antibodies (BD) at 1 μg/ml for T cells. Protein transport inhibitor (brefeldin A; GolgiPlug; BD) was added after 2 h (CD1c+ mDCs) or after 20 h (T cells) at 37°C and 5% CO2. At 6 or 24 h, the cells were stained to identify CD1c+ mDCs (including CD86 [IT2.2]) or T cells (CD4 [OKT4] and CD8 [SK1]). The RBCs were lysed with FACS lysing solution (BD) and washed with 2% FCS/PBS, and the cells were permeabilized with 1× Perm/Wash or Perm 2 (BD) and then stained with intracellular anti-TNF-α (MAB11), IL-12/IL-23p40 (C11.5), IL-10 (JES3-9D7), IFN-γ (B27), IL-2 (MQ1-17H12), or IgG1 isotype controls. FACS data were acquired using a FACSCanto II and LSRFortessa 4 (BD), and the data were analyzed using a Kaluza 1.3 (Beckman Coulter, USA) or FlowJo (FlowJo, LLC, USA).
P. falciparum-infected pRBCs.
P. falciparum K1 (from MR4, part of the BEI Resources Repository, National Institute of Allergy and Infectious Disease, National Institutes of Health [P. falciparum K1, MRA-159, deposited by D. E. Kyle]) was cultured in human RBCs as previously described (32). In brief, the culture supernatant was washed in sterile PBS, and schizonts and trophozoites were isolated using density centrifugation. The washed culture supernatant was layered onto 63% Percoll and 27% Percoll and then centrifuged continuously at 2,000 rpm for 20 min. The upper (and if necessary the lower) layers were removed, and the schizonts and trophozoites were collected from the 63 to 27% interface and washed with PBS. Thin smears were used to check the purity, and the pRBCs or uRBCs were enumerated. Stocks were frozen in glycerol–30% freezing medium at a final concentration of 150 × 106 pRBCs/ml and added to assays immediately after thawing.
Statistics.
Statistical analyses used GraphPad Prism 6 (GraphPad Software, Inc., USA). A Wilcoxon matched-pair test was used to compare longitudinal data. The tests were two-tailed, and results were considered significant if the P values were <0.05.
RESULTS
Effect of infecting pRBC dose on CD1c+ mDCs.
Circulating CD1c+ mDCs were examined in volunteers prior to and during experimental P. falciparum infection with 150 or 1,800 pRBCs. The mean parasitemia levels determined by PCR for subjects infected with either dose are shown in Fig. 1B. Subjects administered 150 pRBCs retained circulating CD1c+ mDCs at peak parasitemia (day 11 median parasitemia, 2,555/ml [IQR = 781 to 4,753]), with a decline 24 h after treatment (Fig. 1C). We observed no change in circulating lymphocytes, CD4+ T cells, CD56+ NK cells, or CD20+ B cells, whereas monocytes significantly increased on day 7 (see Table S1 in the supplemental material). In contrast, subjects administered 1,800 pRBCs had a significant decline in circulating CD1c+ mDCs from day 7 (median parasitemia, 4,577/ml [IQR = 1,645 to 10,066], n = 21; Fig. 1D), with the decline persisting on day 8 (median parasitemia, 9,073/ml [IQR = 4,755 to 21,857], n = 14) and 24 h after drug treatment (Fig. 1D). The higher inoculation dose caused a decline in circulating lymphocytes, CD4+ T cells, and CD56+ NK cells on day 8 and 24 h after treatment but did not impact monocyte counts (see Table S1 in the supplemental material). Circulating B cells were reduced only 24 h after antimalarial drug therapy (see Table S1 in the supplemental material).
Circulating CD1c+ mDCs were examined to determine whether there was active caspase-3 staining. Caspase-3 is an executioner caspase, essential to intrinsic and extrinsic apoptotic pathways and apoptosis occurs upon cleavage of caspase-3 (33). Among subjects administered 150 pRBCs, no active caspase-3+ was detected on day 10 to 11 in CD1c+ mDCs (median, 0.3% [IQR = 0 to 0.7%] caspase-3+) (see Fig. S2 in the supplemental material) at a median parasitemia of 2,555/ml (IQR = 781 to 4,753). In contrast, on day 7 to 8 after infection with 1,800 pRBCs, there was a trend toward increased active caspase-3+ expression by CD1c+ mDCs (median, 5% [IQR = 1.5 to 7.3%]); caspase-3+ [see Fig. S2 in the supplemental material], P = 0.08) at a median parasitemia of 6,877/ml (IQR = 4,182 to 20,398). In the 1,800-pRBC cohort, one participant with 17 times more active caspase-3+ staining was excluded (outlier; caspase-3+ on day 7, 85% versus a cohort median of 5%). When originally included in the analysis, a significant increase in active caspase-3+ expression after 1,800-pRBC infection was observed (P = 0.04).
Reduced HLA-DR and CD86 expression on circulating CD1c+ mDCs.
We next examined the impact of the infecting pRBC dose on HLA-DR expression by CD1c+ mDCs. In accord with previous reports, HLA-DR expression was significantly higher on CD1c+ mDCs compared to other human blood DC subsets (Fig. 2A). In cohorts inoculated with either 150 or 1,800 pRBCs, there was a significant reduction in CD1c+ mDC HLA-DR expression at peak parasitemia (days 10 to 11 for 150 pRBCs, median parasitemia of 2,555/ml [IQR = 781 to 4,753]; days 7 to 8 for 1,800 pRBCs, median parasitemia of 4,577/ml [IQR = 1,645 to 10,066]; Fig. 2B). In a subgroup of participants (n = 14), longitudinal HLA-DR MFI was assessed 24 h before and after antimalarial drug treatment (see Fig. S3A in the supplemental material). Before peak parasitemia, there was no reduction in HLA-DR MFI, and at 24 h after drug treatment, reduced HLA-DR MFI failed to recover (see Fig. S3B and C in the supplemental material). There was no change in monocyte HLA-DR expression at peak parasitemia after infection with 1,800 pRBCs (Fig. 2C).
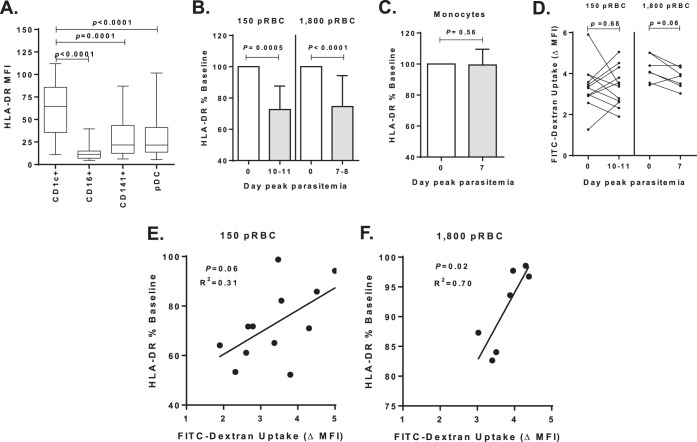
FIG 2.
HLA-DR expression on CD1c+ mDCs. (A) HLA-DR expression on DC subsets: CD1c+ mDCs (n = 21), CD16+ mDCs (n = 26), CD141+ mDCs (n = 33), and plasmacytoid DCs (n = 33) in participants at baseline (day 0). (B) CD1c+ mDC HLA-DR MFI (%) at baseline (day 0) after inoculations of 150 pRBCs (12 participants) or 1,800 pRBCs (21 participants) and peak parasitemia (days 10 and 11 or days 7 and 8, respectively). (C) Monocyte HLA-DR MFI (%) baseline after 1,800-pRBC infection in six participants. (D) Uptake of particulate antigen by CD1c+ mDCs after 150-pRBC infection (12 participants, left graph) or 1,800-pRBC infection (7 participants, right graph). The ΔMFI of FITC-dextran uptake (calculated as the MFI for cells incubated at 37°C – the MFI for cells incubated on ice). (E) Association between day 11 (peak parasitemia) baseline HLA-DR MFI and FITC-dextran uptake after 150-pRBC infection. (F) Association between day 7 (peak parasitemia) baseline HLA-DR MFI and FITC-dextran uptake after 1,800-pRBC infection. Statistics were calculated using the Wilcoxon matched-paired test and linear regression. MFI, median fluorescence intensity.
The impact of 150 or 1,800 pRBCs on circulating CD1c+ mDC phagocytosis was examined using FITC-dextran particles. There was no significant change in particle uptake after 150-pRBC infection (Fig. 2D) and a trend toward reduced uptake by day 7 after 1,800-pRBC infection (Fig. 2D). Importantly, there was a significant positive association between HLA-DR expression and FITC-dextran uptake after inoculation of 1,800 pRBCs (Fig. 2F) and a trend suggesting weak a association for the 150-pRBC cohort (Fig. 2E). These data imply that CD1c+ mDC HLA-DR expression is proportional to phagocytic capacity.
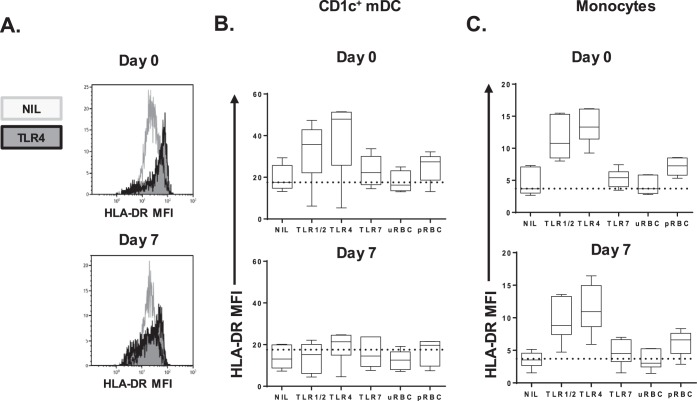
In the 1,800-pRBC cohort, at baseline and peak parasitemia, HLA-DR expression was further assessed ex vivo and after TLR stimulation to determine the capacity of CD1c+ mDC to respond to external stimuli (Fig. 3A). Before infection, CD1c+ mDCs drastically increased HLA-DR expression upon TLR1/2 or TLR4 stimulation and moderately upon TLR7 stimulation or P. falciparum-infected pRBC stimulation (Fig. 3B, top panel). In contrast, at peak parasitemia the CD1c+ mDCs failed to upregulate HLA-DR in response to any TLRs or pRBCs (Fig. 3B, bottom panel). HLA-DR expression was significantly impaired directly ex vivo and across all stimulatory conditions tested (P = 0.03, day 0 versus day 7 for all conditions). In contrast, monocytes from the same blood sample retained the ability to upregulate HLA-DR at peak parasitemia after stimulation with P. falciparum-infected pRBCs or TLR ligands (Fig. 3C, bottom and top panels). The similar magnitudes of HLA-DR expression by monocytes on days 0 and 7 (peak parasitemia) highlight the assay's reproducibility.
FIG 3.
CD1c+ mDC HLA-DR expression after TLR or pRBC stimulation. (A) Representative histograms of HLA-DR MFI on whole-blood CD1c+ mDCs in one individual on days 0 and 7. (B) CD1c+ mDC HLA-DR expression in six participants on day 0 (top graph) and day 7 (bottom graph). The dotted line shows the median HLA-DR MFI on day 0 for the control (NIL) condition (median = 17.6). (C) Monocyte HLA-DR expression in six participants on day 0 (top graph) and day 7 (bottom graph). The dotted line shows the median HLA-DR MFI on day 0 for the control (NIL) condition (median = 4). MFI, median fluorescence intensity; uRBC, uninfected red blood cells; pRBC, parasitized red blood cells.
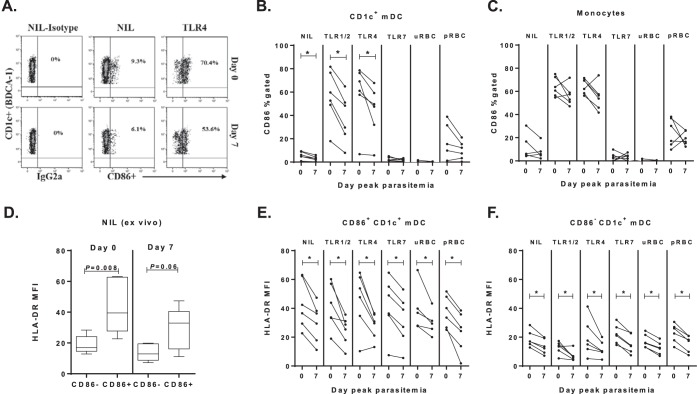
Expression of the costimulatory maturation marker CD86 was next examined on CD1c+ mDCs (Fig. 4A) and monocytes in the 1,800-pRBC cohort. At peak parasitemia (day 7), CD86 expression on circulating CD1c+ mDC was significantly reduced directly ex vivo and after TLR stimulation (Fig. 4B). In contrast, circulating monocytes showed unaltered CD86 expression at peak parasitemia ex vivo or in response to TLR ligands or pRBCs (Fig. 4C). CD86+ and CD86− CD1c+ mDCs were more closely examined for HLA-DR expression. As expected, CD86+ CD1c+ mDCs expressed significantly more HLA-DR than did CD86− CD1c+ mDCs at baseline and at peak parasitemia (Fig. 4D). At peak parasitemia, the HLA-DR expression was significantly reduced on both the CD86+ and CD86− CD1c+ mDCs ex vivo and across stimulatory conditions (Fig. 4E and F). The results show that CD86 expression (albeit reduced) can be induced; however, HLA-DR is clearly impaired on these DCs even after TLR stimulation.
FIG 4.
CD1c+ mDC CD86 expression at peak parasitemia. (A) Representative gating strategy for CD86 on whole-blood CD1c+ mDCs in one individual on day 0 and day 7. (B) The paired frequency of CD86+ CD1c+ mDCs in six participants at day 0 (baseline) and day 7 (peak-parasitemia). The ex vivo (NIL), post-TLR, and uRBC or pRBC stimulation data are shown.(C) The paired frequency of CD86+ CD14+ monocytes in six participants day 0 (baseline) compared to day 7 (peak parasitemia). (D) HLA-DR expression on CD86+ compared to CD86− CD1c+ mDCs at day 0 (baseline) and day 7 (peak parasitemia). A Mann-Whitney test was used for comparison between cell subsets. (E) HLA-DR expression on CD86+ CD1c+ mDCs at day 0 (baseline) and day 7 (peak parasitemia) in six participants. (F) HLA-DR expression on CD86− CD1c+ mDCs at day 0 (baseline) and day 7 (peak parasitemia) in six participants. A Wilcoxon matched-paired test was used for comparison between day 0 and day 7. *, P = 0.03.
Increased TNF and stable IL-12 production by circulating CD1c+ mDCs.
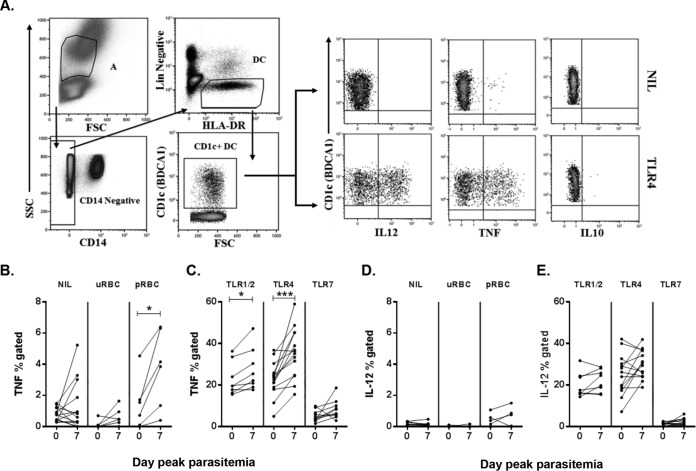
In participants infected with 1,800 pRBCs, the ability of CD1c+ mDCs to produce TNF, IL-12, and IL-10 was simultaneously assessed in response to TLR ligands and P. falciparum-infected pRBCs (Fig. 5A). At peak parasitemia, CD1c+ mDCs significantly increased TNF production in response to P. falciparum-infected pRBC stimulation (Fig. 5B). CD1c+ mDCs also significantly increased TNF production upon combined TLR1/2 or TLR4 stimulation but not upon TLR7 stimulation (Fig. 5C). Ex vivo (nil or uRBC) cells showed no statistically significant change in spontaneous TNF production between baseline and peak parasitemia (Fig. 5B). There was no consistent change in CD1c+ mDC IL-12 production in response to pRBCs (Fig. 5D) or any TLR stimulation (Fig. 5E).
FIG 5.
CD1c+ mDC cytokine responsiveness to TLR or pRBC stimulation. (A) Representative staining of blood CD1c+ mDCs for intracellular cytokines. CD1c+ mDCs were identified as negative for lineage markers (CD14, CD3, CD19, and CD56), HLA-DR+, and CD1c+. Intracellular cytokine production by CD1c+ mDCs on day 0 (IL-12, TNF, and IL-10) under two conditions, ex vivo (NIL; top panel) and TLR4 (bottom panel), was determined. (B) TNF production on day 0 and day 7 ex vivo (NIL) and after uRBC or pRBC (P = 0.03) stimulation. (C) TNF production on day 0 and day 7 after TLR1/2 (P = 0.02), TLR4 (P = 0.0002), or TLR7 stimulation. (D) IL-12 production on day 0 and day 7 ex vivo (NIL) and after uRBC or pRBC stimulation. (E) IL-12 production on day 0 and day 7 after TLR stimulation. A Wilcoxon matched-paired test was used for comparison between days 0 and day 7. *, P < 0.05; ***, P = 0.0002. Line graphs show data for all subjects (n = 14; the exceptions include 8 individuals for TLR1/2, 10 individuals for TLR7, and 6 individuals for uRBCs and pRBCs). FSC, forward scatter; SSC, side scatter; uRBC, uninfected red blood cells; pRBC, parasitized red blood cells.
The frequency of CD1c+ mDCs coproducing TNF and IL-12 in response to TLR1/2 or TLR4 remained stable from days 0 to 7 (TLR1/2 stimulation, day 0, 11% [IQR = 9 to 19%]; day 7, 12% [IQR = 10 to 20%]; P = 0.5; TLR4 stimulation, day 0, 17% [IQR = 10 to 23%]; day 7, 24% [IQR = 21 to 28%]; P = 0.09). No intracellular IL-10 was detected in CD1c+ mDCs either directly ex vivo or after stimulation at baseline or peak parasitemia despite validation of the assay with IL-10 detection in monocytes.
TNF is produced by CD86+ and CD86− CD1c+ mDCs in P. falciparum infection.
The phenotype of TNF-producing CD1c+ mDCs was further evaluated by assessment of CD86 expression and is represented in pie charts (see Fig. S4 in the supplemental material). For all of the stimulations except TLR7 stimulation, the majority of TNF-producing CD1c+ mDCs expressed CD86. At peak parasitemia, CD1c+ mDCs which lacked CD86 increased TNF production after TLR1/2, TLR4, or TLR7 stimulation (P = 0.06; see Fig. S4 in the supplemental material).
Stable CD4+ and CD8+ T cell cytokine responses.
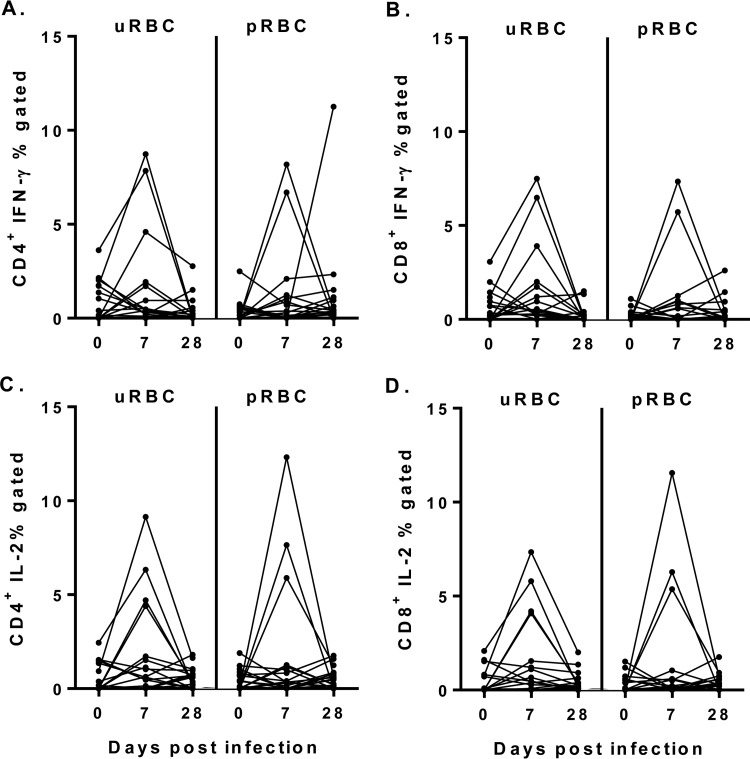
To determine whether P. falciparum impacted T cell cytokine production, CD4+ and CD8+ T cell responses were evaluated after infection with 1,800 pRBCs. There was no significant change in IFN-γ or IL-2 production by CD4+ or CD8+ T cells (Fig. 6) from baseline to peak parasitemia or 3 weeks after antimalarial drug treatment. Furthermore, there was no change in Ki-67 expression by CD4+ T cells (%Ki-67, day 0, 1.4 [IQR = 0.4 to 2.3] and 2.0 [IQR = 0.5 to 2.3] at peak parasitemia) or CD8+ T cells (%Ki-67, day 0, 0.7 [IQR = 0.5 to 2.4] and 1.2 [IQR = 0.7 to 1.8] at peak parasitemia) between baseline and peak parasitemia.
FIG 6.
Cytokine production by T cells after uRBC or pRBC stimulation on day 0, day 7, and day 28. (A) CD4+ T cell IFN-γ production. (B) CD8+ T cell IFN-γ production. (C) CD4+ T cell IL-2 production. (D) CD8+ T cell IL-2 production. A Wilcoxon matched-paired test was used for comparison between days 0 and 7 and days 0 and 28 for 19 participants.
DISCUSSION
This study demonstrates that a single experimental human P. falciparum blood-stage infection leads to downregulation of HLA-DR and CD86 expression on circulating CD1c+ mDC after either a 150- or a 1,800-pRBC inoculating dose. The Plasmodium-impacted CD1c+ mDCs do not upregulate HLA-DR in response to TLR or pRBC stimulation and display an increased propensity for TNF production and impaired phagocytic capacity. Interestingly, monocytes were not similarly impacted and maintained HLA-DR and CD86 expression, suggesting that Plasmodium modulation of HLA-DR is not generic. Taken together, prepatent P falciparum infection subverts CD1c+ mDCs phenotypically and functionally, compromising their ability to prime adaptive immune responses.
CD1c+ mDCs express significantly more HLA-DR than other DC subsets, suggesting a specialized role in antigen uptake and presentation. In prepatent P. falciparum infection, reduced HLA-DR expression on CD1c+ mDCs occurred at comparable parasite densities in both the 150- and the 1,800-pRBC cohorts (days 10 to 11 and days 7 to 8, respectively), indicating the parasite biomass rather than infection duration impacted HLA-DR expression. HLA-DR expression was an indication of phagocytosis ability since there was a significant association between CD1c+ mDC HLA-DR expression and FITC-dextran uptake. Further investigation is required to verify whether similar data are obtained by phagocytosis of P. falciparum-infected pRBCs. The persistent reduction of HLA-DR despite stimulation with different TLR agonists or P. falciparum-infected pRBCs indicates that impaired HLA-DR expression was not reversible or stimulus specific. In the rodent-Plasmodium yoelii model, intact pRBCs induce a similar general inhibition of TLR responsiveness in DCs (34). At peak infection, impaired antigen presentation by splenic DCs has been shown in vivo in the rodent-Plasmodium chabaudi model (35). In addition, the loss of total mDC HLA-DR has been noted in infections with helminths (36), Salmonella (37), and herpes simplex virus (38) and in cases of severe sepsis (39), suggesting this is not a Plasmodium-specific phenomenon. As with helminth infections, where reduced HLA-DR on mDCs results in impaired T cell proliferation and activation (36), we demonstrate the absence of T cell activation, manifested by the lack of increased Ki-67, IFN-γ, or IL-2 production, with altered HLA-DRlo CD1c+ mDCs after P. falciparum infection.
P. falciparum compromised CD86 expression on CD1c+ mDCs, albeit to a lesser degree than did HLA-DR, suggesting different mechanisms and/or recovery of CD86 than MHC class II. Interestingly, both CD86+ CD1c+ mDCs and CD86− CD1c+ mDCs showed impaired HLA-DR expression. Reduction in HLA-DR and CD86 expression on CD1c+ mDCs after blood-stage infection contrasted with stable HLA-DR and CD86 expression during experimental P. falciparum sporozoite infection (40). The method of P. falciparum inoculation, intravenous pRBCs versus intradermal sporozoites, is a possible explanation for this difference. In acute HIV infection (41) and pancreatitis patients (42), CD86 expression is reduced in lymphoid tissue migrating DCs and circulating monocytes. In vitro, P. falciparum pRBCs inhibit monocyte-derived DC maturation of HLA-DR and CD86 expression via contact-dependent (43) or contact-independent (44) mechanisms at high concentrations. Our data support these in vitro studies and demonstrate that P. falciparum compromises CD1c+ mDC CD86 expression in vivo, already at a very low parasite density.
The cytokine profile of CD1c+ mDCs in P. falciparum infection has not previously been reported. In prepatent P. falciparum infection, we characterized a proinflammatory cytokine profile, with stable IL-12, absent IL-10, and increased TNF production. Despite compromised HLA-DR and CD86 expression, CD1c+ mDCs increased TNF production upon TLR1/2, TLR4, or pRBC stimulation. At peak parasitemia, HLA-DRlo CD86− CD1c+ mDCs increased their proportion of TNF production. The consequences of the enhanced TNF remain to be determined. TNF can promote DC maturation and survival in vitro (20, 21). However, proinflammatory cytokines such as TNF are not sufficient for the full functional maturation of DCs (9), defined as the DCs' ability to induce effector T cell responses (26). The increased TNF responses reported in the present study concur with increased PBMC TNF production (plus IL-1β, IL-6, and IL-10) upon TLR1/2 and TLR4 stimulation after experimental P. falciparum sporozoite infection (27). The upregulation of TLR expression may explain the increased TNF production, since acute malaria increases mDC and monocyte TLR2 and TLR4 expression (28, 45). Our IL-12 data support previous reports of absent TLR responses in healthy CD1c+ mDC after stimulation by a single TLR (i.e., TLR2, -3, -4, -7, -8, or -9) (9). The apparent inability of CD1c+ mDCs to increase IL-12 production may impact adaptive immunity since IL-12 is essential for priming of naive CD4+ T cells (15). The design of our study, with a drug cure of parasitemia at a predetermined threshold of 1,000 parasites/ml, precluded the examination of associations between TNF production and clinical symptoms or subsequent parasite biomass.
The decline in circulating CD1c+ mDCs in prepatent P. falciparum infection has not previously been reported. The total blood mDCs are reduced in experimental infections (11), and CD1c+ mDCs are reduced in acute malaria (12, 46), but importantly in areas of malaria endemicity, there is a preservation of blood CD1c+ mDC in asymptomatic carriers of P. falciparum with patent parasitemia (46). Interestingly, after the 150-pRBC infections the circulating CD1c+ mDCs were stable. Caspase-3 staining of CD1c+ mDCs after the 1,800-pRBC infection but not after the 150-pRBC infection indicates apoptosis, which might partially explain differences in peripheral mDC stability. Differences between the 1,800- and 150-pRBC cohorts suggest that the inoculating concentration has considerable impact on the viability of circulating CD1c+ mDCs. Migration is another likely explanation for DC loss from the periphery, since reduced plasmacytoid DCs in uncomplicated malaria is attributed to expression of the lymph node migration chemokine CCR7 (47).
How malaria compromises HLA-DR and CD86 expression remains to be determined. Complexities in MHC class II antigen-processing pathways provide extensive opportunities for pathogen interference (48). Indeed, Salmonella can reduce HLA-DR surface expression by enhancing ubiquitination of MHC class II (49). MARCH I, a ubiquitin ligase, is capable of mediating ubiquitination of MHC class II (50). It has been proposed that TLR stimulation of monocyte-derived DCs downregulates MARCH I and that HLA-DR accumulates on the cell surface (51). However, in early P. falciparum infections, we show that there is reduced HLA-DR expression on CD1c+ mDCs with sustained repression upon TLR stimulation. Additional studies are required to identify the causes of HLA-DR downregulation and to further assess the role of CD1c+ mDCs in the generation and maintenance of effector T cell responses and malaria immunity.
CD1c+ mDCs perform a crucial role in the early steps of the adaptive immune response: they process and present antigens (via MHC classes I and II), deliver costimulation (CD86), signal via TLR, and produce and secrete cytokines to initiate or shape cellular adaptive immune responses. We report here alterations in each of these vital functions by P. falciparum, reduced HLA-DR expression (and failed upregulation), reduced CD86, and increased TNF but not IL-12. P. falciparum even at low parasitemia rapidly and comprehensively compromises CD1c+ mDC maturation and functionality, potentially contributing to the failed enhancement of T cell cytokine responses and immune evasion by P. falciparum. Candidate malaria vaccines should avoid these deleterious responses and instead aim to mature and activate CD1c+ mDC function.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Q-pharm staff who conducted the human infection studies and, in particular, Suzanne Elliot, Nannette Douglas, and Gem Mackenroth for supporting the research. We thank Paul Griffin for advice and technical assistance. We thank Steven Kho, who kindly prepared the pRBCs. We thank the Australian Red Cross Blood Service for their support. The clinical trials from which the samples were drawn were funded by the Medicines for Malaria Venture. We thank the volunteers who participated in all clinical trials.
J.R.L., G.M., and T.W. conceived and designed the experiments and prepared the manuscript. J.R.L., G.M., T.W., and P.E.T. performed the experiments with assistance from K.A.P. and F.H.A. J.R.L., G.M., T.W., and J.B. analyzed the data. N.M.A., M.F.G., C.R.E., and D.L.D. provided intellectual input and assisted with manuscript preparation. J.S.M. conducted the clinical trial and assisted with manuscript preparation.
This study was supported by the Australian National Health and Medical Research Council (NHMRC) (project grant 1021198, program grant 1037304, and fellowships to N.M.A., J.S.M., C.E., D.L.D., M.F.G., and G.M.). The views expressed in this publication are those of the authors and do not reflect the views of the NHMRC. J.R.L. was supported in part by an APA Ph.D. scholarship; J.B. was supported in part by a University of Queensland International Scholarship.
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01522-15.
REFERENCES
- 1.World Health Organization. 2014. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Wykes MN, Good MF. 2008. What really happens to dendritic cells during malaria? Nat Rev Microbiol 6:864–870. doi: 10.1038/nrmicro1988. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Liu YJ. 2002. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 4.Ju X, Clark G, Hart D. 2010. Review of human DC subtypes. Methods Mol Biol 595:3. doi: 10.1007/978-1-60761-421-0_1. [DOI] [PubMed] [Google Scholar]
- 5.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJJ, Dunbar P, Wadley RB, Jeet V. 2010. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 207:1247. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassianos AJ, Hardy MY, Ju X, Vijayan D, Ding Y, Vulink AJE, McDonald KJ, Jongbloed SL, Wadley RB, Wells C. 2012. Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immunoregulatory phenotype and function in response to Escherichia coli. Eur J Immunol 42:1512–1522. doi: 10.1002/eji.201142098. [DOI] [PubMed] [Google Scholar]
- 7.Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, Bianco A, Steckel B, Moro M, Crosti M. 2013. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122:932–942. doi: 10.1182/blood-2013-04-495424. [DOI] [PubMed] [Google Scholar]
- 8.Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. 2011. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol 186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 9.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. 2007. Functional specialization of human circulating CD16 and CD1c myeloid dendritic cell subsets. Blood 109:5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 11.Woodberry T, Minigo G, Piera KA, Amante FH, Pinzon-Charry A, Good MF, Lopez JA, Engwerda CR, McCarthy JS, Anstey NM. 2012. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J Infect Dis 206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]
- 12.Pinzon-Charry A, Woodberry T, Kienzle V, McPhun V, Minigo G, Lampah DA, Kenangalem E, Engwerda C, López JA, Anstey NM. 2013. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med 210:1635–1646. doi: 10.1084/jem.20121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arama C, Giusti P, Bostrom S, Dara V, Traore B, Dolo A, Doumbo O, Varani S, Troye-Blomberg M. 2011. Interethnic differences in antigen-presenting cell activation and TLR responses in Malian children during Plasmodium falciparum malaria. PLoS One 6:e18319. doi: 10.1371/journal.pone.0018319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban BC, Cordery D, Shafi MJ, Bull PC, Newbold CI, Williams TN, Marsh K. 2006. The frequency of BDCA3-positive dendritic cells is increased in the peripheral circulation of Kenyan children with severe malaria. Infect Immun 74:6700–6706. doi: 10.1128/IAI.00861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 16.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T. 2009. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 17.Moormann AM, Sumba PO, Chelimo K, Fang H, Tisch DJ, Dent AE, John CC, Long CA, Vulule J, Kazura JW. 2013. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis 208:149–158. doi: 10.1093/infdis/jit134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bejon P, Mwacharo J, Kai O, Todryk S, Keating S, Lowe B, Lang T, Mwangi TW, Gilbert SC, Peshu N. 2007. The induction and persistence of T cell IFN-γ responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol 179:4193–4201. doi: 10.4049/jimmunol.179.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCall MB, Sauerwein RW. 2010. Interferon-γ- central mediator of protective immune responses against the pre-erythrocytic and blood-stage of malaria. J Leukoc Biol 88:1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludewig B, Graf D, Gelderblom HR, Becker Y, Kroczek RA, Pauli G. 1995. Spontaneous apoptosis of dendritic cells is efficiently inhibited by TRAP (CD40-ligand) and TNF-α, but strongly enhanced by interleukin-10. Eur J Immunol 25:1943–1950. doi: 10.1002/eji.1830250722. [DOI] [PubMed] [Google Scholar]
- 22.Couper KN, Blount DG, Riley EM. 2008. IL-10: the master regulator of immunity to infection. J Immunol 180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 23.Engwerda CR, Minigo G, Amante FH, McCarthy JS. 2012. Experimentally induced blood-stage malaria infection as a tool for clinical research. Trends Parasitol 28:515–521. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson E, Sampaio N, Schofield L. 2013. Toll-like receptors and malaria: sensing and susceptibility. J Trop Dis 2:2. [Google Scholar]
- 25.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. 2005. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maney NJ, Reynolds G, Krippner-Heidenreich A, Hilkens CM. 2014. Dendritic cell maturation and survival are differentially regulated by TNFR1 and TNFR2. J Immunol 193:4914–4923. doi: 10.4049/jimmunol.1302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall MB, Netea MG, Hermsen CC, Jansen T, Jacobs L, Golenbock D, van der Ven AJ, Sauerwein RW. 2007. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol 179:162–171. doi: 10.4049/jimmunol.179.1.162. [DOI] [PubMed] [Google Scholar]
- 28.Franklin BS, Parroche P, Ataíde MA, Lauw F, Ropert C, de Oliveira RB, Pereira D, Tada MS, Nogueira P, da Silva LHP. 2009. Malaria primes the innate immune response due to interferon-γ induced enhancement of Toll-like receptor expression and function. Proc Natl Acad Sci U S A 106:5789–5794. doi: 10.1073/pnas.0809742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockett R, Tozer S, Peatey C, Bialasiewicz S, Whiley D, Nissen M, Trenholme K, McCarthy J, Sloots T. 2011. A real-time, quantitative PCR method using hydrolysis probes for the monitoring of Plasmodium falciparum load in experimentally infected human volunteers. Malaria J 10:48. doi: 10.1186/1475-2875-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, Peatey C, Rockett R, O'Rourke P, Marquart L, Hermsen C. 2011. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 6:e21914. doi: 10.1371/journal.pone.0021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerome K, Sloan D, Aubert M. 2003. Measurement of CTL-induced cytotoxicity: the caspase 3 assay. Apoptosis 8:563–571. doi: 10.1023/A:1026123223387. [DOI] [PubMed] [Google Scholar]
- 32.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 33.Cagnol S, Chambard JC. 2010. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J 277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 34.Bettiol E, Carapau D, Galan-Rodriguez C, Ocaña-Morgner C, Rodriguez A. 2010. Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation. Malar J 9:64. doi: 10.1186/1475-2875-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sponaas AM, Cadman ET, Voisine C, Harrison V, Boonstra A, O'Garra A, Langhorne J. 2006. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J Exp Med 203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. 2010. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis 4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell EK, Mastroeni P, Kelly AP, Trowsdale J. 2004. Inhibition of cell surface MHC class II expression by Salmonella. Eur J Immunol 34:2559–2567. doi: 10.1002/eji.200425314. [DOI] [PubMed] [Google Scholar]
- 38.Neumann J, Eis-Hübinger AM, Koch N. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol 171:3075–3083. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi D, Louis S, Pene F, Sirgo G, Rousseau C, Claessens Y, Vimeux L, Cariou A, Mira J, Hosmalin A. 2011. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med 37:1438–1446. doi: 10.1007/s00134-011-2306-1. [DOI] [PubMed] [Google Scholar]
- 40.Teirlinck AC, Roestenberg M, Bijker EM, Hoffman SL, Sauerwein RW, Scholzen A. 2015. Plasmodium falciparum infection of human volunteers activates monocytes and CD16+ dendritic cells and induces up-regulation of CD16 and CD1c expression. Infect Immun 83:3732–3739. doi: 10.1128/IAI.00473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loré K, Sönnerborg A, Broström C, Goh L-E, Perrin L, McDade H, Stellbrink H-J, Gazzard B, Weber R, Napolitano LA. 2002. Accumulation of DC-SIGN+ CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16:683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 42.Wolk K, Höflich C, Zuckermann-Becker H, Döcke W-D, Volk H-D, Sabat R. 2007. Reduced monocyte CD86 expression in postinflammatory immunodeficiency. Crit Care Med 35:458–467. doi: 10.1097/01.CCM.0000254724.54515.2F. [DOI] [PubMed] [Google Scholar]
- 43.Urban BC, Ferguson DJP, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 44.Elliott SR, Spurck TP, Dodin JM, Maier AG, Voss TS, Yosaatmadja F, Payne PD, McFadden GI, Cowman AF, Rogerson SJ. 2007. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun 75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loharungsikul S, Troye-Blomberg M, Amoudruz P, Pichyangkul S, Yongvanitchit K, Looareesuwan S, Mahakunkijcharoen Y, Sarntivijai S, Khusmith S. 2008. Expression of Toll-like receptors on antigen-presenting cells in patients with falciparum malaria. Acta Trop 105:10–15. doi: 10.1016/j.actatropica.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Kho S, Marfurt J, Noviyanti R, Kusuma A, Piera KA, Burdam FH, Kenangalem E, Lampah DA, Engwerda CR, Poespoprodjo JR. 2015. Preserved dendritic cell HLA-DR expression and reduced regulatory T cell activation in asymptomatic Plasmodium falciparum and P. vivax infection. Infect Immun 83:3224–3232. doi: 10.1128/IAI.00226-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, Stewart V, Hasegawa H, Looareesuwan S. 2004. Malaria blood-stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol 172:4926. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 48.Brodsky FM, Lem L, Solache A, Bennett EM. 1999. Human pathogen subversion of antigen presentation. Immunol Rev 168:199–215. doi: 10.1111/j.1600-065X.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 49.Lapaque N, Hutchinson JL, Jones DC, Méresse S, Holden DW, Trowsdale J, Kelly AP. 2009. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Natl Acad Sci U S A 106:14052–14057. doi: 10.1073/pnas.0906735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, Koseki H, Ohara O, Nakayama M. 2007. Novel regulation of MHC class II function in B cells. EMBO J 26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. 2008. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I downregulation. Proc Natl Acad Sci U S A 105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.